Arteméter para el paludismo grave
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010678.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 septiembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Enfermedades infecciosas
- Clasificada:
-
- Pendiente de actualización
Authors currently updating
The update is due to be published in 2019.Evaluada: 22 March 2019
- Pendiente de actualización
- Copyright:
-
- Copyright © 2014 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
- This is an open access article under the terms of the Creative Commons Attribution‐Non‐Commercial Licence, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Ekpereonne Esu (EE) and Emmanuel E. Effa (EEE) identified and extracted data from eligible trials for this review. EE entered data into Review Manager (RevMan). EEE and EE performed risk of bias assessment and analysed data. EE prepared the 'Summary of findings' tables and the first draft of the review. All authors read, gave input to all sections and approved the final version.
Sources of support
Internal sources
-
University of Calabar, Nigeria.
-
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Declarations of interest
None known.
Acknowledgements
The academic editor for this review was Dr Michael Eisenhut; Dr David Sinclair provided support in the preparing the 'Summary of findings' tables.
The protocol for this review was developed during the Reviews for Africa Fellowship Programme organized by the Nigerian Branch of the South Africa Cochrane Centre in July 2012. The UK Department for International Development (DFID) supports this programme through the Effective Health Care Research Consortium (EHCRC) at the Liverpool School of Tropical Medicine (LSTM). This document is an output from a project funded by UKaid for the benefit of developing countries. The views expressed are not necessarily those of UKAid or the Department for International Development (DFID).
The editorial base for the Cochrane Infectious Diseases Group is funded by UKaid from the UK Government for the benefit of developing countries.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Jun 18 | Artemether for severe malaria | Review | Ekpereonne B Esu, Emmanuel E Effa, Oko N Opie, Martin M Meremikwu | |
