Sulodeksid za liječenje venskih ulkusa nogu
Abstract
Background
Venous leg ulcers are common, chronic wounds caused by venous diseases, with a high recurrence rate and heavy disease burden. Compression therapy (bandages or stockings) is the first choice treatment for venous leg ulcers. However, when ulcers remain unhealed, medication can also be used with or without compression therapy. Sulodexide, a highly purified glycosaminoglycan (a naturally occurring molecule) has antithrombotic and profibrinolytic properties (it reduces the formation of blood clots) as well as anti‐inflammatory effects. Sulodexide has been studied as a potential treatment for venous leg ulcers.
Objectives
To assess the efficacy and safety of sulodexide for treating venous leg ulcers.
Search methods
In July 2015 we searched: The Cochrane Wounds Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; EBSCO CINAHL; Chinese Biomedical Literature Database (CBM); China National Knowledge Infrastructure Database (CNKI); Wan Fang and VIP. We also searched clinical trials registries to identify ongoing studies, as well as references listed in relevant publications. There were no restrictions based on date of publication, language or study setting.
Selection criteria
Randomised controlled trials (RCTs) involving people with a diagnosis of venous leg ulcers which compared sulodexide with placebo or any other drug therapy (such as pentoxifylline, flavonoids, aspirin), with or without compression therapy.
Data collection and analysis
We used standard Cochrane methodological procedures. The authors independently selected studies, extracted data and assessed risk of bias. We pooled data to present the risk ratio (RR) with 95% confidence interval (CI), or presented a narrative summary. We assessed overall evidence quality according to the GRADE approach.
Main results
We included four RCTs with a total of 463 participants (aged 42 years to 93 years); one report was only available as a published abstract.
Meta‐analysis of three RCTs suggests an increase in the proportion of ulcers completely healed with sulodexide as an adjuvant to local treatment (including wound care and compression therapy) compared with local treatment alone (rate of complete healing with sulodexide 49.4% compared with 29.8% with local treatment alone; RR 1.66; 95% CI 1.30 to 2.12). This evidence for sulodexide increasing the rate of complete healing is low quality due to risk of bias. It is unclear whether sulodexide is associated with any increase in adverse events (4.4% with sulodexide versus 3.1% with no sulodexide; RR 1.44; 95% CI 0.48 to 4.34). The evidence for adverse events is very low quality, downgraded twice for risk of bias and once for imprecision.
Authors' conclusions
Sulodexide may increase the healing of venous ulcers, when used alongside local wound care, however the evidence is only low quality and the conclusion is likely to be affected by new research. It is not clear whether sulodexide is associated with adverse effects. The standard dosage, route and frequency of sulodexide reported in the trials was unclear. Further rigorous, adequately powered RCTs examining the effects of sulodexide on healing, ulcer recurrence, quality of life and costs are necessary.
PICO
Laički sažetak
Sulodeksid za liječenje venskih ulkusa nogu
Istraživačko pitanje
U ovom Cochrane sustavnom pregledu analizirani su dokazi o učinku sulodeksida na liječenje ljudi s venskim ulkusima nogu.
Dosadašnje spoznaje
Venski ulkusi na nogama su čest oblik dugotrajnih (kroničnih) rana koje se opetovano javljaju. Uzrokuju ih bolesti vena, posebice u starijih. Za liječenje venskih ulkusa noge koristi se kompresijska terapija (elastični zavoji ili čarape). Međutim, ta terapija za neke nije pogodna, a neki ulkusi ne zacijele uz kompresiju. Sulodeksid (lijek koji smanjuje zgrušavanje krvi) istraživan je kao mogući lijek za venske ulkuse nogu. U ovom Cochrane sustavnom pregledu literature analizirano je postoje li dobri dokazi da sulodeksid poboljšava liječenje ulkusa.
Obilježja uključenih istraživanja
Ovaj pregled literature uključio je 4 istraživanja, s ukupno 463 bolesnika u dobi između 42 i 93 godine. Istraživanja su uspoređivala sulodeksid korišten u kombinaciji s lokalnom terapijom (kompresija i njega rana) sa samom lokalnom terapijom. Istraživanja su trajala je od jednog do tri mjeseca.
Ključni rezultati
Tri istraživanja (438 sudionika) upućuju na to da bi sulodeksid mogao poboljšati cieljenje rana, jer se udio ulkusa koji su potpuno zacijelili povećao s 29,8% (samo s lokalnom terapijom) na 49,4% (kada se koristi i sulodeksid). Niej jasno je je li sulodeksid povezan s većim brojem nuspojava (4,4% s njim naspram 3,1% bez njega).
Kvaliteta dokaza
Ukupna kvaliteta dokaza za svaki ishod varira između niske i vrlo niske, zbog rizika od pogrešaka i nepreciznosti (koji je, za neke ishode, rezultat malog broja istraživanja koji su bili dostupni).
Ovaj Cochrane sustavni pregled uključuje studije objavljene do 1. srpnja 2015. godine.
