Antidepresivos para pacientes con epilepsia y depresión
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Depression] explode all trees
#2 MeSH descriptor: [Depressive Disorder] explode all trees
#3 depression* or depressive*:ti,ab,kw (Word variations have been searched)
#4 "respiratory depression":ti,ab,kw (Word variations have been searched)
#5 (#1 or #2 or #3) not #4
#6 MeSH descriptor: [Antidepressive Agents] explode all trees
#7 (antidepressant or antidepressive or "af 1161" or "ba 34276" or "bc 105" or "brl 29060" or "brl 29060" or "cl 67772" or "cp 15467 61" or "du 23000" or "fg 7051" or "ici 58834" or "l deprenyl" or "leo 640" or "lilly 110140" or "lu 10 171" or "ma 1291" or "nsc 16895" or "org gb 94" or "r 55667" or "ro 11 1163" or "trans 2 phenylcyclopropylamine" or "ym 35 995" or "ym 992" or "zk 62711" or *amitriptyline or *doxepin or *moclobemide or *nortriptyline or *phenylethylhydrazine or *sertraline or *trimip or *trimipramine or *tripramine or *tryptophan or abilify or adapin or adaptol or adderall or agomelatine or aiglonyl or allegron or altruline or amfebutamone or amineptine or amineurin or amisulpride or amitrip or amitriptylin* or amitrol or amiz?l or amoxapine or amphetamine or anafranil or anapsique or aponal or ardeydorm or ardeytropin or aremis or arima or aripiprazole or arminol or aropax or asenapine or asendin or astyl or atomoxetine or auror?x or aventyl or axiomin or benactyzine or benzeneacetic acid or besitran or bolvidon or bosnyl or brofaramine or bupropion or buspar or buspirone or butriptyline or carbamazepine or celexa or chlomipramine or chlorgyline or cipralex or cipramil or citalopram or clomipramine or clorgilin* or clorgyline or concerta or cymbalta or cytalopram or dalcipran or damilen or de*methylimipramine or deanol or defanyl or deftan or deman?l or demolox or depakote or deponerton or deprax or deprenorm or deprilept or deptran or desidox or desiflu or desipramine or desisulpid or desitriptyline or desmethylamitriptylin or desmethylloxapine or desvenlafaxine or desyrel or dexedrine or dexmethylphenidate or dextroamphetamine or dibencycladine or digton or dilithium carbonate or dimethylaminoethanol or dimethylethanolamine or dogmatil or dolmatil or domical or doneurin or dosulepin or dothiepin or doxepia or doxepin* or duloxetine or dumirox or edronax or ef*exor or eglonyl or ekilid or elavil or eldepryl or eldoral or emovit or emsam or endep or escitalopram or eskalith or espadox or espiride or etonin or etoperidone or evadene or favarin or fenelzin or feprapax or feraken or fevarin or floxyfral or fluoxetin* or fluvoxadura or fluvoxamin* or focalin or gam?nil or gladem or guastil or herphonal or hydiphen or imidobenzyle or imipramine or imizin or insidon or iprazid or iprindole or iproniazid or isocarboxazid or ixel or janimine or jatrosom or lamictal or lamotrigine or laroxyl or lebopride or lentizol or lerivon or lexapro or lisdexamfetamine or lithane or lithium or lithobid or lofepramine or lomont or lopramine or lubazodone or lucidil or ludiomil or lustral or luvox or lyphan or manerix or maprolu or