| 2014 Sep 11 | Artemether for severe malaria | Review | Ekpereonne Esu, Emmanuel E Effa, Oko N Opie, Amirahobu Uwaoma, Martin M Meremikwu | |
| 2013 Aug 12 | Artemether intramuscular injection for severe malaria in children | Protocol | Ekpereonne Esu, Emmanuel E Effa, Oko N Opie, Amirahobu Uwaoma, Martin M Meremikwu | |
Differences between protocol and review
In the protocol, we specified that we would include only trials in children (aged < 15 years). However, we amended the inclusion criteria to include trials in adults and children.
We said we would explore data by drug regimen, type of severe malaria (cerebral versus non‐cerebral malaria), time since admission to hospital, length of follow‐up and geographical region, but data were insufficient.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Africa;
- Age Factors;
- Antimalarials [*administration & dosage, adverse effects];
- Artemether [*administration & dosage, adverse effects];
- Artesunate [administration & dosage, adverse effects];
- Asia;
- Coma [drug therapy];
- Fever [drug therapy];
- Injections, Intramuscular [mortality];
- Malaria, Cerebral [drug therapy, mortality];
- Malaria, Falciparum [*drug therapy, mortality];
- Oceania;
- Quinine [administration & dosage, adverse effects];
- Randomized Controlled Trials as Topic;
- Treatment Outcome;
Medical Subject Headings Check Words
Adolescent; Adult; Child; Child, Preschool; Humans; Infant;
PICO
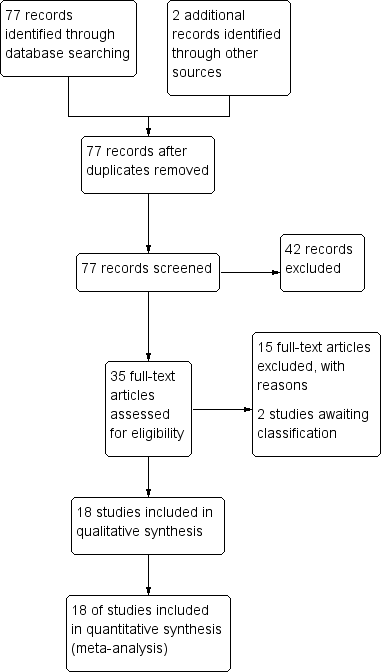
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
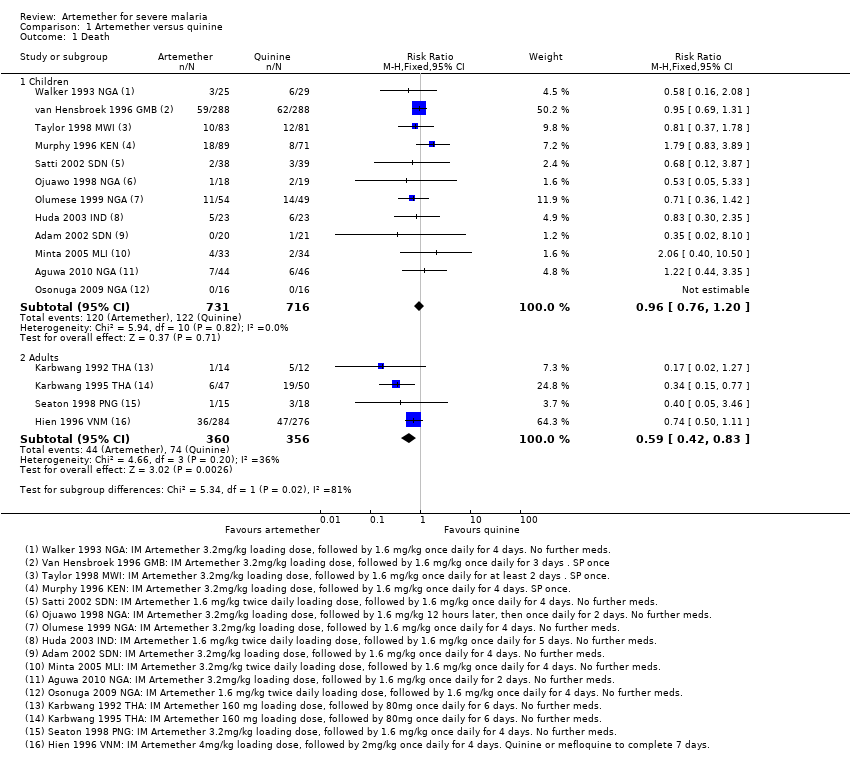
Comparison 1 Artemether versus quinine, Outcome 1 Death.
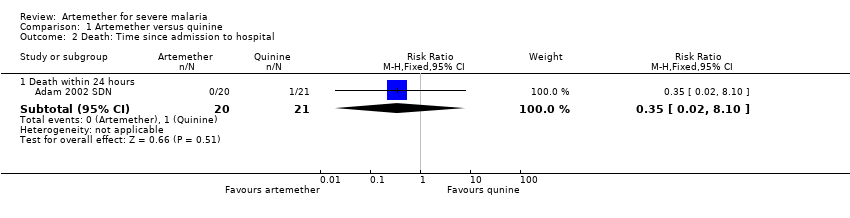
Comparison 1 Artemether versus quinine, Outcome 2 Death: Time since admission to hospital.
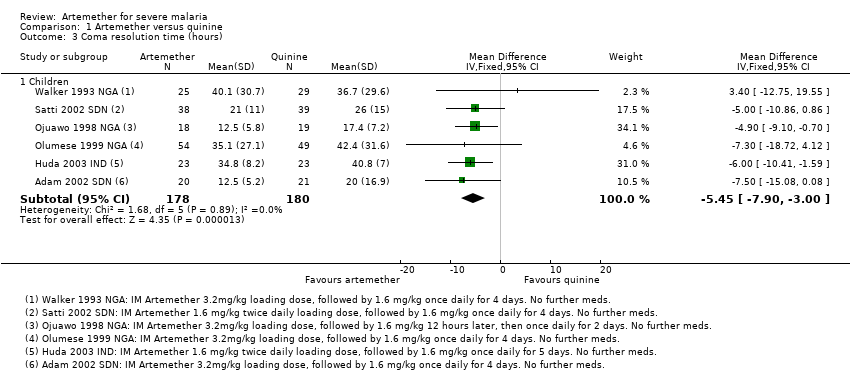
Comparison 1 Artemether versus quinine, Outcome 3 Coma resolution time (hours).

Comparison 1 Artemether versus quinine, Outcome 4 Neurological sequelae at discharge.
