Pokonsolidacyjna terapia kwasem retinowym chorych na agresywną postać nerwiaka zarodkowego (neuroblastoma), leczonych autologicznym przeszczepem hematopoetycznych komórek macierzystych
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Setting
Duration of enrollment
Randomisation
Median follow‐up time
| |
| Participants | Eligibility criteria
Number of patients eligible for this review
Age
Gender
Stage of disease
Remission status
Compliance with randomisation
Previous treatment, except initial and consolidation chemotherapy
Comorbidity
| |
| Interventions | All participants
First randomisation BMT arm (patients in the continuation chemotherapy arm were not eligible for this review)
2nd randomisation Retinoic acid arm
Control arm
| |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A permuted‐block design was used for the random assignment of approximately equal numbers of participants from each of 2 strata (those with and those without metastatic disease) to transplantation or continuation chemotherapy. The 2nd randomisation was similarly balanced with respect to the numbers of participants from each group of the first randomisation and non‐randomised participants who were ineligible for transplantation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants, physicians and nurses was not reported |
| Blinding of outcome assessment (detection bias) | Low risk | The study committee and investigators were unaware of participants' treatment assignments, and the study was monitored by an independent committee according to a group sequential monitoring plan |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not reported if all reported outcomes for all participants were assessed |
| Selective reporting (reporting bias) | Low risk | Reporting was in agreement with the protocol with regard to the outcome measures. We were concerned with the possibility that data for the same participants were included in several publications. However, we did not include duplicate data in the review |
| Other bias | Unclear risk | Analysis was conducted using the data of the randomised participants, which should be consistent with the ITT principle. It was unclear if all 98 participants received the treatment to which they were randomised. Also, not all participants treated with transplantation in the first randomisation and without progressive disease afterwards were included in the 2nd randomisation. It is unclear how many eligible patients did not undergo the 2nd randomisation. The consequences of 2 randomisations in 1 study for the risk of bias are unclear. |
AIDS: acquired immunodeficiency syndrome; BMT: bone marrow transplantation; cGy: centi‐Gray; CR: complete response; N: number.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not intervention of interest: not HDCT followed by autologous HSCT | |
| Not comparator of interest | |
| Not comparator of interest | |
| Not intervention of interest: not HDCT followed by autologous HSCT, only title/abstract available | |
| Not intervention of interest: not retinoic acid | |
| Not comparator of interest: all participants received retinoic acid | |
| Not publication type of interest | |
| Not publication type of interest | |
| Not intervention of interest: not HDCT followed by autologous HSCT | |
| Not an intervention of interest: not a HDCT followed by autologous HSCT | |
| Not comparator of interest: no comparator | |
| Not comparator of interest: no comparator | |
| Not comparator of interest | |
| Not intervention of interest: not HDCT followed by autologous HSCT, only title/abstract available | |
| Not publication type of interest | |
| Not intervention of interest: not retinoic acid, only title/abstract available | |
| Not intervention of interest: not HDCT followed by autologous HSCT | |
| Not intervention of interest: not HDCT followed by autologous HSCT | |
| Not intervention of interest: not HDCT followed by autologous HSCT | |
| Not outcome of interest or not reported separately | |
| Not intervention of interest: not retinoic acid | |
| Not comparator of interest, only title/abstract available | |
| Not comparator of interest | |
| Not comparator of interest | |
| Not comparator of interest: no comparator | |
| Not comparator of interest: no comparator | |
| Not comparator of interest: all participants received retinoic acid | |
| Not comparator of interest: no comparator | |
| Not diagnosis of interest, only title/abstract available | |
| Not comparator of interest: no comparator | |
| Not intervention of interest: not HDCT followed by autologous HSCT | |
| Not comparator of interest: pharmacokinetic study | |
| Not publication type of interest: not RCT | |
| Not comparator of interest | |
