Pokonsolidacyjna terapia kwasem retinowym chorych na agresywną postać nerwiaka zarodkowego (neuroblastoma), leczonych autologicznym przeszczepem hematopoetycznych komórek macierzystych
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010685.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 25 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer infantil
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All review authors approved the final version of the review.
FP: concept, selecting and appraising studies (both for the original version of the review and the update), extracting and analysing data, interpretation of results, primary manuscript preparation.
ECVD: appraising studies (for the original version), extracting and analysing data, interpretation of results, manuscript review.
HE: selecting and appraising studies (for the update), manuscript review.
FB: selecting and appraising studies (for the original version), providing a clinical perspective, interpretation of results, manuscript review.
Sources of support
Internal sources
-
University of Cologne, Germany.
Provision of the full‐text articles
External sources
-
Stichting Kinderen Kankervrij (KiKa), Netherlands.
The salary of ECVD is paid by KiKa; KiKa was not involved in the design and execution of this Cochrane Review.
Declarations of interest
FP: none known
ECVD: none known
HE: none known
FB: none known
Acknowledgements
We would like to acknowledge the Editorial Base of Cochrane Childhood Cancer for their advice and support. The editorial base of Cochrane Childhood Cancer is funded by Stichting Kinderen Kankervrij (KiKa). We thank Jan Kohler for a timely and appropriate response to an inquiry.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 25 | Retinoic acid postconsolidation therapy for high‐risk neuroblastoma patients treated with autologous haematopoietic stem cell transplantation | Review | Frank Peinemann, Elvira C van Dalen, Heike Enk, Frank Berthold | |
| 2015 Jan 29 | Retinoic acid post consolidation therapy for high‐risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation | Review | Frank Peinemann, Elvira C van Dalen, Doreen A Kahangire, Frank Berthold | |
| 2013 Jul 31 | Retinoic acid post consolidation therapy for high‐risk neuroblastoma | Protocol | Frank Peinemann, Carmen Bartel, Ulrich Grouven, Frank Berthold | |
Differences between protocol and review
In the protocol, we stated that we would present five outcomes in the 'Summary of findings' table: overall survival, treatment‐related mortality, early toxicity, late toxicity, and health‐related quality of life. In the 'Summary of findings' table, up to seven outcomes may be chosen for inclusion that should represent the most important outcomes to patients. Progression‐free survival and event‐free survival lead the list of genuinely important secondary outcomes in the protocol. We added those two outcomes to the 'Summary of findings' table to present more completely the seven most important outcomes. The types of primary and secondary outcomes in the review remain unchanged from the protocol.
Differences between the previous version and this version of the review
The search terms for the ongoing trials database ClinicalTrials.gov have been slightly changed. We specified the search to find study types 'interventional studies' and clinical study phase '2' or '3' to improve precision. In the protocol, we stated that we would search for abstracts presented at the last five consecutive annual meetings of the American Society of Clinical Oncology (ASCO), the International Society of Paediatric Oncology (SIOP) and the Advances in Neuroblastoma Research (ANR). We did not do this for the original version of the review, but did so during the update for ASCO, SIOP, and the meetings of the American Society of Hematology (ASH), but not for ANR.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Consolidation Chemotherapy;
- Adrenal Gland Neoplasms [mortality, *therapy];
- Antineoplastic Agents [administration & dosage, *therapeutic use];
- Bone Marrow Transplantation;
- Disease‐Free Survival;
- Hematopoietic Stem Cell Transplantation [*methods];
- Neuroblastoma [mortality, *therapy];
- Randomized Controlled Trials as Topic;
- Selection Bias;
- Tretinoin [administration & dosage, *therapeutic use];
Medical Subject Headings Check Words
Humans; Infant;
PICO

1Study flow diagram of current review version.
Abbreviations. CT.gov: ClinicalTrials.gov; ICTRP: International Clinical Trials Registry Platform

Flow of patients in the Matthay 1999 study (as prepared by review author FP).
Abbreviations. BMT: bone marrow transplantation; ContCT: continuation chemotherapy; CT: chemotherapy; HDCT: high‐dose chemotherapy; RA: retinoic acid.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
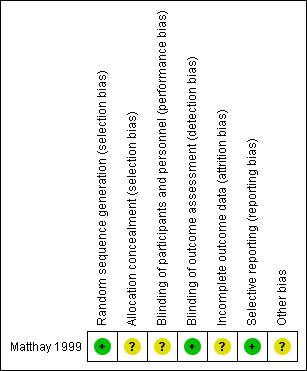
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Retinoic acid versus no further therapy, outcome: 1.1 Overall survival.