maprotilin* or mareen or marplan or melitracen or meresa or meridia or methylphenidate or mianserin or micalith or midalcipran or milnacepra* or mirpan or mirtazapine or moclamine or moclix or moclob?mide or moclobemid* or moclobeta or moclodura or moclonorm or modal or molipaxin or nardelzine or nardil or naturruhe or nefadar or nefazodone or neogama or nialamide or norfenazin or norpramin or nortrilen or norval or novoprotect or olanzapine or opipramol or optimax or oxitriptan or pamelor or parnate or paroxetine or paxil or paxtibi or pertofran* or pertrofran or petylyl or phenelzine or phenethylhydrazine or pirazidol or pirlindole or pizotifen or pizotyline or polomigran or pontiride or pramolan or priadel or pristiq or prondol or prothiaden or protriptyline or prozac or prudoxin or pryleugan or psicocen or psymion or quetiapine or quilinorm* or quipazine or quitaxon or quomen or r55667 or reboxetine or reductil or remeron or rhotrimine or rimoc or ritalin or ritanserin or rolipram or sandomigran or saphris or sarafem or saroten or sarotex or savella or sealdin or sediel or selegiline or sendis or seroquel or seroxat or serzone or sibutramine or sin*quan or solian or stangyl or strattera or sulp or sulpiride or sulpitil or sulpivert or sulpor or surmontil or sycrest or symbyax or synedil or syneudon or tandospirone or tegretol or tepavil or thombran or tianeptine or tofranil or toledomin or tolvon or tonibral or tradozone or tramadol or tramal or transamine or tranylcypromine or trazodon* or trimeprimin* or trimidura or trimineurin or triptafen or trittico or trofan or tryptacin or tryptan or tryptanol or tryptine or tryptizol or tyrima or ultram or valdoxan or valproic acid or venlafaxine or viibryd or vilazodone or viloxazine or vivactil or vivalan or vyvanse or wellbutrin or xepin or yentreve or zelapar or zimelidine or zispin or zoloft or zonalon or zyban or zyntabac):ti,ab,kw (Word variations have been searched)
#8 #6 or #7
#9 (epilep* or seizure* or convuls*):ti,ab,kw (Word variations have been searched)
#10 MeSH descriptor: [Epilepsy] explode all trees
#11 MeSH descriptor: [Seizures] explode all trees
#12 (#9 or #10 or #11) in Trials
#13 #5 and #8 and #12
#14 MeSH descriptor: [Electroconvulsive Therapy] explode all trees
#15 #13 not #14
Appendix 2. MEDLINE search strategy
1. (validation studies or clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv or comparative study or evaluation studies or multicenter study).pt.
2. ((observation$ or cohort or case$ or cross?section$ or "cross section$" or "time‐series" or "time series" or "before and after" or "before‐and‐after" or retrospective) adj2 (study or trial or method)).mp.
3. (randomized controlled trial or controlled clinical trial).pt. or (randomized or placebo or randomly).ab.
4. clinical trials as topic.sh.
5. trial.ti.
6. 1 or 2 or 3 or 4 or 5
7. exp animals/ not humans.sh.
8. 6 not 7
9. exp Depression/ or exp Depressive Disorder/ or exp Dysthymic Disorder/ or (depression$ or depressive$).tw.
10. "respiratory depression".tw.
11. 9 not 10
12. exp Antidepressive Agents/ or anti?depress$.tw.