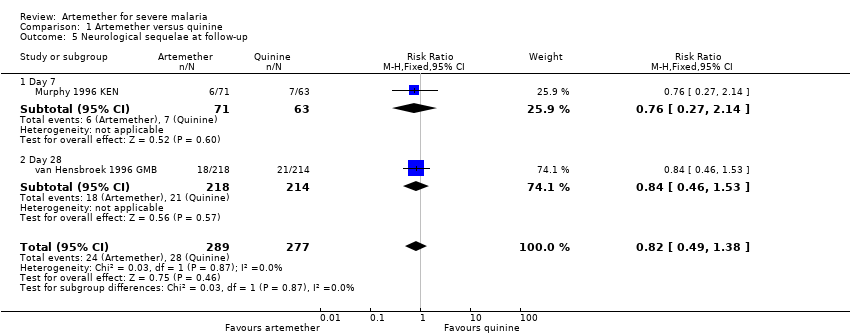
Comparison 1 Artemether versus quinine, Outcome 5 Neurological sequelae at follow‐up.

Comparison 1 Artemether versus quinine, Outcome 6 Parasite clearance time.
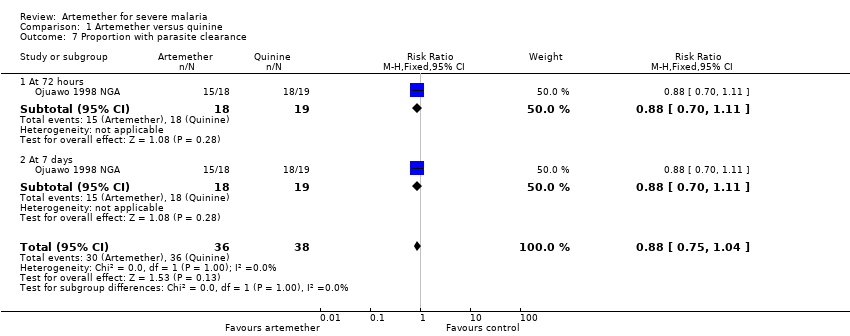
Comparison 1 Artemether versus quinine, Outcome 7 Proportion with parasite clearance.

Comparison 1 Artemether versus quinine, Outcome 8 Fever clearance time (hours).

Comparison 1 Artemether versus quinine, Outcome 9 Need for blood transfusion.

Comparison 1 Artemether versus quinine, Outcome 10 Episodes of hypoglycaemia.

Comparison 1 Artemether versus quinine, Outcome 11 Adverse events.

Comparison 2 Artemether versus artesunate, Outcome 1 Death.

Comparison 2 Artemether versus artesunate, Outcome 2 Need for blood transfusion.
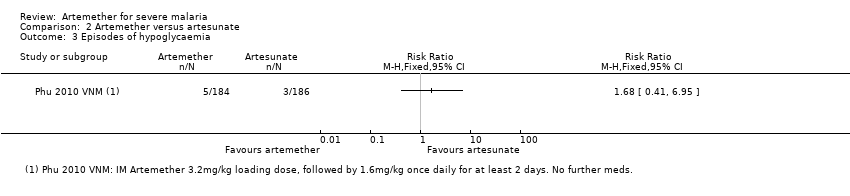
Comparison 2 Artemether versus artesunate, Outcome 3 Episodes of hypoglycaemia.

Comparison 2 Artemether versus artesunate, Outcome 4 Adverse events.