| Not intervention of interest: 28% (49/175) patients did not receive high‐dose chemotherapy and bone marrow transplantation confirmed by author inquiry and after contacting the first author of this study, it became clear that separate data on the eligible participants were not available | |
| Not comparator of interest | |
| Not intervention of interest: not consolidation therapy | |
| Not intervention of interest: not HDCT followed by autologous HSCT | |
| Not publication type of interest: not RCT | |
| Not comparator of interest: no comparator | |
| Not comparator of interest: no comparator | |
| Not publication type of interest: not RCT | |
| Not a comparator of interest: both arms received retinoic acid | |
| Not comparator of interest | |
| Not comparator of interest | |
| Not publication type of interest: not RCT | |
| Not diagnosis of interest | |
| Not publication type of interest: narrative review | |
| Not comparator of interest: no comparator | |
| Not comparator of interest | |
| Not comparator of interest: no comparator | |
| Not publication type of interest: not RCT | |
| Not publication type of interest: narrative review | |
| Not intervention of interest, only title/abstract available | |
| Not publication type of interest: editorial | |
| Not intervention of interest | |
| Not publication type of interest: comment | |
| Not comparator of interest | |
| Not comparator of interest | |
| Not diagnosis of interest, only title/abstract available | |
| Not publication type of interest: not RCT | |
| Not publication type of interest: not RCT | |
| Not intervention of interest, only title/abstract available | |
| Not comparator of interest: no comparator | |
| not comparator of interest: no comparator | |
| Not intervention of interest: not retinoic acid, only title/abstract available | |
| Not comparator of interest | |
| Not publication type of interest: duplicate data | |
| Not comparator of interest | |
| Not comparator of interest: all participants received retinoic acid | |
| Not comparator of interest: no comparator | |
| Not diagnosis of interest: not high‐risk neuroblastoma | |
| Not publication type of interest: narrative review | |
| Not diagnosis of interest: melanoma | |
| Not diagnosis of interest: not neuroblastoma | |
| Not comparator of interest | |
| Not comparator of interest: no comparator | |
| Not outcome of interest or not reported separately: pharmacokinetics | |
| Not intervention of interest: not retinoic acid | |
| Not comparator of interest: all participants received retinoic acid | |
| Not comparator of interest: no comparator | |
| Not comparator of interest | |
| Not comparator of interest | |
| Not comparator of interest: no comparator | |
| Not outcome of interest or not reported separately: pharmacokinetics | |
| Not outcome of interest or not reported separately: pharmacokinetics | |
| Not outcome of interest or not reported separately: pharmacokinetics | |
| Not comparator of interest: no comparator | |
| Not comparator of interest: no comparator | |
| Not comparator of interest: no comparator | |
| Not comparator of interest: retinoic acid independent of HDCT followed by autologous HSCT | |
| Not comparator of interest: neuroblastoma not reported separately | |
| Not comparator of interest: retinoic acid independent of not HDCT followed by autologous HSCT, only title/abstract available | |
| Not comparator of interest: all participants received retinoic acid | |
| Not diagnosis of interest: glioma | |
| Not intervention of interest: not retinoic acid, only title/abstract available |
HDCT: high‐dose chemotherapy; HSCT: hematopoietic stem cell transplantation; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | Patients with neuroblastoma |
| Interventions | 13‐cis‐retinoic acid after intensive consolidation therapy |
| Outcomes | Event‐free survival |
| Notes | Title of an abstract of the annual meeting of the American Society of Clinical Oncology in 1998. Presumably associated with the study Matthay 1999. |
| Methods | RCT |
| Participants | Patients with high‐risk neuroblastoma |
| Interventions | 13‐cis‐retinoic acid following myeloablative therapy |
| Outcomes | Overall survival |
| Notes | Title of an abstract of the annual meeting of the American Society of Clinical Oncology in 2002. Presumably associated with the study Matthay 1999. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.87 [0.46, 1.63] | |
| Analysis 1.1 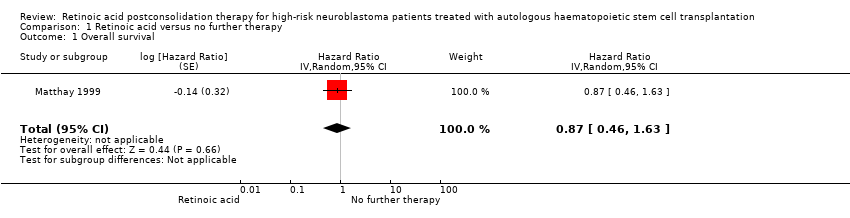 Comparison 1 Retinoic acid versus no further therapy, Outcome 1 Overall survival. | ||||
| 2 Event‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.86 [0.50, 1.49] | |
| Analysis 1.2  Comparison 1 Retinoic acid versus no further therapy, Outcome 2 Event‐free survival. | ||||

1Study flow diagram of current review version.
Abbreviations. CT.gov: ClinicalTrials.gov; ICTRP: International Clinical Trials Registry Platform

Flow of patients in the Matthay 1999 study (as prepared by review author FP).