13. ("af 1161" or "bc 105" or "brl 29060" or "cl 67772" or "cp 15467 61" or "du 23000" or "ici 58834" or "leo 640" or "lilly 110140" or "ma 1291" or "nsc 16895" or "org gb 94" or "r 55667" or "ro 11‐1163" or "trans 2 phenylcyclopropylamine" or "ym‐35,995" or "zk 62711" or abilify or adapin or adaptol or adderall or af?1161 or agomelatine or aiglonyl or allegron or altruline or amfebutamone or amineptine or amineurin or amisulpride or amitrip or amitriptylin$ or amitrol or amiz?l or amoxapine or amphetamine or anafranil or anapsique or apo?doxepin or apo?moclob?mide or apo?nortriptyline or apo?sertraline or apo?trimip or apoamitriptyline or aponal or ardeydorm or ardeytropin or aremis or arima or aripiprazole or arminol or aropax or asenapine or asendin or astyl or atomoxetine or auror?x or aventyl or axiomin or ba?34276 or bc?105 or benactyzine or benzeneacetic acid or besitran or beta?phenylethylhydrazine or bolvidon or bosnyl or brl?29060 or brofaramine or bupropion or buspar or buspirone or butriptyline or carbamazepine or celexa or chlomipramine or chlorgyline or cipralex or cipramil or citalopram or cl?67772 or clomipramine or clorgilin$ or clorgyline or concerta or cp?15467?61 or cymbalta or cytalopram or dalcipran or damilen or de?methylimipramine or deanol or defanyl or deftan or deman?l or demolox or depakote or deponerton or deprax or deprenorm or deprilept or deptran or desidox or desiflu or desipramine or desisulpid or desitriptyline or desmethylamitriptylin or desmethylloxapine or desvenlafaxine or desyrel or dexedrine or dexmethylphenidate or dextroamphetamine or dibencycladine or digton or dilithium carbonate or dimethylaminoethanol or dimethylethanolamine or dogmatil or dolmatil or domical or doneurin or dosulepin or dothiepin or doxepia or doxepin$ or du?23000 or duloxetine or dumirox or edronax or ef?exor or eglonyl or ekilid or elavil or eldepryl or eldoral or emovit or emsam or endep or escitalopram or eskalith or espadox or espiride or etonin or etoperidone or evadene or favarin or fenelzin or feprapax or feraken or fevarin or fg?7051 or floxyfral or fluoxetin$ or fluvoxadura or fluvoxamin$ or focalin or gam?nil or gen?nortriptyline or gen?sertraline or gladem or guastil or herphonal or hydiphen or ici?58834 or imidobenzyle or imipramine or imizin or insidon or iprazid or iprindole or iproniazid or isocarboxazid or ixel or janimine or jatrosom or lamictal or lamotrigine or laroxyl or l‐deprenyl or lebopride or lentizol or lerivon or levo?tryptophan or lexapro or lilly?110140 or lisdexamfetamine or lithane or lithium or lithobid or lofepramine or lomont or lopramine or l‐tryptophan or lu?10?171 or lubazodone or lucidil or ludiomil or lustral or luvox or lyphan or ma?1291 or manerix or maprolu or maprotilin$ or mareen or marplan or melitracen or meresa or meridia or methylphenidate or mianserin or micalith or midalcipran or milnacepram or milnacipra? or mirpan or mirtazapine or moclamine or moclix or moclob?mide or moclobemid$ or moclobeta or moclodura or moclonorm or modal or molipaxin or nardelzine or nardil or naturruhe or nefadar or nefazodone or neogama or nialamide or nor?nortriptyline or norfenazin or norpramin or nortrilen or nortriptyline or norval or novo?doxepin or novo?moclob?mide or novo?nortriptyline or novo?sertraline or novo?tripramine or novoprotect or nsc?16895 or nu?moclob?mide or nu?nortriptyline or nu?trimipramine or nu?tripramine or numo?moclob?mide or olanzapine or opipramol or optimax or oxitriptan or pamelor or parnate or paroxetine or paxil or paxtibi or pert?ofran$ or petylyl or phenelzine or phenethylhydrazine or phenylethylhydrazine or pirazidol or pirlindole or pizotifen or pizotyline or pms?moclob?mide or pms?nortriptyline or pms?tryptophan or polomigran or pontiride or pramolan or priadel or pristiq or prondol or prothiaden or protriptyline or prozac or prudoxin or pryleugan or psicocen or psymion or quetiapine or quilinorm?retard or quipazine or quitaxon or quomen or r55667 or r‐55667 or ratio?nortriptyline or ratio?sertraline or ratio?tryptophan or reboxetine or reductil or remeron or rhotrimine or rhoxal?sertraline or rimoc or ritalin or ritanserin or ro‐11‐1163 or rolipram or sandomigran or saphris or sarafem or saroten or sarotex or savella or sealdin or sediel or selegiline or sendis or seroquel or seroxat or sertraline or serzone or sibutramine or sin?quan or solian or stangyl or strattera or sulp or sulpiride or sulpitil or sulpivert or sulpor or surmontil or sycrest or symbyax or synedil or syneudon or tandospirone or tegretol or tepavil or thombran or tianeptine or tofranil or toledomin or tolvon or tonibral or tradozone or tramadol or tramal or trans‐2‐phenylcyclopropylamine or transamine or tranylcypromine or trazodon$ or trim?pr?min$ or trimidura or trimineurin or trimip or tripramine or triptafen or trittico or trofan or tryptacin or tryptan or tryptanol or tryptine or tryptizol or tryptophan or tyrima or ultram or valdoxan or valproic acid or venlafaxine or viibryd or vilazodone or viloxazine or vivactil or vivalan or vyvanse or wellbutrin or xepin or yentreve or ym‐992 or zelapar or zimelidine or zispin or zk?62711 or zoloft or zonalon or zyban or zyntabac).mp.