Authors' conclusions
Summary of findings
| Sulodexide + local treatment compared to local treatment for treating venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| local treatment | Sulodexide + local treatment | |||||
| Proportion of ulcers completely healed (overall) | 298 per 1000 | 494 per 1000 | RR 1.66 | 438 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 30 days | 189 per 1000 | 360 per 1000 | RR 1.91 | 114 | ⊕⊝⊝⊝ | |
| Proportion of ulcers completely healed at 60 days | 250 per 1000 | 412 per 1000 | RR 1.65 | 324 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 90 days | 327 per 1000 | 524 per 1000 | RR 1.6 | 230 | ⊕⊕⊝⊝ | |
| Time to complete ulcer healing | Available data were limited and not analysed | |||||
| Change in absolute ulcer size | Available data were limited and not analysed | |||||
| Ulcer recurrence | Not reported | |||||
| Adverse effects | 31 per 1000 | 44 per 1000 | RR 1.44 | 344 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | Not reported | |||||
| Direct costs | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors). 2 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (single study with very wide confidence intervals). 3 Downgraded one level for risk of bias (lack of allocation concealment) and one level for imprecision (single study with very wide confidence intervals). 4 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (wide confidence intervals). | ||||||
Background
Description of the condition
Leg ulcers are usually defined as a loss of skin on the lower limb that takes more than six weeks to heal (Nelson 2011). Leg ulceration is a common chronic disease and the prevalence ranges from 0.62 to 5 per 1000 people, varying from country to country, for example, the prevalence of leg ulcers is 0.62/1000 in Australia (Baker 1991), 1/1000 in China (Fu 1998), 1.6/1000 in Sweden (Nelzen 1994), 1.5 to 3/1000 in the United Kingdom (NHS CRD 1997), and 5/1000 for the proportion of the population in the United States that is over 20 years of age (Coon 1973). Moreover, leg ulcer prevalence increases with age, rising to a peak prevalence between 60 years and 80 years old, with women suffering from ulcers about 1.6 times more frequently than men (Valencia 2001). High risk factors for leg ulcers include prolonged standing (McCulloch 2001), obesity, a sedentary lifestyle and family history (Beebe‐Dimmer 2005).
Approximately, 80% to 85% of all leg ulcers occur as a result of venous diseases (Simon 2004). Venous leg ulcers, also known as varicose ulcers or stasis ulcers, are a chronic and recurrent disease. The estimated recurrence rate for healed ulcers ranges from 26% to 69% in the first year (Mayer 1994; Franks 1995), rising to 75% after two years (Mayer 1994). Leg ulcers have become a big financial burden to both patients and health services (Hareendran 2005). The annual expenditure on leg ulceration is GBP 230 million to 400 million (1991 prices) according to the National Health Service in the United Kingdom (Bosanquet 1992), and the cost is estimated to be as high as USD 1.9 billion to 2.5 billion per year in the United States (Valencia 2001). Moreover, leg ulcers can have a serious impact on patients' quality of life (González‐Consuegra 2011; Herberger 2011).
Venous leg ulcers are strongly associated with chronic venous insufficiency (CVI) (White 2005). The venous blood flow system in the lower extremities consists of deep veins, superficial veins and perforator veins, all of which have one‐way open valves to prevent reflux (flow in the opposite direction). Deep veins are distributed within the calf muscle, and possess high blood pressure due to contraction of the calf muscle. By contrast, blood pressure within the superficial veins is lower. Perforator veins communicate between the deep and superficial veins. Normally, the valves of the perforator veins close when the calf muscle contracts in order to separate the superficial veins from the high pressure of the deep veins. The valves open while the calf muscle relaxes to let blood from the superficial veins flow through the perforator veins into the deep veins. Abnormally, CVI occurs when the normal venous system is disturbed by diseases of the venous system (incompetent vein valves, loss of vein wall elasticity, lesions obstructing the venous tract, etc.) or failure of the calf muscle pumping system (Valencia 2001; White 2005), which lead to an increase in venous pressure, which is termed 'venous hypertension'. Venous hypertension is responsible for most venous disease symptoms of the leg, such as oedema (swelling due to fluid retention), lipodermatosclerosis (painful inflammation and discolouration of skin), varicose veins and ulceration. (Andreozzi 2012).
Despite the above, the pathogenic steps between venous hypertension and leg ulcers are not fully understood. One theory, called the 'Pericapillary fibrin cuffs and fibrinolytic abnormalities hypothesis' (Valencia 2001), proposes that sustained venous hypertension increases capillary permeability, leading to leakage of fibrinogen out of the capillaries. Leaked fibrinogen polymerises to form a barrier to the diffusion of oxygen and nutrients, that is, the 'pericapillary fibrin cuff'. The lack of oxygen and nutrients leads to local tissue cell death and the formation of ulcers (Browse 1982). Another theory, the 'White cell trapping hypothesis' (Valencia 2001), proposes that venous hypertension decreases the pressure gradient between the arterial and venous systems of the lower extremity, leading to a reduction in the flow rate of capillary blood that results in white blood cells (leukocytes) becoming trapped. These white blood cells can form a direct physical barrier, and also release certain mediators (such as collagenase, elastase, cytokines, free radicals and chemotactic factors), resulting in an increase in capillary permeability, leakage of fibrinogen, and the inflammatory reaction that leads to ulceration (Pascarella 2005).
As there is no gold standard for the diagnosis of venous leg ulcers, it is difficult to apply a standard definition. Nonetheless, it is important to distinguish the origin of a leg ulcer i.e. whether it is due to venous, arterial or neuropathic disease, or any other causes (Velasco 2011). Arterial leg ulcers can be excluded through measuring the ankle‐brachial pressure index (ABPI) by doppler ultrasonography. An ABPI less than or equal to 0.5 indicates that leg ulcers are caused by arterial disease. Neuropathic ulcers are more common in patients suffering from diabetes mellitus (Valencia 2001).