| Artemether compared with quinine for treating children with severe malaria | |||||
| Patient or population: Children with severe malaria | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Quinine | Artemether | ||||
| Death | 170 per 1000 | 164 per 1000 | RR 0.96 | 1447 | ⊕⊕⊕⊝ |
| Coma resolution time | The mean coma resolution time ranged across control groups from | The mean coma resolution time in the intervention groups was | ‐ | 358 | ⊕⊕⊝⊝ |
| Neurological sequelae at discharge | 220 per 1000 | 185 per 1000 | RR 0.84 | 968 | ⊕⊕⊝⊝ |
| Parasite clearance time | The mean parasite clearance time ranged across control groups from | The mean parasite clearance time in the intervention groups was | ‐ | 420 | ⊕⊕⊕⊝ |
| Fever clearance time | The mean fever clearance time ranged across control groups from | The mean fever clearance time in the intervention groups was | ‐ | 457 | ⊕⊕⊝⊝ |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 No serious risk of bias: Trials were variable in their risk of bias, but exclusion of the trials at high or unclear risk of selection bias did not change this result. | |||||
| Artemether compared with quinine for treating adults with severe malaria | |||||
| Patient or population: Adults with severe malaria | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Quinine | Artemether | ||||
| Death | 208 per 1000 | 123 per 1000 | RR 0.59 | 716 | ⊕⊕⊕⊝ |
| Coma resolution time | ‐ | ‐ | Not pooled. Little difference. | 657 | ⊕⊕⊝⊝ |
| Neurological sequelae at discharge | 4 per 1000 | 12 per 1000 (1 to 111) | RR 2.92 | 560 | ⊕⊕⊝⊝ |
| Parasite clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 | ⊕⊕⊕⊝ |
| Fever clearance time | ‐ | ‐ | Not pooled. Little difference apparent. | 716 | ⊕⊕⊝⊝ |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 No serious risk of bias: Trials are generally well conducted and at low risk of bias. 7 No serious risk of bias: This single trial was at low risk of bias. 8 Downgraded by 1 for serious imprecision: Neurological sequelae in adults were uncommon. This trial is underpowered to detect or exclude clinically important differences. | |||||
| Artemether compared with artesunate for treating adults with severe malaria | |||||
| Patient or population: Adults with severe malaria | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Artesunate | Artemether | ||||
| Death | 87 per 1000 | 156 per 1000 | RR 1.80 | 494 | ⊕⊕⊕⊝ |
| Coma resolution time | ‐ | ‐ | Not pooled. No significant difference | 494 | ⊕⊕⊕⊝ |
| Neurological sequelae at discharge | ‐ | ‐ | ‐ | 0 | ‐ |
| Parasite clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 | ⊕⊕⊕⊝ |
| Fever clearance time | ‐ | ‐ | Not pooled. No significant difference | 494 | ⊕⊕⊝⊝ |
| *The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 No serious risk of bias: Trials were generally well conducted and at low risk of bias. | |||||
| Search set | CIDG SR1 | CENTRAL | MEDLINE2 | Embase2 | LILACS2 | ISI Web of Science |
| 1 | malaria | Malaria ti, ab, MeSH | Malaria ti, ab, MeSH | Malaria ti, ab, Emtree | malaria | malaria |
| 2 | artemether | Artemether ti, ab | Artemether ti, ab | Artemether ti, ab, Emtree | artemether | artemether |
| 3 | Artemisinin* | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* ti, ab | Artemisinin* | Artemisinin* |
| 4 | intramuscular | Intramuscular ti, ab | Intramuscular ti, ab | Intramuscular ti, ab | intramuscular | intramuscular |
| 5 | parenteral | Injections, Intramuscular [MeSH] | Injections, Intramuscular [MeSH] | Intramuscular drug administration [Emtree] | parenteral | parenteral |
| 6 | 2 or 3 | Parenteral ti, ab | Parenteral ti, ab | Parenteral drug administration [Emtree] | 2 or 3 | 2 or 3 |
| 7 | 4 or 5 | 2 or 3 | 2 or 3 | 2 or 3 | 4 or 5 | 4 or 5 |
| 8 | 1 and 5 and 7 | 4 or 5 or 6 | 4 or 5 or 6 | 4 or 5 or 6 | 1 and 5 and 7 | 1 and 5 and 7 |
| 9 | ‐ | 1 and 7 and 8 | 1 and 7 and 8 | 1 and 7 and 8 | ‐ | Randomised clinical trial* |
| 10 | ‐ | ‐ | ‐ | ‐ | ‐ | 8 and 9 |
| 1Cochrane Infectious Diseases Group Specialized Register. | ||||||
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| 2002 | 'Children' | 20 mg/kg IV | 10 mg/kg IV every eight hours for 72 hours | Oral quinine for 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| 2007 | 6 months to 12 yrs | 20 mg/kg IV or IM | 10 mg/kg IV/IM every eight hours | None | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days | None | |
| 2001 | < 14 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 5 days | None | |