14. 12 or 13
15. exp Epilepsy/
16. exp Seizures/
17. (epilep$ or seizure$ or convuls$).tw.
18. 15 or 16 or 17
19. exp Pre‐Eclampsia/ or exp Eclampsia/
20. 18 not 19
21. 8 and 11 and 14 and 20
22. exp Electroconvulsive Therapy/
23. 21 not 22
24. 23 not case reports.pt.
Appendix 3. Extended 'Risk of bias' tool for non‐randomised studies
Studies for which the 'Risk of bias' tool is intended
Only suitable for 'cohort‐like' studies, individually or cluster‐allocated. This can include secondary analyses of clinical databases providing the analysis is clearly structured as a comparison of control and intervention participants:
Individually allocated study designs
-
Randomised controlled trial
-
Quasi‐randomised controlled trial
-
Non‐randomised controlled trial
-
Controlled before and after study (not common use of this label, see controlled cohort before and after study below)
-
Prospective cohort study
-
Retrospective cohort study
Cluster‐allocated study designs
-
Cluster randomised controlled trial
-
Cluster quasi‐randomised controlled trial
-
Cluster non‐randomised controlled trial
-
Controlled interrupted time series
-
Controlled cohort before and after study
Assessment of risk of bias
Issues when using the modified 'Risk of bias' tool to assess cohort‐like non‐randomised studies:
-
Follows principle for existing Cochrane Collaboration tool for risk of bias: score judgement and provide information (preferably direct quote) to support judgement.
-
Modified 'Risk of bias' tool includes an additional item on confounding.
-
Five‐point scale for some items (to distinguish 'unclear' from intermediate risk of bias).
-
Keep in mind the general philosophy – assessment is not about whether researchers could have done better but about risk of bias; the assessment tool must be used in a standard way whatever the difficulty/circumstances of investigating the research question of interest and whatever study design features were used.
-
Use of a five‐point scale is uncharted territory
-
Anchors for five‐point scale: '1/No/low risk' of bias should correspond to a high quality RCT. '5/high risk' of bias should correspond to a risk of bias that means the findings should not be considered (too risky, too much bias, more likely to mislead than inform).
Sequence generation
-
Low/high/unclear risk of bias item.
-
Always high risk of bias (not random) for a non‐randomised study.
-
Might argue that this item is redundant for non‐randomised studies since they are always at high risk of bias – but important to include in 'Risk of bias' table ('level playing field' argument).
Allocation concealment
-
Low/high/unclear risk of bias item.
-
Potentially low risk of bias for a non‐randomised study, e.g. quasi‐randomised (high risk of bias due to sequence generation) but concealed (author judges that the people making decisions about including participants did not know how allocation was being done, e.g. odd/even date of birth/hospital number).
Risk of bias from confounding (additional item for non‐randomised studies; assess for each outcome)
-
Assumes a prespecified list of potential confounders defined in the protocol for the systematic review.
-
Low (1) / 2 / 3 / 4 / high (5) / unclear risk of bias item.
-
Judgement needs to factor in (see 'worksheet'):
-
proportion of confounders (from pre‐specified list) that were considered;
-
whether most important confounders (from pre‐specified list) were considered;
-
resolution/precision with which confounders were measured;
-
extent of imbalance between groups at baseline;
-
care with which adjustment was done (typically a judgement about the statistical modelling carried out by authors).