Abbreviations. BMT: bone marrow transplantation; ContCT: continuation chemotherapy; CT: chemotherapy; HDCT: high‐dose chemotherapy; RA: retinoic acid.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
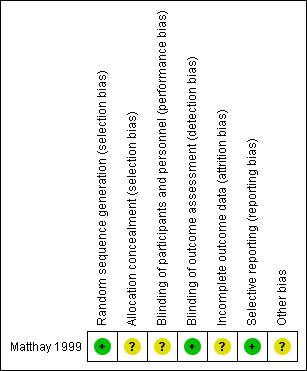
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Retinoic acid versus no further therapy, outcome: 1.1 Overall survival.
Abbreviations. CI: confidence interval; IV: inverse variance; SE: standard error.

Forest plot of comparison: 1 Retinoic acid versus no further therapy, outcome: 1.2 Event‐free survival.
CI: confidence interval; IV: inverse variance; SE: standard error.

Comparison 1 Retinoic acid versus no further therapy, Outcome 1 Overall survival.

Comparison 1 Retinoic acid versus no further therapy, Outcome 2 Event‐free survival.
| Retinoic acid postconsolidation therapy compared to no further treatment for high‐risk neuroblastoma patients treated with autologous HSCT | ||||||
| Patient or population: high‐risk neuroblastoma patients treated with autologous HSCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No further treatment | Retinoic acid post‐consolidation therapy | |||||
| Overall survival (reported as mortality) | 583 per 10001 | 533 per 1000 | HR 0.87 | 98 | ⊕⊕⊝⊝ | The length of follow‐up was not mentioned for the 98 participants eligible for this review |
| Treatment‐related mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided |
| Progression‐free survival ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on this outcome was provided |
| Event‐free survival (reported as relapse, disease progression, death from any cause, or second neoplasm) | 604 per 10001 | 549 per 1000 | HR 0.86 | 98 | ⊕⊕⊝⊝ | The length of follow‐up was not mentioned for the 98 participants eligible for this review |
| Early toxicity ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided |
| Late toxicity including secondary malignancies ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided |
| Health‐related quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on this outcome was provided |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The assumed risk is based on the number of events in the control group at the final time point of the survival curve presented in the included study. | ||||||
| INRG stage | Age (months) | Histologic category | Grade of tumour differentiation | MYCN | 11q aberration | Ploidy | Pretreatment risk group | |
| Code | Interpretation | |||||||
| L1/L2 | – | Ganglioneuroma maturing; ganglioneuroblastoma intermixed | ‐ | – | – | – | A | Very low |
| L1 | – | Any, except ganglioneuroma or ganglioneuroblastoma | – | Not amplified | – | – | B | Very low |
| Amplified | – | – | K | High | ||||
| L2 | < 18 | Any, except ganglioneuroma or ganglioneuroblastoma | – | Not amplified | No | – | D | Low |
| Yes | – | G | Intermediate | |||||
| ≥ 18 | Ganglioneuroblastoma nodular; neuroblastoma | Differentiating | Not amplified | No | – | E | Low | |
| Yes | – | H | Intermediate | |||||
| Poorly differentiated or undifferentiated | Not amplified | – | – | H | Intermediate | |||
| – | Amplified | – | – | N | High | |||
| M | < 18 | – | – | Not amplified | – | Hyperdiploid | F | Low |
| < 12 | – | – | Not amplified | – | Diploid | I | Intermediate | |
| 12 to < 18 | – | – | Not amplified | – | Diploid | J | Intermediate | |
| < 18 | – | – | Amplified | – | – | O | High | |
| ≥ 18 | – | – | – | – | – | P | High | |
| MS | < 18 | – | – | Not amplified | No | – | C | Very low |
| Yes | – | Q | High | |||||
| Amplified | – | – | R | High | ||||
| Reference: Cohn 2009. The INRG consensus classification schema includes the criteria INRG stage, age, histologic category, grade of tumour differentiation, MYCN status, presence/absence of 11q aberrations, and tumour cell ploidy. Sixteen statistically or clinically different pretreatment groups of patients (lettered A through R), or both, were identified using these criteria. The categories were designated as very low (A, B, C), low (D, E, F), intermediate (G, H, I, J), or high (K, N, O, P, Q, R) pretreatment risk subsets. | ||||||||