| 2004 | 3 months to 15 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine 10 mg/kg every eight hours | 3.2mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None | |
| 1996 | 5 months to 12 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | SP once | |
| 1998 | Mean age about 4 yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM 12 hrs later, then once daily for 2 days | None | |
| 1999 | 11 months to 5 yrs | 20mg/kg IV | 10mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| 2009 | 1 to 12yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None | |
| 1996 | 3 months to 15yrs | 10 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 1.6 mg/kg IM twice daily | 1.6 mg/kg IM once daily for 4 days | None | |
| 1994 | Mean age of 3 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours for at least 2 doses | SP once | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 2 days at least | SP once | |
| 1994 | 1 to 9yrs | 20 mg/kg IV | 10 mg/kg IV every twelve hours | Quinine to complete 5 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 3 days | SP once1 | |
| 1993 | 1 to 5yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| IM = intramuscular; IV = intravenous; SP = sulphadoxine‐pyrimethamine. 1Only in the second and third years of the study. | ||||||||
| Trial ID | Year of study | Age limits | Quinine dosing schedule | Artemether dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| 1996 | 15 to 79 yrs | 20 mg/kg IM | 10 mg/kg IM every eight hours | Quinine or mefloquine to complete 7 days | 4 mg/kg IM | 2 mg/kg IM once daily for 4 days | Quinine or mefloquine to complete 7 days | |
| 1991 | 15 to 45 yrs | 20 mg/kg IV | 10 mg/kg every eight hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None | |
| 1994 | 15 to 55 yrs | 20 mg/kg IV | 10 mg/kg every eight hours for 7 days | Quinine to complete 7 days | 160 mg IM | 80 mg IM once daily for 6 days | None | |
| 1995 | > 12 yrs | 20 mg/kg IV | 10 mg/kg IV every eight hours | Quinine to complete 7 days | 3.2 mg/kg IM | 1.6 mg/kg IM once daily for 4 days | None | |
| IM = intramuscular; IV = intravenous. | ||||||||
| Trial ID | Year of study | Age limits | Artemether dosing schedule | Artesunate dosing schedule | ||||
| Loading dose | Maintainance | Follow‐on therapy | Loading dose | Maintainance | Follow‐on therapy | |||
| 2003 | 15 to 77 yrs | 3.2 mg/kg IM | 1.6 mg/kg IM daily | None | 2.4 mg/kg IM | 1.2 mg/kg IM once daily | 2 mg/kg of artesunate to complete 7 days | |
| 1994 | 15 to 66 yrs | 200 mg IM | 100 mg IM once daily for 3 days | Mefloquine once | 120 mg IM or IV | 60 mg IM or IV once daily for 3 days | Mefloquine once | |
| IM = intramuscular; IV = intravenous. | ||||||||
| Trial ID | Coma resolution time | Fever clearance time | Parasite clearance time | Hypoglycaemia |
| Mean value (h) reported and defined as a Blantyre coma score of 5 recorded for at least 24 hours | Mean value (h) reported and defined as the time after which the temperature remained normal (axillary temperature < 37.5°C) | Mean value (h) reported and defined as the time passed from admission and start of treatment until two consecutive negative smears. Blood films repeated every 8 hours. | Number of episodes (n/N) reported but not defined | |
| Proportions with coma resolution on D3 reported but not defined | Proportions with fever clearance on D3 and D14 reported and defined as body temperature ≤ 37.5°C after commencement of treatment | Proportions with parasite clearance on D3 and D14. Parasite clearance was taken as adequate clinical and parasitological response (ACPR) | Not reported | |
| Median value (h) reported and defined as the time to reach a score of 15 on the Glasgow Coma Scale | Median value (h) reported but not defined. | Median value (h) reported and defined as the time to Assessed every 4 hours for the first 24 hours and every 6 hours until three consecutive negative blood smears | Number of episodes (n/N) reported but not defined | |
| Glasgow coma scale was used in grading the level of consciousness of the patients every eight hours | Mean value (h) reported and defined as time to clearance of fever | Mean value (h) reported but not defined | Not reported | |
| Unclear if values reported are means or medians (h) | Mean value (h) reported and defined as time for the temperature to fall below 37.5°C and remain that value for 72 hours | Mean value (h) reported and defined as the time for the parasite count to fall below the level of microscopic detection (thick film) | Not reported | |