Abbreviations. CI: confidence interval; IV: inverse variance; SE: standard error.

Forest plot of comparison: 1 Retinoic acid versus no further therapy, outcome: 1.2 Event‐free survival.
CI: confidence interval; IV: inverse variance; SE: standard error.

Comparison 1 Retinoic acid versus no further therapy, Outcome 1 Overall survival.

Comparison 1 Retinoic acid versus no further therapy, Outcome 2 Event‐free survival.
| Retinoic acid postconsolidation therapy compared to no further treatment for high‐risk neuroblastoma patients treated with autologous HSCT | ||||||
| Patient or population: high‐risk neuroblastoma patients treated with autologous HSCT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No further treatment | Retinoic acid post‐consolidation therapy | |||||
| Overall survival (reported as mortality) | 583 per 10001 | 533 per 1000 | HR 0.87 | 98 | ⊕⊕⊝⊝ | The length of follow‐up was not mentioned for the 98 participants eligible for this review |
| Treatment‐related mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided |
| Progression‐free survival ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on this outcome was provided |
| Event‐free survival (reported as relapse, disease progression, death from any cause, or second neoplasm) | 604 per 10001 | 549 per 1000 | HR 0.86 | 98 | ⊕⊕⊝⊝ | The length of follow‐up was not mentioned for the 98 participants eligible for this review |
| Early toxicity ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided |
| Late toxicity including secondary malignancies ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adequate information on this outcome was provided |
| Health‐related quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on this outcome was provided |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The assumed risk is based on the number of events in the control group at the final time point of the survival curve presented in the included study. | ||||||
| INRG stage | Age (months) | Histologic category | Grade of tumour differentiation | MYCN | 11q aberration | Ploidy | Pretreatment risk group | |
| Code | Interpretation | |||||||
| L1/L2 | – | Ganglioneuroma maturing; ganglioneuroblastoma intermixed | ‐ | – | – | – | A | Very low |
| L1 | – | Any, except ganglioneuroma or ganglioneuroblastoma | – | Not amplified | – | – | B | Very low |
| Amplified | – | – | K | High | ||||
| L2 | < 18 | Any, except ganglioneuroma or ganglioneuroblastoma | – | Not amplified | No | – | D | Low |
| Yes | – | G | Intermediate | |||||
| ≥ 18 | Ganglioneuroblastoma nodular; neuroblastoma | Differentiating | Not amplified | No | – | E | Low | |
| Yes | – | H | Intermediate | |||||
| Poorly differentiated or undifferentiated | Not amplified | – | – | H | Intermediate | |||
| – | Amplified | – | – | N | High | |||
| M | < 18 | – | – | Not amplified | – | Hyperdiploid | F | Low |
| < 12 | – | – | Not amplified | – | Diploid | I | Intermediate | |
| 12 to < 18 | – | – | Not amplified | – | Diploid | J | Intermediate | |
| < 18 | – | – | Amplified | – | – | O | High | |
| ≥ 18 | – | – | – | – | – | P | High | |
| MS | < 18 | – | – | Not amplified | No | – | C | Very low |
| Yes | – | Q | High | |||||
| Amplified | – | – | R | High | ||||
| Reference: Cohn 2009. The INRG consensus classification schema includes the criteria INRG stage, age, histologic category, grade of tumour differentiation, MYCN status, presence/absence of 11q aberrations, and tumour cell ploidy. Sixteen statistically or clinically different pretreatment groups of patients (lettered A through R), or both, were identified using these criteria. The categories were designated as very low (A, B, C), low (D, E, F), intermediate (G, H, I, J), or high (K, N, O, P, Q, R) pretreatment risk subsets. | ||||||||
| Stage | Definition |