Description of the intervention
The goals of interventions for venous leg ulcers include promoting ulcer healing, reducing recurrence of ulcers, improving quality of life and reducing adverse effects (De Araujo 2003; Nelson 2011). Among the variety of types of therapy, compression therapy has emerged as the standard treatment for venous leg ulcers (NHS CRD 1997; O'Meara 2012; Nelson 2011), however, it usually takes a long time for ulcer healing (Erickson 1995), and compression is not suitable for patients with arterial disease (NHS CRD 1997; Nelson 2011). Adjuvant (additional) drug therapies have also been studied (e.g. pentoxifylline (Jull 2012), flavonoids (Scallon 2013), and aspirin (de Oliveira Carvalho 2016)). Sulodexide has also been suggested as a potential candidate for adjuvant treatment of venous leg ulcers (Nelson 2011).
Sulodexide is a highly purified glycosaminoglycan (GAG) consisting of 80% fast‐moving heparin (FMH) and 20% dermatan sulphate (DS). The FMH (7000 Da) is composed of unfractionated heparin and a fraction with a lower electrophoretic mobility. The DS (25,000 Da) is a polydisperse polysaccharide (Cosmi 2003). Compared with heparin alone, sulodexide shows a longer half‐life and lower risk of haemorrhage (Lasierra‐Cirujeda 2010).
Sulodexide is primarily used in patients with thrombotic risk diseases, and is administered either orally or parenterally (e.g. via infusion).
How the intervention might work
Sulodexide is claimed to have antithrombotic, profibrinolytic and anti‐inflammatory effects (Andreozzi 2012). A systematic review has shown it to lower blood pressure (Olde Engberink 2015).
Sulodexide has an antithrombotic effect by inhibiting thrombin activity and thrombin formation (Cosmi 2003). Thrombin is an essential part of the coagulation (blood clotting) system as it helps to convert fibrinogen to fibrin. Sulodexide has a positive effect on various blood components that inhibit thrombin activity and also has a negative effect on blood components that promote the conversion of prothrombin into thrombin.
Sulodexide stimulates fibrinolytic activity by promoting the conversion of plasminogen to plasmin, which results in fibrinolysis (the breaking up of fibrin).
Moreover, recent studies demonstrate that sulodexide has anti‐inflammatory effect (Andreozzi 2012). Karoń 2007 demonstrated that systemic (whole body) administration of sulodexide reduced intraperitoneal and vascular inflammation in rats. Ciszewicz 2009 demonstrated that sulodexide exerts an anti‐inflammatory effect in human endothelial cells by suppressing the generation of oxygen‐derived free radicals, and the release of monocyte chemotactic protein‐1 (MCP‐1) and interleukin‐6 (IL‐6).
Why it is important to do this review
Venous leg ulcers are a common, chronic, recurrent disease. Although the activity of sulodexide has been investigated in clinical trials treating venous leg ulcers, there has been no rigorous systematic review of its effects on venous ulcer healing.
Objectives
To assess the effects of sulodexide on the healing of venous leg ulcers along with any side effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of sulodexide were eligible for inclusion.
Types of participants
We included studies involving people of any age, gender, and in any care setting, described as having a venous leg ulcer.
Types of interventions
We included RCTs which evaluated oral or parenteral administration of sulodexide (any strength and over any period of administration) compared with placebo or any other drug therapy (such as pentoxifylline, flavonoids, aspirin), with or without compression therapy. Any co‐interventions (e.g. other drug therapy or compression therapy) had to be used in a standardised way across all arms in a trial.
Types of outcome measures
Primary outcomes
-
Proportion of ulcers completely healed during follow up.
-
Time to complete ulcer healing.
-
Percentage, or absolute, change in ulcer size.
Secondary outcomes
-
Ulcer recurrence.
-
All reported adverse events, such as bleeding, allergic reaction, injection site pain, gastrointestinal reaction, etc.
-
Health‐related quality of life (measured using a validated standardised generic questionnaire such as EQ‐5D (Herdman 2011), SF‐36 (Ware 2000), or validated disease‐specific questionnaire (Augustin 1997)).
-
Direct costs.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify relevant RCTs:
-
The Cochrane Wounds Specialised Register (searched 01 July 2015);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2015, Issue 6);
-
Ovid MEDLINE (1946 to 01 July 2015);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 01 July 2015);
-
Ovid EMBASE (1974 to 01 July 2015);
-
EBSCO CINAHL (1982 to 01 July 2015);
-
Chinese Biomedical Literature Database (CBM) (1978 to 13 August 2015);
-
China National Knowledge Infrastructure Database (CNKI) (1979 to 13 August 2015);
-
Wan Fang Database (1986 to 13 August 2015);
-
VIP Database (1989 to 13 August 2015).
The search strategies used for CENTRAL, MEDLINE, EMBASE and CINAHL can be found in Appendix 1, Appendix 2, Appendix 3, and Appendix 4. The search strategies was modified and translated appropriately for each Chinese database search (Appendix 5; Appendix 6; Appendix 7; Appendix 8). We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter terms developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the trial filter terms developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). There were no restrictions based on date of publication, language or study setting.