-
-
Low risk of bias requires that all important confounders are balanced at baseline, i.e.:
-
not primarily/not only a statistical judgement; or
-
measured 'well' and 'carefully' controlled for in the analysis.
-
We have provided an optional 'worksheet' to help reviewers to focus on the task (rows = confounders and columns = factors to consider). Authors should make a risk of bias judgement about each factor first and then combine these (by eyeballing rather than quantitatively) to make the judgement in the main 'Risk of bias' table.
Risk of bias from lack of blinding (assess for each outcome, as per the existing risk of bias tool)
-
Low (1) / 2 / 3 / 4 / high (5) / unclear risk of bias item.
-
Judgement needs to factor in:
-
nature of outcome (subjective/objective; source of information);
-
who was/was not blinded and the risk that those who were not blinded could introduce performance or detection bias.
-
Risk of bias from incomplete outcome data (assess for each outcome, as per the existing risk of bias tool)
-
Low (1) / 2 / 3 / 4 / high (5) / unclear risk of bias item.
-
Judgement needs to factor in:
-
reasons for missing data;
-
whether amount of missing data balanced across groups, with similar reasons;
-
whether group comparison appropriate (e.g. 'analysed in allocated group' issue).
-
Risk of bias from selective reporting (assess for each outcome)
-
More wide‐ranging than existing assessment recommendation. Key issue is whether outcomes were clearly defined, and methods of analysis were pre‐specified and adhered to.
-
Low (1) / 2 / 3 / 4 / high (5) / unclear risk of bias item.
-
Judgement needs to factor in:
-
existing risk of bias guidance on selective outcome reporting;
-
also, extent to which analyses (and potentially other choices) could have been manipulated to bias the findings reported, e.g. choice of method of model fitting, potential confounders considered/included;
-
look for evidence that there was a protocol in advance of doing any analysis/obtaining the data (difficult unless explicitly reported); non‐randomised studies are very different from RCTs. RCTs must have a protocol in advance of starting to recruit (for research ethics committee/institutional review board/other regulatory approval); non‐randomised studies need not (especially older studies);
-
hence, separate yes/no items asking reviewers whether they think the researchers had a prespecified protocol and analysis plan?
-
Appendix 4. Assessment of confounding variables
| Assessment of how researchers dealt with confounding Method for identifying relevant confounders described by researchers: Yes No If yes, describe the method used:
Relevant confounders described: Yes No
List confounders described below |
| Method used for controlling for confounding At design stage: matching by characteristics of subjects (see below for matching by propensity score) Variables on which subjects matched: …………………………………. …………………………………. …………………………………. …………………………………. At analysis stage: stratification multivariable regression propensity scores (matching) propensity scores (multivariable regression) Describe confounders controlled for below |
| Confounders described by researchers Enter/preprint prespecified list of confounders (rank order in importance? Important in bold?) |
| Confounder | Considered | Precision | Imbalance | Adjustment |
|
| ||||
|
| ||||
|
|

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
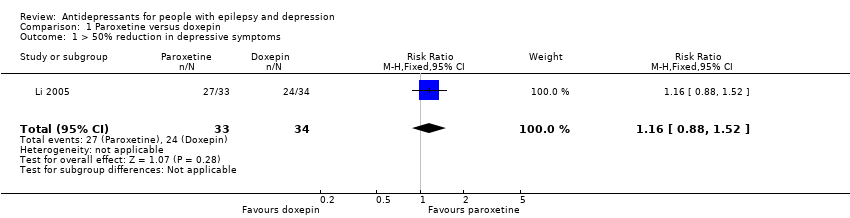
Comparison 1 Paroxetine versus doxepin, Outcome 1 > 50% reduction in depressive symptoms.