| Stage | Definition |
| 1 | Localised tumour with complete gross excision, with or without microscopic residual disease; representative ipsilateral lymph nodes negative for tumour microscopically (nodes attached to and removed with the primary tumour may be positive) |
| 2A | Localised tumour with incomplete gross excision; representative ipsilateral nonadherent lymph nodes negative for tumour microscopically |
| 2B | Localised tumour with or without complete gross excision, with ipsilateral nonadherent lymph nodes positive for tumour. Enlarged contralateral lymph nodes must be negative microscopically |
| 3 | Unresectable unilateral tumour infiltrating across the midlinea, with or without regional lymph node involvement; or localised unilateral tumour with contralateral regional lymph node involvement; or midline tumour with bilateral extension by infiltration (unresectable) or by lymph node involvement |
| 4 | Any primary tumour with dissemination to distant lymph nodes, bone, bone marrow, liver, skin and/or other organs (except as defined for stage 4S) |
| 4S | Localised primary tumour (as defined for stage 1, 2A or 2B), with dissemination limited to skin, liver, and/or bone marrowb (limited to infants < 1 year of age) |
| Reference: Brodeur 1993. Note: Multifocal primary tumours leg, bilateral adrenal primary tumours should be staged according to the greatest extent of disease, as defined above, and followed by a subscript letter M e.g. 3M. | |
| Response | Primary tumour | Metastatic sites |
| Complete response | No tumour | No tumour; catecholamines normal |
| Very good partial response | Decreased by 90% to 99% | No tumour; catecholamines normal; residual 99Tc bone changes allowed |
| Partial response | Decreased by more than 50% | All measurable sites decreased by > 50%. Bones and bone marrow: number of positive bone sites decreased by > 50%; no more than 1 positive bone marrow site allowed |
| Minimal response | No new lesions; > 50% reduction of any measurable lesion (primary or metastases) with < 50% reduction in any other; < 25% increase in any existing lesion | |
| No response | No new lesions; < 50% reduction but < 25% increase in any existing lesion | |
| Progressive disease | Any new lesion; increase of any measurable lesion by > 25%; previous negative marrow positive for tumour | |
| Reference: Brodeur 1993. | ||
| INSS stage | Age | MYCN | INPC classification | DNA index | Risk group |
| 1 | 0 to 21 y | Any | Any | Any | Low |
| 2A/2B | < 365 d | Any | Any | Any | Low |
| ≥ 365 d to 21 y | Nonamplified | Any | ‐ | Low | |
| ≥ 365 d to 21 y | Amplified | Favorable | ‐ | Low | |
| ≥ 365 d to 21 y | Amplified | Unfavorable | ‐ | High | |
| 3 | < 365 d | Nonamplified | Any | Any | Intermediate |
| < 365 d | Amplified | Any | Any | High | |
| ≥ 365 d to 21 y | Nonamplified | Favorable | ‐ | Intermediate | |
| ≥ 365 d to 21 y | Nonamplified | Unfavorable | ‐ | High | |
| ≥ 365 d to 21 y | Amplified | Any | ‐ | High | |
| 4 | < 548 d | Nonamplified | Any | Any | Intermediate |
| < 365 d | Amplified | Any | Any | High | |
| ≥ 548 d to 21 y | Any | Any | ‐ | High | |
| 4S | < 365 d | Nonamplified | Favorable | > 1 | Low |
| < 365 d | Nonamplified | Any | = 1 | Intermediate | |
| < 365 d | Nonamplified | Unfavorable | Any | Intermediate | |
| < 365 d | Amplified | Any | Any | High | |
| Reference: NCI PDQ 2017 | |||||
| Study | Study design | Type of HSCT | Type of RA | Dose1 | Pat2 | Type of adverse event (N of affected participants) |
| CR | PBSCT | CRA | 160 | 1 | Pneumocystis carinii pneumonia (1) | |
| CR | NR | CRA | 160 | 1 | Hypercalcaemia (1), osteoblastic lesions (1) | |
| SA‐IS | BMT or PBSCT | CRA | 160 | 44 | NR | |
| SA‐IS | PBSCT | CRA | 160 | 33 | NR | |
| SA‐IS | PBSCT | CRA | 120 to 160 | 14 | NR | |
| CR | BMT | CRA | 160 | 2 | Bone marrow transplant nephropathy (2) | |