We also searched the following clinical studies registries for any ongoing studies:
-
ClinicalTrials.gov (http://clinicaltrials.gov/);
-
International Standard Randomised Controlled Trial Number Register (ISRCTN) Registry (http://www.isrctn.com/);
-
WHO International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/),
-
Chinese Clinical Trial Registry (ChiCTR) (http://www.chictr.org.cn/searchprojen.aspx).
Searching other resources
We searched the references listed in relevant trial reports and reviews to identify any further relevant RCTs.
Data collection and analysis
Selection of studies
We used EndNote X7 software (EndNote 2014) to merge retrieved reports of each database and to remove duplicate records of the same study. Two review authors (BW, JL) assessed titles and abstracts of studies independently to exclude obviously irrelevant reports. We retrieved full‐text copies of all potentially eligible reports, and reviewed them in the light of the inclusion criteria. Two review authors (BW, JL) made final decisions on the inclusions by cross‐checking the results, and a third review author (MY) was consulted when there were any disagreements. Where we identified multiple reports of the same trial, we extracted the maximum amount of data from the multiple reports and identified one report as the primary reference. We set out a study flow diagram as recommended by the PRISMA statement to illustrate the process of screening and selecting studies for inclusion in the review (Figure 1) (Liberati 2009).

PRISMA flow diagram of literature screening
Data extraction and management
We extracted study data based on the data extraction sheet provided by Cochrane Wounds, which included the following information: general data (authors, publication year and contact information), baseline data (participants' number, age and gender), risk of bias assessment information (randomisation, allocation concealment, blinding and incomplete outcome data), interventions, duration of follow up, outcome measures and results. We planned to contact the study authors for more information, if necessary.
Independently, two review authors (BW, JL) extracted and managed data from all included trials, and discussed any disagreements. A third review author (MY) acted as arbiter when BW and JL failed to reach an agreement.
Assessment of risk of bias in included studies
We assessed risk of bias of included studies by the methods recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (BW, MY) independently evaluated the following seven items for each study: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias. We judged risk of bias for each item as 'Low risk', 'High risk' and 'Unclear risk' following the assessment criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Appendix 9). Finally, we presented our assessment of risk of bias in a 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3).
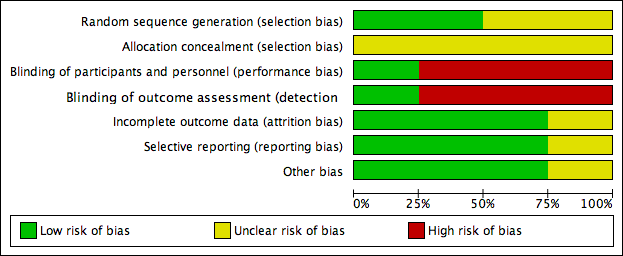
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For dichotomous outcomes, we presented the risk ratio (RR) with 95% confidence interval (CI) for each individual study. For continuous outcomes, we used the mean difference (MD) with 95% CI for each individual study. For time‐to‐event data, we planned to report estimates of time to complete ulcer healing and to plot hazard ratio (HR) estimates with 95% CI when available from study reports (Deeks 2011), however, HR was not reported in Niglio 1991 or Scondotto 1999 and the mean time to complete ulcer healing was presented in Scondotto 1999.
Unit of analysis issues
We considered individual participants as the unit of analysis. We intended to present a narrative summary if the unit of randomisation or analysis of included study was ulcer or limb. We planned to re‐analyse any cluster‐randomised studies identified by calculating the effective sample sizes with the intra‐cluster coefficient (ICC) estimated externally from similar studies (Deeks 2011), however, we did not include any cluster‐randomised studies in this review. The outcome of 'proportion of ulcers completely healed' was reported at different time points; we firstly performed an overall analysis by pooling all the included studies, then conducted subgroup analyses at 30 days', 60 days' and 90 days' follow up.
Dealing with missing data
We attempted to contact the original study authors when essential data were missing. We tried to contact authors of the included RCT published as an abstract only (Niglio 1991) by email, unfortunately, we got no replies. We assumed the missing participants had both positive and negative outcomes (e.g. missing participants assumed for ulcers healed and ulcers not healed), and undertook an analysis based on these assumptions (best case/worst case scenario) respectively, performing a sensitivity analysis.
Assessment of heterogeneity
Firstly, if clinical diversity existed between the trials (e.g. ulcers of different severity, different types of interventions, or different durations of follow up), we planned not to combine data from these trials and to present a narrative summary. Secondly, for clinically homogeneous (similar) studies, we intended to perform a Chi2 test, with P values less than 0.1 indicating significant statistical heterogeneity. In order to quantify heterogeneity not due to chance, we used the I2 statistic (Higgins 2003). A rough guide for the interpretation of I2 was as follows: 0% to 40% represented heterogeneity that might not be important; 30% to 60% might represent moderate heterogeneity; 50% to 90% might represent substantial heterogeneity; 75% to 100% represented considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We performed a comprehensive search for eligible RCTs to minimise reporting bias. We planned to use funnel plots to assess publication bias (Sterne 2011) when more than 10 studies were included; however, there were insufficient studies to conduct this analysis. To evaluate selective reporting of outcomes, we planned to compare the study protocols with the final study reports, however, none of the protocols was available. Then we compared the 'Methods' section of the published studies with the 'Results' section to identify any outcomes that were measured but not reported.