| 1 | Localised tumour with complete gross excision, with or without microscopic residual disease; representative ipsilateral lymph nodes negative for tumour microscopically (nodes attached to and removed with the primary tumour may be positive) |
| 2A | Localised tumour with incomplete gross excision; representative ipsilateral nonadherent lymph nodes negative for tumour microscopically |
| 2B | Localised tumour with or without complete gross excision, with ipsilateral nonadherent lymph nodes positive for tumour. Enlarged contralateral lymph nodes must be negative microscopically |
| 3 | Unresectable unilateral tumour infiltrating across the midlinea, with or without regional lymph node involvement; or localised unilateral tumour with contralateral regional lymph node involvement; or midline tumour with bilateral extension by infiltration (unresectable) or by lymph node involvement |
| 4 | Any primary tumour with dissemination to distant lymph nodes, bone, bone marrow, liver, skin and/or other organs (except as defined for stage 4S) |
| 4S | Localised primary tumour (as defined for stage 1, 2A or 2B), with dissemination limited to skin, liver, and/or bone marrowb (limited to infants < 1 year of age) |
| Reference: Brodeur 1993. Note: Multifocal primary tumours leg, bilateral adrenal primary tumours should be staged according to the greatest extent of disease, as defined above, and followed by a subscript letter M e.g. 3M. | |
| Response | Primary tumour | Metastatic sites |
| Complete response | No tumour | No tumour; catecholamines normal |
| Very good partial response | Decreased by 90% to 99% | No tumour; catecholamines normal; residual 99Tc bone changes allowed |
| Partial response | Decreased by more than 50% | All measurable sites decreased by > 50%. Bones and bone marrow: number of positive bone sites decreased by > 50%; no more than 1 positive bone marrow site allowed |
| Minimal response | No new lesions; > 50% reduction of any measurable lesion (primary or metastases) with < 50% reduction in any other; < 25% increase in any existing lesion | |
| No response | No new lesions; < 50% reduction but < 25% increase in any existing lesion | |
| Progressive disease | Any new lesion; increase of any measurable lesion by > 25%; previous negative marrow positive for tumour | |
| Reference: Brodeur 1993. | ||
| INSS stage | Age | MYCN | INPC classification | DNA index | Risk group |
| 1 | 0 to 21 y | Any | Any | Any | Low |
| 2A/2B | < 365 d | Any | Any | Any | Low |
| ≥ 365 d to 21 y | Nonamplified | Any | ‐ | Low | |
| ≥ 365 d to 21 y | Amplified | Favorable | ‐ | Low | |
| ≥ 365 d to 21 y | Amplified | Unfavorable | ‐ | High | |
| 3 | < 365 d | Nonamplified | Any | Any | Intermediate |
| < 365 d | Amplified | Any | Any | High | |
| ≥ 365 d to 21 y | Nonamplified | Favorable | ‐ | Intermediate | |
| ≥ 365 d to 21 y | Nonamplified | Unfavorable | ‐ | High | |
| ≥ 365 d to 21 y | Amplified | Any | ‐ | High | |
| 4 | < 548 d | Nonamplified | Any | Any | Intermediate |
| < 365 d | Amplified | Any | Any | High | |
| ≥ 548 d to 21 y | Any | Any | ‐ | High | |
| 4S | < 365 d | Nonamplified | Favorable | > 1 | Low |
| < 365 d | Nonamplified | Any | = 1 | Intermediate | |
| < 365 d | Nonamplified | Unfavorable | Any | Intermediate | |
| < 365 d | Amplified | Any | Any | High | |
| Reference: NCI PDQ 2017 | |||||
| Study | Study design | Type of HSCT | Type of RA | Dose1 | Pat2 | Type of adverse event (N of affected participants) |
| CR | PBSCT | CRA | 160 | 1 | Pneumocystis carinii pneumonia (1) | |
| CR | NR | CRA | 160 | 1 | Hypercalcaemia (1), osteoblastic lesions (1) | |
| SA‐IS | BMT or PBSCT | CRA | 160 | 44 | NR | |
| SA‐IS | PBSCT | CRA | 160 | 33 | NR | |
| SA‐IS | PBSCT | CRA | 120 to 160 | 14 | NR | |
| CR | BMT | CRA | 160 | 2 | Bone marrow transplant nephropathy (2) | |
| CR | BMT | CRA | 33 to 1023 | 1 | Growth failure (1) | |