| Median value (h) reported and defined as the time taken for the patients to recover completely from unconciousness | Mean value (h) reported and defined as time for the temperature to fall below 37.5°C and remain that value for 72 hours | Median value (h) reported and defined as the time taken for parasite count to fall below the level of microscopic detection (thick film) | Not reported | |
| Mean value (h) reported and defined as the time to normalization of consciousness | Mean value (h) reported but not defined | Mean value (h) reported and defined as time till negative parasitaemia result | Not reported | |
| Median value (h) reported but not described | Median value (h) reported but not described | Median value (h) reported but not described. Every four hours until clearance | Not reported | |
| Mean value (h) reported and defined as the interval between onset of therapy and the attainment of full consciousness | Mean value (h) reported and defined as the interval between the onset of therapy and the time the body temperature is ≤ 37°C and remained so | Defined as two successive thick blood films done at 12 hours interval are negative for asexual forms of plasmodium species | Not reported | |
| Mean value (h) reported and defined as time to regain full consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5°C and remain so for at least 48 hours | Mean value (h) reported and defined as the time from start of drug administration to the first of two consecutive negative thick smears remaining negative until day 7 | Not reported | |
| Mean value (h) reported and defined as time to attainment of a Blantyre score of 5 for at least 24 hours from initiation of treatment | Mean value (h) reported but not defined | Mean value (h) reported but not defined. Thick and thin film done on D0 and repeated on Days 3, 7 and 14 | Not reported | |
| Median value (h) reported and defined as time to Glasgow coma score of 15. | Median value (h) reported and defined as the time for temperature to fall below 37.5°C and remain so | Median value (h) reported and defined as the time to clear all parasites | Number of episodes (n/N) reported but not defined | |
| Mean value (h) reported and defined as time to regaining consciousness | Mean value (h) reported and defined as the time for temperature to fall below 37.5°C | Mean value (h) reported and defined as time to clear parasites measured every six hours till clearance | Not reported | |
| Median value (h) reported but not defined | Median value (h) reported and defined as a temperature <37.5 °C on two successive readings | Median value (h) reported and defined as as the time at which the blood films were negative for P. falciparum for at least eight hours | Number of episodes (n/N) reported but not defined | |
| Median value (h) reported and defined as time required for a child to achieve a Blantyre Coma Score of 5 | Median value (h) reported and defined as the time at which the rectal or axillary temperature dropped below 37.5°C and remained < 37.5°C for 24 consecutive hours | Median value (h) reported and defined as the time at which the first of two negative (0 parasites/200 WBC) thick blood films was prepared. Every four hours till clearance | Not reported | |
| Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time needed for the rectal temperature to fall below | Median value (h) reported and defined as time needed for all parasites to clear relative to parasite density at admission and assessed every 12 hours till clearance | Number of episodes (n/N) reported and defined as a blood glucose level below 40 mg/dL (2.2 mmol/L) | |
| Median value (h) reported and defined as time to regain full consciousness | Median value (h) reported and defined as time for axillary temperature to fall to, and remain for ≥ 24 hours at 37.5°C or lower | Median value (h) reported and defined as time to clear parasites | Not reported | |
| Mean value (h) reported but not defined | Mean value (h) reported | Mean value (h) reported and defined as the time for parasitaemia to be cleared and to remain so up to Day 7. Assessed every six hours during period of coma and then every 12 hours. | Not reported | |
| WBC = white blood cell. | ||||
| Outcome | Type of test | Proportion in control group3 | Proportion in Intervention group | Estimated RR | Total sample size1,2 |
| Death | Superiority | 0.17 | 0.136 | 0.80 | 3514 |
| Equivalence | 0.17 | 0.14 to 0.204 | ‐ | 6592 | |