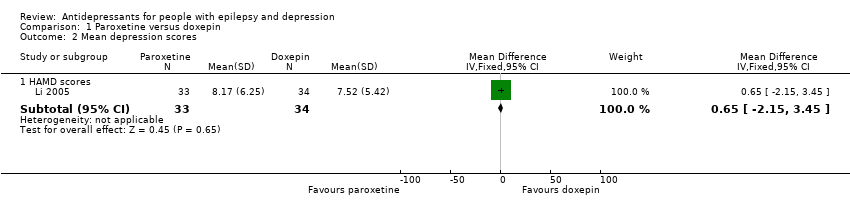
Comparison 1 Paroxetine versus doxepin, Outcome 2 Mean depression scores.
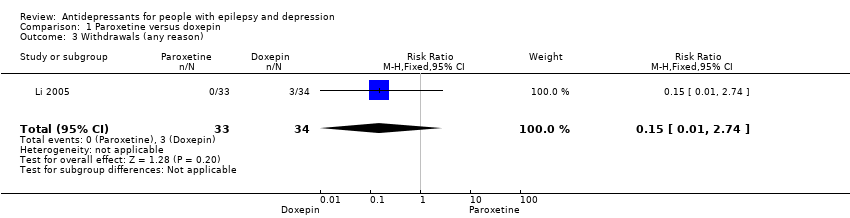
Comparison 1 Paroxetine versus doxepin, Outcome 3 Withdrawals (any reason).

Comparison 1 Paroxetine versus doxepin, Outcome 4 Adverse effects.
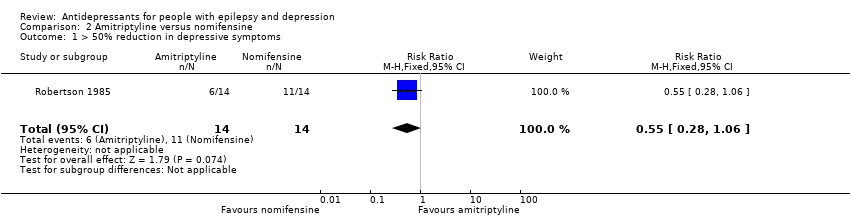
Comparison 2 Amitriptyline versus nomifensine, Outcome 1 > 50% reduction in depressive symptoms.
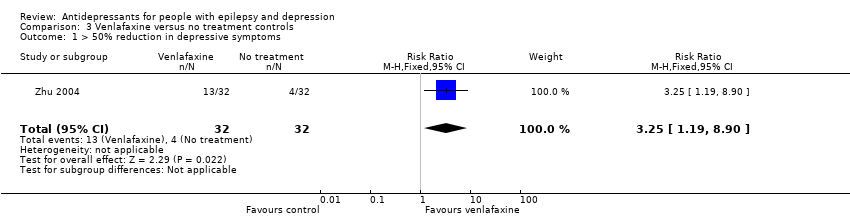
Comparison 3 Venlafaxine versus no treatment controls, Outcome 1 > 50% reduction in depressive symptoms.
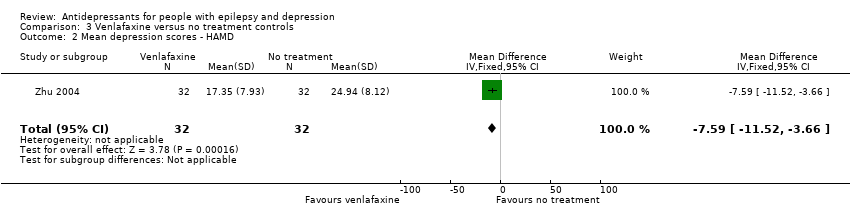
Comparison 3 Venlafaxine versus no treatment controls, Outcome 2 Mean depression scores ‐ HAMD.

Comparison 4 Citalopram (before and after), Outcome 1 Mean depression scores HAMD‐21.