| CR | BMT | CRA | 33 to 1023 | 1 | Growth failure (1) | |
| SA‐IS | BMT | CRA | 100 to 200 | 31 | Grade 3/4 toxicity of skin, liver and hypercalcaemia correlated with peak serum levels of CRA | |
| SA‐IS | PBSCT | CRA | 160 | 12 | Ataxia (1) | |
| RCT | BMT | CRA | NR | 12 | NR | |
| RCT | NR | CRA | 15 to 224 | NR | Dry skin (47), cheilitis (24), bone pain (16), other (13) | |
| RCT | PBSCT | CRA | 160 | 192 | Grade 3 toxic effects: hypertension (4), hematuria (2), elevated serum creatinine (2), proteinuria (3); purged and non‐purged transplantation group combined | |
| CS | NR | CRA | 160 | 1 | Cheilitis (1) | |
| CS | NR | CRA | NR | 20 | NR | |
| CR | NR | CRA | 160 | 3 | Hypercalcaemia (3) | |
| CR | NR | Fenretinide | 666 to 20515 | 2 | Rod electroretinogram suppression (2) | |
| SA‐IS | PBSCT | CRA | 160 | 8 | NR | |
| SA‐IS | BMT | CRA | 160 | 22 | NR | |
| CR | BMT | CRA | 130 to 400 | 2 | Hypercalcaemia (2) | |
| CR | BMT | CRA | 33 to 1023 | 1 | Generalised metaphyseal modification (1) | |
| SA‐IS | PBSCT | CRA | 160 | 30 | NR | |
| CR | NR | Fenretinide | 2210 | 1 | Langerhans cell histiocytosis (1) | |
| SA‐IS | BMT or PBSCT | CRA | NR | 36 | NR | |
| SA‐IS | NR | CRA | 160 | 75 | NR | |
| CR | NR | CRA | 140 | 1 | NR | |
| SA‐IS | PBSCT | CRA | 125 | 44 | Skin eruption, particularly face | |
| CR | BMT | CRA | 160 | 2 | Bone marrow transplant nephropathy (2) | |
| SA‐IS | NR | CRA | 160 | 28 | Mild skin toxicity (9), cheilitis (1), hypercalcaemia (2) | |
| SA‐IS | NR | CRA | 160 | 103 | Grade 3 ‐ 4 skin toxicity or cheilitis (5) | |
| SA‐IS | BMT | CRA | 100 to 200 | 49 | Dose‐limiting toxicity of hypercalcaemia (3), arthralgia and myalgia (1), grade 1 to 3 hypercalcaemia (9) | |
| SA‐IS | BMT | CRA | 200 | 51 | Hypercalcaemia (3), rash (2) | |
| SA‐IS | NR | Fenretinide | 1800 to 2475 | 626 | Rash (1), diarrhoea (1), nausea (2), vomiting (1), nyktalopia (1), abdominal pain (4) | |
| RCT | NR | CRA | 160 | 108 | "Few toxic effects" | |
| Notice: Studies presented in this table were not eligible for inclusion in this review. 1Dose in mg/m2/day. The unit mg/kg may be transformed to mg/m2. The average body weight, body length, and body surface of a 6‐month‐old child may be 8 kg, 67 cm, and 0.39 m2, thus 8 kg divided by 0.39 m2 is roughly equalto a conversion factor of 20 (CDC 2000a). This factor increases continuously with age. The average body weight, body length, body surface of an 11‐year‐old child may be 36 kg, 143 cm, and 1.20 m2, thus 36 kg divided by 1.20 m2 is roughly equal to a conversion factor of 30 (CDC 2000b). The unit mg per day may be transformed to m2 per day. For example, 300 mg per day may vary on average between 769 mg/m2 (300 mg/0.39 m2) and 250 m2 (300 mg/1.20 m2) 2Participants treated with retinoic acid after HDCT followed by autologous HSCT acid and evaluated for toxicity. 3Inamo 1999; Nishimura 1997: Patients received retinoic acid at a dose of 40 mg per day. The daily dose may vary on average between 102 mg/m2 (40 mg/0.39 m2) and 33 m2 (40 mg/1.20 m2) 4Kohler 2000: Participants received retinoic acid at a dose of 0.75 mg/kg per day. The daily dose may vary on average between 15 mg/m2 (0.75 mg/kg * 20) and 22 mg/m2 (0.75 mg/kg * 30). 5Marmor 2008: Participants received retinoic acid at a dose of 800 mg per day. The daily dose may vary on average between 2051 mg/m2 (800 mg/0.39 m2) and 666 m2 (800 mg/1.20 m2). 6Villablanca 2011: 51 of the 62 participants received HSCT. BMT: bone marrow transplantation; CR: case report; CS: case series; CRA: 13‐cis‐retinoic acid; HDCT: high‐dose chemotherapy; HSCT: haematopoietic stem cell transplantation; N: number of participants; NR: not reported; PBSCT: peripheral blood stem cell transplantation; RA: retinoic acid; SA‐IS: single‐arm intervention study such as phase‐1 or phase‐2 clinical trial | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.87 [0.46, 1.63] | |
| 2 Event‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.86 [0.50, 1.49] | |