| Neurological sequelae | Superiority | 0.25 | 0.20 | 0.80 | 2184 |
| Equivalence | 0.25 | 0.22 to 0.284 | ‐ | 8760 | |
| 1 These calculation were performed using a power calculator available at: http://www.sealedenvelope.com/power/ | |||||
| Outcome | Type of test | Mean in control group3 | Mean in Intervention group4 | SD of outcome | Total sample size1,2 |
| Coma resolution time | Superiority | 25 | 19 | 20 | 350 |
| Equivalence | 25 | 19 to 31 | 20 | 382 | |
| Parasite clearance time | Superiority | 42 | 36 | 20 | 350 |
| Equivalence | 42 | 36 to 48 | 20 | 382 | |
| Fever clearance time | Superiority | 48 | 42 | 20 | 350 |
| Equivalence | 48 | 36 to 54 | 20 | 382 | |
| 1 These calculations were performed using a power calculator available at: http://www.sealedenvelope.com/power/ | |||||
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (h) | Median (IQR) | 160 | 12 | 13 | Not significantly different | |
| Median (IQR) | 576 | 26 | 20 | P = 0.046 | ||
| Median (IQR) | 164 | 18 | 20 | Not significantly different | ||
| Coma recovery (%) on Day 3 | 90 | 15.9% | 21.4% | RR = 0.763 (95% CI 0.065 to 9.015) | ||
| Mean (SD) | 32 | 4.5 (13.05) | 9 (24.59) | P = 0.523 | ||
| Mean (SD) | 67 | 30.57 (29.02) | 25.15 (31.62) | P = 0.53 | ||
| Time to hospital discharge | % spending less than one week in hospital | 90 | 61.76% | 71.74% | P = 0.829 | |
| Fever clearance (h) | Median (IQR) | 160 | 32 | 32 | Not significantly different | |
| 576 | 30 | 33 | P = 0.8 | |||
| 164 | 31 | 45 | "Significant" | |||
| Fever clearance (%) on Day 3 | 90 | 90.0% | 87.7% | P = 0.753 | ||
| Parasite clearance (h) | Median (IQR) | 160 | 39.5 | 48 (37 to 56) | P < 0.001 | |
| 576 | 48 | 60 | P < 0.001 | |||
| 164 | 32 | 40 | 'significant' | |||
| Parasite clearance (%) on Day 3 | 90 | 99.0% n = 44 | 96.8% n = 46 | P = 0.422 | ||
| Needing blood transfusion | ‐ | ‐ | ‐ | ‐ | "The two groups were similar in terms of the need for blood transfusions,and the incidence of secondary bacterial infections (data not shown)." | |
| IQR = interquartile range. | ||||||
| Study ID | Sample size | Clinical symptoms monitoring | Biochemistry | Haematological | Electrocardiogram | Additional comments on adverse events |
| 41 | Not reported | Not reported | Not reported | Not reported | "Neurological deficits were not observed in any patient during the follow‐up period" | |
| 90 | Not reported | Not reported | Not reported | Not reported | None | |
| 560 | Clinical assessment every 4 hours for the first 24 hours and 6 hourly afterwards | Blood glucose, lactate and cytokine levels measured 4, 8, 12 and 24 hours after admission | Full blood count on admission | Pre‐treatment and 12 hours after initiation of treatment on Day 0, 4 hours after last dose and at discharge | None | |
| 46 | Lumbar puncture Chest x‐ray on day 0 | Blood Glucose, Renal Function Test, Liver Function Test and Serum Electrolyte on Days 0 and 3 | Full Blood Count on Days 0 and 3 | Day 0 | "No serious side effects of either of the drugs were observed in our study...... Closer and more frequent monitoring and larger sample size would have probably revealed more subtle adverse drug effects." | |
| 26 | Clinical evaluation daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 | Biochemistry on Days 0, 2, 4 and 7 | Full Blood Count on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every six hours for quinine and artemether patients respectively | "The side effects in the quinine group were dizziness and vertigo. No side effects were detected with artemether". | |
| 102 | Clinical evaluation on admission and twice daily for at least 7 days Lumbar puncture Chest x‐ray on day 0 | Biochemistry on Days 0, 2, 4 and 7 | FBC on Days 0, 2, 4 and 7 | On admission for all patients; then once daily and every six hours for quinine and artemether patients respectively | QTc prolongation and tinnitus were the major adverse events in Quinine arm. Mild transient pain at injection site for approximately 15 mins after artemether treatment. | |
| 67 | Clinical examination daily on Days 1 to7, and 14 | Blood glucose on Days 1, 2, 3, 5, 7 and 14 Urea and Serum Electrolyte, transaminases, phosphatases on Days 1, 3, 5, 7 and 14 | FBC on Days 1, 3, 5, 7 and 14 | Once daily on Days 1, 3, 5, 7 and 14 | None | |
| 160 | Clinical assessment on admission, then at six, then 12 hour intervals till discharge | Blood glucose, urea, electrolytes, blood gases and when clinically indicated | FBC on Day 0 and when clinically indicated Blood cultures on Day 0 | On admission and at 6, 24, 30, 48 and 54 hours | None | |