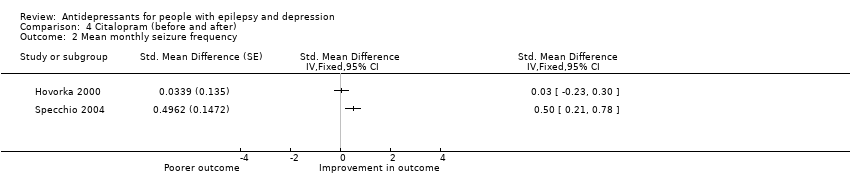
Comparison 4 Citalopram (before and after), Outcome 2 Mean monthly seizure frequency.
| Paroxetine compared to doxepin for people with epilepsy and depression | ||||||
| Patient or population: people with epilepsy and depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxepin | Paroxetine | |||||
| > 50% reduction in depressive symptoms | 706 per 1000 | 819 per 1000 | RR 1.16 | 67 | ⊕⊕⊕⊝1 | Only 1 study examined the influence of paroxetine versus doxepin on reduction in depression and it found no significant difference between the 2 drugs |
| Mean depression scores ‐ HAMD scores | The mean HAMD depression score in the intervention groups was | 67 | ⊕⊕⊕⊝1 | In the same study no differences were found between mean depression scores in patients taking paroxetine compared to those taking doxepin | ||
| Seizure frequency | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Withdrawals (specific reasons) | 88 per 1000 | 13 per 1000 | RR 0.15 | 67 | ⊕⊕⊕⊝1 | In this study 0 patients withdrew from the paroxetine group and 3 withdrew from the doxepin group. No significant difference was found between the 2 groups |
| Cognitive functioning | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Quality of life | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Adverse effects | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded for imprecision due to only one study contributing to the outcomes and it was a small study. | ||||||
| Amitriptyline compared to nomifensine for people with epilepsy and depression | ||||||
| Patient or population: people with epilepsy and depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nomifensine | Amitriptyline | |||||
| > 50% reduction in depressive symptoms | 786 per 1000 | 432 per 1000 | RR 0.55 | 28 | ⊕⊕⊕⊝1 | 1 study compared amitriptyline and nomifensine in reducing seizures and there was no significant difference found between the 2 groups |
| Seizure frequency | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Withdrawals | ‐ | ‐ | ‐ | 0 | ‐ | No data contributed to this outcome |
| Cognitive functioning | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Quality of life | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Adverse effects | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded for imprecision due to only one study contributing to the outcomes and it was a small study. | ||||||
| Venlafaxine compared to no treatment for people with epilepsy and depression | ||||||
| Patient or population: people with epilepsy and depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Venlafaxine | |||||
| > 50% reduction in depressive symptoms | 125 per 1000 | 406 per 1000 | RR 3.25 | 64 | ⊕⊕⊕⊝1 moderate | 1 study compared venlafaxine to a no treatment control group and found that venlafaxine was more than 3 times more effective in reducing seizures compared to controls |
| Mean depression scores ‐ HAMD | The mean HAMD depression score in the intervention groups was | 64 | ⊕⊕⊕⊝1 | The same study found mean depression scores to be significantly lower in the venlafaxine group compared to the control group | ||
| Seizure frequency | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Withdrawals | ‐ | ‐ | ‐ | 0 | ‐ | No data contributed to this outcome |
| Cognitive functioning | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Quality of life | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| Adverse effects | ‐ | ‐ | ‐ | 0 (0 studies) | ‐ | No data contributed to this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded for imprecision due to only one study contributing to the outcomes and it was a small study. | ||||||
| Citalopram (before and after) for people with epilepsy and depression | ||||
| Patient or population: people with epilepsy and depression | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants | Quality of the evidence | Comments |
| Corresponding risk | ||||
| Citalopram (before and after) | ||||
| Mean depression scores ‐ HAMD | The mean HAMD depression score in the intervention groups was | 88 | ⊕⊕⊝⊝ | 2 before and after studies investigated citalopram and found that depression scores were significantly lower after treatment |
| Mean monthly seizure frequency | 88 | ⊕⊝⊝⊝ | 2 studies found mixed evidence for the effect of citalopram on seizure frequency. Due to high heterogeneity the overall effect estimate is not presented | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1Across the studies there were concerns about bias with regards to the methods of blinding and methods to deal with confounding variables. | ||||
| 1 Low risk | 2 | 3 | 4 | 5 High risk | |