Data synthesis
We analysed the data using Review Manager (RevMan) 5.3 software provided by the Cochrane Collaboration (RevMan 2014). We used a fixed‐effect model for meta‐analysis in the absence of clinical, methodological and statistical heterogeneity. If the I2 statistic was greater than zero, we also applied a random‐effects model to see whether the conclusions differed, and any difference was noted. If pooling was not possible or appropriate, we intended to present a narrative summary (Deeks 2011).
'Summary of findings' tables
We used GRADE Profiler 3.6 (GRADEpro GDT 2015) to import data from RevMan 5.3 to create 'Summary of findings' tables for each outcome evaluated in this review. Summary of findings tables evaluated the overall quality of evidence on the primary and secondary outcomes. The GRADE system classified the quality of evidence in the following four grades: high, moderate, low and very low (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
If clinical heterogeneity was investigated and there were sufficient RCTs included, we planned to conduct subgroup analyses as follows.
-
Different types of interventions, determined by the type of control group (placebo or other therapies) or combination of compression therapy (with or without compression).
-
Different durations of follow up.
There were sufficient RCTs included to allow us to undertake the second planned subgroup analysis for the primary outcome of proportion of ulcers completely healed.
Sensitivity analysis
We intended to perform a sensitivity analysis for the primary outcomes to investigate the robustness of our findings. We planned to conduct a sensitivity analysis by comparing meta‐analysis results of:
-
RCTs with low risk of bias (adequate sequence generation, adequate allocation concealment and an adequate method of outcome assessor blinding) compared with all included RCTs.
-
the assumption that missing participants had a positive outcome versus a negative outcome.
We were able to undertake the second planned sensitivity analysis for one of the studies comparing sulodexide to placebo (Coccheri 2002).
Results
Description of studies
Results of the search
See: Characteristics of included studies; Characteristics of excluded studies.
The search strategies yielded a total of 133 articles after de‐duplication. Twelve remained after title and abstract screening, and four trials met the inclusion criteria after full text screening (Niglio 1991; Scondotto 1999; Coccheri 2002; Zou 2007). The flow of literature screening process of the review is shown in Figure 1.
Of the four studies included, three compared sulodexide plus local treatment (including wound care and compression therapy) with local treatment alone (Niglio 1991; Scondotto 1999; Zou 2007), one compared sulodexide plus local treatment with placebo plus local treatment (Coccheri 2002).
Included studies
Study design
Four RCTs were included in this review. Three studies were conducted in Italy (Niglio 1991; Scondotto 1999; Coccheri 2002), and one in China (Zou 2007). Three were single‐centre studies (Niglio 1991; Scondotto 1999; Zou 2007), one was a multi‐centre study (31 Italian centres) (Coccheri 2002).
Participants
A total of 463 patients with venous leg ulcers were randomised in the four included RCTs. Sample sizes ranged from 20 (Niglio 1991) to 235 (Coccheri 2002) participants. One RCT described sample size calculations (Scondotto 1999). All four studies reported the age of the participants; the youngest was 42 years old (Scondotto 1999) and the oldest was 93 years old (Scondotto 1999).
Interventions
All four RCTs evaluated sulodexide interventions in combination with local treatment in clinics. However, the sulodexide was given in different ways. Three RCTs started with sulodexide 600 lipasemic units (LSU) intramuscular injection, once daily for the first 20 days (Coccheri 2002) or 30 days (Scondotto 1999; Zou 2007), followed by sulodexide 250 LSU (Scondotto 1999) or 500 LSU (Coccheri 2002) orally, twice daily for another 30 days (Scondotto 1999) or 70 days (Coccheri 2002). One RCT, Niglio 1991, gave sulodexide 500 LSU orally, once daily in the morning.
Local treatment consisted of wound care and compression therapy. Reported wound care mainly included cleansing of ulcers (Scondotto 1999; Coccheri 2002; Zou 2007), removal of debris (Coccheri 2002; Zou 2007), antisepsis (Coccheri 2002; Zou 2007) and dressing of the wound (Coccheri 2002; Zou 2007). Compression therapy usually used stretch elastic bandages (Scondotto 1999; Coccheri 2002; Zou 2007) and stretch socks (Zou 2007).
Outcomes
Three RCTs reported the primary outcome of 'proportion of ulcers completely healed' (Scondotto 1999; Coccheri 2002; Zou 2007). Two RCTs reported 'time to complete ulcer healing' (Niglio 1991; Scondotto 1999), however Niglio 1991 was only an abstract and the time data were not available. Two RCTs, Coccheri 2002 and Zou 2007 reported 'absolute ulcer size' and also the secondary outcome, 'adverse effects'. None of the included studies reported the secondary outcomes, 'ulcer recurrence', 'health‐related quality of life', and 'direct costs'.
Excluded studies
Eight studies were excluded for the following reasons: two studies were review articles (Krasinski 2010; Andreozzi 2012); four did not use sulodexide as a study intervention (Incandela 2001; Colletta 2003; Meaume 2008; Dereure 2012); one included participants with mixed venous and arterial leg ulceration within the same individual and results from those with venous ulcers alone could not be extracted (Serra 2014) and one was a quasi‐RCT (Kucharzewski 2003).
Risk of bias in included studies
Details are described in the risk of bias section of the Characteristics of included studies, and shown by the risk of bias graph (Figure 2) and the risk of bias summary (Figure 3).
Allocation
Sequence generation
Two studies stated that the sequence was generated by random number table (Scondotto 1999; Zou 2007), with low risk of bias. Two did not describe the allocation of sequence generation (Niglio 1991; Coccheri 2002), with unclear risk of bias.