| SA‐IS | BMT | CRA | 100 to 200 | 31 | Grade 3/4 toxicity of skin, liver and hypercalcaemia correlated with peak serum levels of CRA | |
| SA‐IS | PBSCT | CRA | 160 | 12 | Ataxia (1) | |
| RCT | BMT | CRA | NR | 12 | NR | |
| RCT | NR | CRA | 15 to 224 | NR | Dry skin (47), cheilitis (24), bone pain (16), other (13) | |
| RCT | PBSCT | CRA | 160 | 192 | Grade 3 toxic effects: hypertension (4), hematuria (2), elevated serum creatinine (2), proteinuria (3); purged and non‐purged transplantation group combined | |
| CS | NR | CRA | 160 | 1 | Cheilitis (1) | |
| CS | NR | CRA | NR | 20 | NR | |
| CR | NR | CRA | 160 | 3 | Hypercalcaemia (3) | |
| CR | NR | Fenretinide | 666 to 20515 | 2 | Rod electroretinogram suppression (2) | |
| SA‐IS | PBSCT | CRA | 160 | 8 | NR | |
| SA‐IS | BMT | CRA | 160 | 22 | NR | |
| CR | BMT | CRA | 130 to 400 | 2 | Hypercalcaemia (2) | |
| CR | BMT | CRA | 33 to 1023 | 1 | Generalised metaphyseal modification (1) | |
| SA‐IS | PBSCT | CRA | 160 | 30 | NR | |
| CR | NR | Fenretinide | 2210 | 1 | Langerhans cell histiocytosis (1) | |
| SA‐IS | BMT or PBSCT | CRA | NR | 36 | NR | |
| SA‐IS | NR | CRA | 160 | 75 | NR | |
| CR | NR | CRA | 140 | 1 | NR | |
| SA‐IS | PBSCT | CRA | 125 | 44 | Skin eruption, particularly face | |
| CR | BMT | CRA | 160 | 2 | Bone marrow transplant nephropathy (2) | |
| SA‐IS | NR | CRA | 160 | 28 | Mild skin toxicity (9), cheilitis (1), hypercalcaemia (2) | |
| SA‐IS | NR | CRA | 160 | 103 | Grade 3 ‐ 4 skin toxicity or cheilitis (5) | |
| SA‐IS | BMT | CRA | 100 to 200 | 49 | Dose‐limiting toxicity of hypercalcaemia (3), arthralgia and myalgia (1), grade 1 to 3 hypercalcaemia (9) | |
| SA‐IS | BMT | CRA | 200 | 51 | Hypercalcaemia (3), rash (2) | |
| SA‐IS | NR | Fenretinide | 1800 to 2475 | 626 | Rash (1), diarrhoea (1), nausea (2), vomiting (1), nyktalopia (1), abdominal pain (4) | |
| RCT | NR | CRA | 160 | 108 | "Few toxic effects" | |
| Notice: Studies presented in this table were not eligible for inclusion in this review. 1Dose in mg/m2/day. The unit mg/kg may be transformed to mg/m2. The average body weight, body length, and body surface of a 6‐month‐old child may be 8 kg, 67 cm, and 0.39 m2, thus 8 kg divided by 0.39 m2 is roughly equalto a conversion factor of 20 (CDC 2000a). This factor increases continuously with age. The average body weight, body length, body surface of an 11‐year‐old child may be 36 kg, 143 cm, and 1.20 m2, thus 36 kg divided by 1.20 m2 is roughly equal to a conversion factor of 30 (CDC 2000b). The unit mg per day may be transformed to m2 per day. For example, 300 mg per day may vary on average between 769 mg/m2 (300 mg/0.39 m2) and 250 m2 (300 mg/1.20 m2) 2Participants treated with retinoic acid after HDCT followed by autologous HSCT acid and evaluated for toxicity. 3Inamo 1999; Nishimura 1997: Patients received retinoic acid at a dose of 40 mg per day. The daily dose may vary on average between 102 mg/m2 (40 mg/0.39 m2) and 33 m2 (40 mg/1.20 m2) 4Kohler 2000: Participants received retinoic acid at a dose of 0.75 mg/kg per day. The daily dose may vary on average between 15 mg/m2 (0.75 mg/kg * 20) and 22 mg/m2 (0.75 mg/kg * 30). 5Marmor 2008: Participants received retinoic acid at a dose of 800 mg per day. The daily dose may vary on average between 2051 mg/m2 (800 mg/0.39 m2) and 666 m2 (800 mg/1.20 m2). 6Villablanca 2011: 51 of the 62 participants received HSCT. BMT: bone marrow transplantation; CR: case report; CS: case series; CRA: 13‐cis‐retinoic acid; HDCT: high‐dose chemotherapy; HSCT: haematopoietic stem cell transplantation; N: number of participants; NR: not reported; PBSCT: peripheral blood stem cell transplantation; RA: retinoic acid; SA‐IS: single‐arm intervention study such as phase‐1 or phase‐2 clinical trial | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.87 [0.46, 1.63] | |
| 2 Event‐free survival Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | 0.86 [0.50, 1.49] | |