| Confounding | All important1 confounders considered2 and suitable method of adjustment3 employed. Outcome unlikely to be affected | Most important4 confounders considered and suitable method of adjustment employed. Outcome unlikely to be affected | Some confounders5 considered and full or partial adjustment employed6. Possible implication for outcome | Some confounders considered and no adjustment employed. Likely to affect outcome | No important confounders considered and no adjustment employed. Likely to affect outcome |
| Blinding | Assessors blinded to participant's drug regime and participants blinded to drug regime. Outcome unlikely to be affected | Assessors blinded to participant's drug regime. Outcome unlikely to be affected | Partial blinding7 involved in study. Possible implication for outcome | Partial or no blinding involved in study. Outcome likely to be affected | No blinding involved in study. Outcome likely to be affected |
| Incomplete outcome data | No missing data and/or appropriate analysis8 used to deal with missing data. Unlikely to affect outcome | Smaller amount (< 25%) of missing data with reasons given, balanced across groups. Unlikely to affect outcome | Larger amount of missing data (> 25%) with or without reasons given, balanced across groups. Possible implication for outcome | Larger amount (> 25%) of missing data, imbalance across groups. Outcome likely to be affected | No information provided regarding missing data. Likely to affect outcome |
| Selective outcome reporting | A priori outcomes measured, analysed and reported in main report. Protocol available. Unlikely to affect outcome | A priori outcomes measured, analysed and reported in main report9. Protocol not available. Unlikely to affect outcomes | Limited information regarding a priori outcomes and measures. Possible implication for outcome | Outcomes measured but not analysed or reported | Outcomes measured but not analysed or reported and clinical judgement infers the presence of an unreported measured outcome10 |
| Other bias | No bias identified | Bias identified. Unlikely to affect outcome | Bias identified. Possible implication for outcome | Bias identified. Likely to affect outcome | Bias identified. Extremely likely to affect outcome |
| 1Important confounders include:
2Reported demographic information and other confounders. 3Matching scores, multiple regression, analysis of co‐variance, stratification. 4At least four out of six of important confounders including: mean baseline depression score and mean baseline seizure frequency. 5At least two out of six of the important confounders. 6Full adjustment of confounding variables, e.g. see footnote 2, or partial adjustment, e.g. researchers select limited number of variables to adjust for. 7Assessors of outcome are only blinded to certain groups, e.g. blinded to intervention group but not controls. 8Intention‐to‐treat analysis. 9An a priori statement is made in the methods section of the main report regarding measurement and analysis of outcome. 10For example, failure to report full‐scale depression score when all other indices are reported. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 > 50% reduction in depressive symptoms Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.88, 1.52] |
| 2 Mean depression scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 HAMD scores | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐2.15, 3.45] |
| 3 Withdrawals (any reason) Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.74] |
| 4 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.1 Blurred vision | 1 | 67 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.34 [0.09, 1.32] |
| 4.2 Dizziness | 1 | 67 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.21 [0.03, 1.37] |
| 4.3 Dry mouth | 1 | 67 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.26 [0.06, 1.20] |
| 4.4 Sleep disorders | 1 | 67 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.32 [0.08, 1.20] |
| 4.5 Urinary retention | 1 | 67 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.34 [0.01, 21.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 > 50% reduction in depressive symptoms Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.28, 1.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 > 50% reduction in depressive symptoms Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.19, 8.90] |
| 2 Mean depression scores ‐ HAMD Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐7.59 [‐11.52, ‐3.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean depression scores HAMD‐21 Show forest plot | 2 | 176 | Std. Mean Difference (Fixed, 95% CI) | 1.17 [0.96, 1.38] |
| 2 Mean monthly seizure frequency Show forest plot | 2 | Std. Mean Difference (Fixed, 95% CI) | Totals not selected | |