Allocation concealment
None of the four RCTs provided enough description of the allocation concealment process, so we judged the risk of bias as unclear.
Blinding
Coccheri 2002 reported using a double‐blind procedure of participants and outcome assessment, so the risk of bias was low. Blinding of participants, personnel and outcome assessment was not performed in the other three studies (Niglio 1991; Scondotto 1999; Zou 2007), so we judged the risk of bias with respect to blinding as high.
Incomplete outcome data
Two of the included RCTs, Scondotto 1999 and Zou 2007 reported that there were no participants lost to follow‐up, and one study, Coccheri 2002 reported the withdrawal of some participants during treatment, however, the authors performed an intention‐to‐treat analysis, so we judged the risk of bias was low in these three RCTs. Niglio 1991 was reported in an abstract only, so we could not confirm the risk of attrition bias.
Selective reporting
None of the four studies could be evaluated through the protocols, however, three RCTs reported all outcomes predefined in the 'Methods' section (Scondotto 1999; Coccheri 2002; Zou 2007), so we judged the risk of bias of this domain as low. Again, Niglio 1991 was reported in an abstract only, so we could not confirm the risk of reporting bias.
Other potential sources of bias
There was no other source of bias identified in three RCTs, Scondotto 1999, Coccheri 2002 and Zou 2007. Niglio 1991 reported as an abstract only, so other sources of bias could not be identified.
Effects of interventions
See: summary of findings Table for the main comparison for sulodexide and local treatment combined versus local treatment for treating venous leg ulcers.
Sulodexide plus local wound care compared with placebo or local wound care alone
Primary outcomes
Proportion of ulcers completely healed.
Three RCTs with a total of 438 participants (233 participants in the sulodexide group and 205 in the control) reported numbers of participants completely healed (Scondotto 1999; Coccheri 2002; Zou 2007). Firstly we performed an overall analysis based on outcome data of the participants' last follow‐up. The meta‐analysis showed an increase in the proportion of people whose ulcers completely healed with sulodexide compared with people in the control groups: RR 1.66; 95% CI 1.30 to 2.12 (Analysis 1.1), compared with control groups. This was low quality evidence (downgraded twice for risk of bias due to lack of allocation concealment and blinded participants, personnel and outcome assessment).
One study reported 55 participants (31 in the sulodexide group and 24 in the control) withdrawn with reasons (Coccheri 2002) and for the sensitivity analysis, we assumed that the ulcers of these 55 withdrawn participants were all completely healed. The result of the sensitivity analysis indicated that the overall result was robust to this alternative assumption about the outcome of the missing participants: RR 1.53; 95% CI 1.27 to 1.83 (Analysis 1.2).
We then performed subgroup analyses according to the follow‐up duration of up to 30 days, 31 to 60 days, and 61 to 90 days.
Up to 30 days' follow‐up
One RCT, Zou 2007, with a total of 114 participants (61 participants in the sulodexide group and 53 in the control) reported the proportion of ulcers completely healed up to 30 days. It is unclear whether sulodexide improved numbers of participants completely healed: RR 1.91; 95% CI 1.00 to 3.66 (Analysis 1.3), (very low quality evidence downgraded twice for risk of bias and once for imprecision).
31 to 60 days' follow‐up
Two studies, Scondotto 1999 and Coccheri 2002, with a total of 324 participants (172 participants in the sulodexide group and 152 in the control group) reported this outcome. Pooling of these studies showed increased healing of the ulcers of people treated with sulodexide: RR 1.65; 95% CI 1.20 to 2.28 (Analysis 1.4), (low quality evidence downgraded twice for risk of bias).
61 to 90 days' follow‐up
One RCT, Coccheri 2002, with a total of 230 participants (120 participants in the sulodexide group and 110 in the control group) reported this outcome. Sulodexide was associated with an increase in the proportion of participants completely healed: RR 1.60; 95% CI 1.17 to 2.20 (Analysis 1.5), (low quality evidence downgraded once for risk of bias and once for imprecision).
Time to complete ulcer healing.
Three RCTs reported 'time to complete ulcer healing' (Niglio 1991; Scondotto 1999; Coccheri 2002).
Niglio 1991 randomised 10 participants each to the sulodexide (500 LSU orally, once daily) group and to the control group. Participants in each group received wound care and compression therapy. The reported healing time might be earlier in the sulodexide group (P < 0.01), however, exact time data were not available as this study was reported in an abstract only.
Scondotto 1999 randomised 52 participants to the sulodexide (600 LSU intramuscular injection, once daily for 30 days; then 250 LSU orally, twice a day for 30 days) group and 42 patients to the control group. Participants in each group received wound care and compression therapy. The overall mean (SD) time to complete ulcer healing for all study participants was 72 (64) days in the sulodexide group compared with 110 (137) days in the control group (P = 0.08). In the subgroup analysis for participants with epidermal ulcers, the mean (SD) time to complete ulcer healing was 25 (20) days in the sulodexide group and 51 (21) days in the control group (P = 0.0001). For participants with dermal ulcers, the mean (SD) time to complete ulcer healing was 86 (66) days in the sulodexide group and 133 (51) days in the control group (P = 0.02).
Coccheri 2002randomised 121 participants to the sulodexide (600 LSU intramuscular injection, once daily for 20 days; then 500 LSU orally, twice daily for 70 days) group and 114 patients to the control group. Participants in each group received wound care and compression therapy. The interaction of sulodexide combined with compression therapy on time to complete ulcer healing was reported as significant (P = 0.011) by multivariate analysis, in favour of sulodexide.
Change in absolute ulcer size.
Two studies reported 'change in absolute ulcer size' during follow‐up (Coccheri 2002; Zou 2007).
Coccheri 2002 reported the ulcer size on day 0, day 20, day 60 and day 90. The mean (SD) ulcer size was 12.25 (23.12) cm2 in the sulodexide group versus 13.38 (26.00) cm2 in the control group for day 0; 8.81 (19.84) cm2 versus 10.59 (25.25) cm2 for day 20; 7.19 (21.11) cm2 versus 9.97 (26.16) cm2 for day 60; and 5.39 (14.91) cm2 versus 8.35 (24.21) cm2 for day 90. The changes in ulcer size with time were reported as significant for the sulodexide group (P = 0.004), but not for the control group.
Zou 2007 randomised 61 participants to the sulodexide (600 LSU intramuscular injection, once daily until the ulcer healed or maximum 30 days) group and 53 participants to the control group. Participants in each group received wound care and compression therapy . The ulcer size was 12.25 cm2 in the sulodexide group versus 13.38 cm2 in the control group for day 0; 8.81 versus 10.59 cm2 for day 20; and 5.39 versus 8.35 cm2 for day 30; however, the P value for the between‐group difference of each comparison phase was not reported.
Secondary outcomes
Ulcer recurrence.
No studies examined this outcome.
Adverse effects.
Two studies, Coccheri 2002 and Zou 2007, with a total of 344 participants (181 patients in the sulodexide group and 163 in the control) reported adverse effects during the treatment period. There was no clear evidence of a difference in rates of adverse effects when the data from these studies were pooled: RR 1.44; 95% CI 0.48 to 4.34. The reported adverse effects included cutaneous rash, epigastric pain and headache. This was very low quality evidence downgraded twice for risk of bias and once for imprecision.
Health‐related quality of life.
No studies examined this outcome.
Direct costs.
No studies examined this outcome.
Discussion
Summary of main results
Four RCTs were included in this review. All four RCTs investigated sulodexide as an adjuvant to local treatment, including wound care and compression therapy, for treating venous leg ulcers.
For the primary outcome measurements, three RCTs reported 'proportion of ulcers completely healed' overall (Scondotto 1999; Coccheri 2002; Zou 2007), and there were more ulcers completely healed in the sulodexide and local treatment combination group compared with the local treatment group. Three RCTs reported 'time to complete ulcer healing' (Niglio 1991; Scondotto 1999; Coccheri 2002). There is some (very) low quality evidence that patients treated with sulodexide may experience faster healing of their venous ulcers than those who do not receive sulodexide however this estimate of effect is likely to change with new research. Two RCTs which reported change 'change in absolute ulcer size' (which is an intermediate outcome) (Coccheri 2002; Zou 2007) also individually reported greater reductions in size with sulodexide .
For the secondary outcome measurements, only two RCTs reported 'adverse effects' (Coccheri 2002; Zou 2007). There was no clear difference in rates of adverse effects and this finding is very low quality due to risk of bias and imprecision.
No other secondary outcomes, such as ulcer recurrence, quality of life or costs, were reported.
Overall completeness and applicability of evidence
We were unable to evaluate all the interventions we had expected to in this review. All the included RCTs compared sulodexide with placebo or no treatment as an adjuvant therapy to local treatment; no studies compared sulodexide with other drugs, such as pentoxifylline, flavonoids or aspirin.
The included studies did not assess all the previously defined outcome measures. Three out of four RCTs reported the proportion of ulcers completely healed, three out of four trials reported time to complete ulcer healing, two out of four trials reported change in absolute ulcer size and adverse effects. Niglio 1991 reported their study as an abstract only. The only outcome of time to complete ulcer healing was mentioned but time data were not available so the review authors tried to contact Niglio for more study details. None of the included RCTs reported the planned outcomes ulcer recurrence, health‐related quality of life and direct costs.
Quality of the evidence
Four RCTs with a total of 463 patients were included in the review. The reported outcomes were likely to be influenced by lack of blinding, so the risk of bias with respect to blinding (including blinding of participants and personnel, and blinding of outcome assessment) was high in three studies (Niglio 1991; Scondotto 1999; Zou 2007). Niglio 1991 was published as abstract only so could be properly assessed (the risk of bias of each domain except blinding was unclear).
The quality of the evidence for each outcome was either low or very low. The main reasons for downgrading the quality of the evidence were risk of bias and imprecision (summary of findings Table for the main comparison).
Potential biases in the review process
We comprehensively searched databases and trial registries, as well as reference lists of relevant studies for published RCTs with no restrictions on language. However, we did not identify or intend to include any unpublished studies, so the possibility of publication bias cannot be excluded. We identified and excluded one RCT which included participants with mixed venous and arterial leg ulcers within the same individual and results from those with venous ulcers alone could not be extracted (Serra 2014). In addition, we included a study published as an abstract only and although we tried to contact the authors for more information, we did not hear back (Niglio 1991). It is possible that there may be additional unpublished data we were unable to access.
Agreements and disagreements with other studies or reviews
Systematic review and meta‐analyses of sulodexide for venous leg ulcers based on RCTs have not been performed previously, and RCTs on this topic are rare. The existing reviews (Nelson 2011; Andreozzi 2012; Andreozzi 2014) on sulodexide for venous leg ulcers were based on the same studies we identified (Scondotto 1999; Coccheri 2002; Kucharzewski 2003; Zou 2007) plus one quasi‐RCT (Kucharzewski 2003). Nelson 2011 conducted a systematic review to evaluate interventions for venous leg ulcers. Among various interventions, the four studies Scondotto 1999, Coccheri 2002, Kucharzewski 2003 and Zou 2007 were included in Nelson's review to evaluate the efficacy and safety of sulodexide. However, no pooled analysis was conducted in Nelson's review. The conclusion was that sulodexide plus compression could increase healing rates at two to three months compared with compression alone (Nelson 2011). Andreozzi reported two reviews on sulodexide in the management of chronic venous disease (Andreozzi 2012; Andreozzi 2014), and the evaluation of sulodexide on venous leg ulcers was based on the same four studies (Scondotto 1999; Coccheri 2002; Kucharzewski 2003; Zou 2007). The quasi‐RCT we excluded concluded that sulodexide could accelerate the healing process for patients with chronic venous leg ulcers (Kucharzewski 2003).

PRISMA flow diagram of literature screening
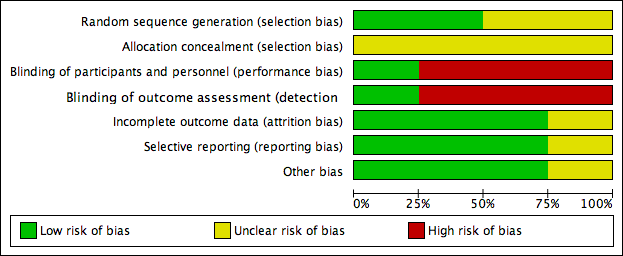
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
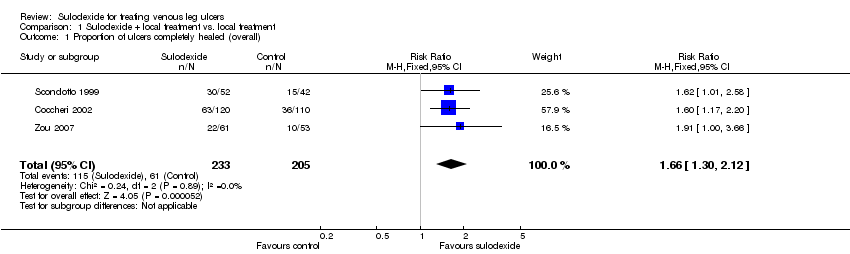
Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 1 Proportion of ulcers completely healed (overall).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 2 Proportion of ulcers completely healed (sensitivity analysis).

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 3 Proportion of ulcers completely healed at 30 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 4 Proportion of ulcers completely healed at 60 days.
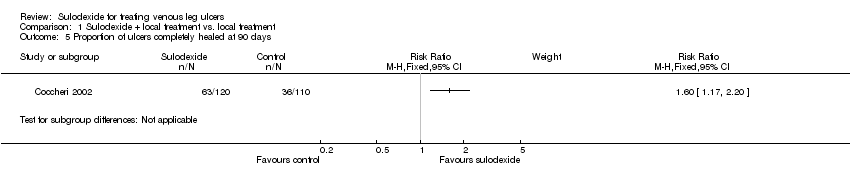
Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 5 Proportion of ulcers completely healed at 90 days.

Comparison 1 Sulodexide + local treatment vs. local treatment, Outcome 6 Adverse effects.
| Sulodexide + local treatment compared to local treatment for treating venous leg ulcers | ||||||
| Patient or population: patients with venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| local treatment | Sulodexide + local treatment | |||||
| Proportion of ulcers completely healed (overall) | 298 per 1000 | 494 per 1000 | RR 1.66 | 438 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 30 days | 189 per 1000 | 360 per 1000 | RR 1.91 | 114 | ⊕⊝⊝⊝ | |
| Proportion of ulcers completely healed at 60 days | 250 per 1000 | 412 per 1000 | RR 1.65 | 324 | ⊕⊕⊝⊝ | |
| Proportion of ulcers completely healed at 90 days | 327 per 1000 | 524 per 1000 | RR 1.6 | 230 | ⊕⊕⊝⊝ | |
| Time to complete ulcer healing | Available data were limited and not analysed | |||||
| Change in absolute ulcer size | Available data were limited and not analysed | |||||
| Ulcer recurrence | Not reported | |||||
| Adverse effects | 31 per 1000 | 44 per 1000 | RR 1.44 | 344 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | Not reported | |||||
| Direct costs | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors). 2 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (single study with very wide confidence intervals). 3 Downgraded one level for risk of bias (lack of allocation concealment) and one level for imprecision (single study with very wide confidence intervals). 4 Downgraded two levels for risk of bias (risk of selection bias due to lack of allocation concealment; risk of performance bias due to lack of blinding of participants, personnel and outcome assessors) and one level for imprecision (wide confidence intervals). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers completely healed (overall) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.30, 2.12] |
| 2 Proportion of ulcers completely healed (sensitivity analysis) Show forest plot | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.27, 1.83] |
| 3 Proportion of ulcers completely healed at 30 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Proportion of ulcers completely healed at 60 days Show forest plot | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.20, 2.28] |
| 5 Proportion of ulcers completely healed at 90 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Adverse effects Show forest plot | 2 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.48, 4.34] |