| 37 | Clinical assessment on Day 0 | Urea and electrolyte Blood sugar and liver function test on Day 0 | FBC on Day 0 | None | None | |
| 103 | Clinical assessments on Days 0, 3, 7, 14, 28 | Blood glucose, Urea and creatinine, electrolytes on Days 0, 3, 7, 14, 28 | WBC count on Days 0, 3, 7, 14, 28 | None | "No adverse reactions to the two drugs were recorded during the study". | |
| 32 | Clinical examination on Days 0 to 7 and 14 | None | None | None | None | |
| 370 | Clinical examination on admission Chest x‐ray on admission Lumbar puncture | Blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine transaminase, plasma lactate | Full blood count on admission | None | None | |
| 77 | Clinical evaluation on admission and every six hours on Days 0 to 4, and then once daily on Days 14, 21 and 28 | Blood glucose, serum creatinine, serum aspartate, aminotransferase on Day 0 | WBC, Haemoglobin on Days 0 and 3 | None | None | |
| 33 | Chest X‐ray on admission | Renal and Liver function tests on admission, Days 3 and 7 | Full Blood Count on Days 0, 3 and 7 | None | None | |
| 183 | CSF collected on admission | Blood glucose, Blood pH, on D0 (every four hours for the first 24 hours | Haematocrit every eight hours Full Blood Count, urea and electrolytes on Days 0, 3, 7 and 28 | On admission, 6, 48,54 and 96 hours | "Of the initial 127 patients on whom serial electrocardiographic tracings were made, more patients in the quinine group | |
| 576 | Clinical examination on Day 0 Lumbar puncture on admission | Blood glucose on admission, after 4hours and 12 hours | PCV, Haemoglobin, Blood culture on Day 0 | None | None | |
| 124 | Clinical examination on admission | Blood glucose, serum creatinine, serum bilirubin on admission | Full blood count on admission | None | None | |
| 54 | Clinical examination twice daily Spinal taps | Urea and Electrolyte, on days 3, 7, 14, 28 | PCV on days 3, 7, 14, 28 | On admission, at 4 and 6 hours | None |
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Quinine | Comparative results reported in article |
| Coma resolution time (h) | Median (IQR) | 560 | 66 (30 to 132) | 48 (20 to 84) | P = 0.003 | |
| Median (Range) | 97 | 48 (6 to 144) | 48 (6 to 144) | Not significantly different | ||
| Fever clearance (h) | Median (IQR) | 560 | 127 (60 to 216) | 90 (54 to 144) | < 0.001 | |
| Median (Range) | 33 | 32 (20 to 112) | 48 (28 to 88) | P = 0.034 | ||
| Median (Range) | 97 | 79 (16 to 147) | 84 (36 to 144) | Not significantly different | ||
| Parasite clearance (h) | Median (IQR) | 560 | 72 (54 to 102) | 90 (66 to 108) | < 0.001 | |
| Median (range) | 33 | 48(4 to 72) | 52 (12 to112) | P = 0.381 | ||
| Median (Range) | 97 | 54 (30 to 164) | 78 (18 to 168) | P = 0.007 | ||
| IQR = interquartile range. | ||||||
| Pre‐specified outcome | Trial reported outcome | Trial | No. of participants | Artemether | Artesunate IM | Artesunate IV | Comparative results reported in article |
| Coma resolution time (h) | Median (range) | 370 | 72(2 to 2232) n = 184 | 60(4 to 2136) n = 186 | ‐ | P = 0.11 | |
| Median (95% CI) | 124 | 47 (31 to 63) | 30 (18 to 42) | 24 (4 to 44) | ‐ | ||
| Fever clearance (h) | Median (range) | 370 | 108 (0 to 888) n = 184 | 108 (0 to 888) n = 186 | ‐ | P = 0.27 | |
| Median (95% CI) | 124 | 48 (38 to 58) | 36 (30 to 42) | 30 (18 to 42) | ‐ | ||
| Parasite clearance (h) | Median (range) | 370 | 72 (2 to 204) | 72 (7 to 330) | ‐ | P = 0.97 | |
| Median (95% CI) | 124 | 30 (26 to 34) | 24 (15 to 33) | 24 (15 to 33) | Not statistically significant | ||
| IM = intramuscular; IV = intravenous. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 12 | 1447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.20] |
| 1.2 Adults | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.83] |
| 2 Death: Time since admission to hospital Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Death within 24 hours | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 3 Coma resolution time (hours) Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Children | 6 | 358 | Mean Difference (IV, Fixed, 95% CI) | ‐5.45 [‐7.90, ‐1.00] |
| 4 Neurological sequelae at discharge Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Children | 7 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 4.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.31, 27.86] |
| 5 Neurological sequelae at follow‐up Show forest plot | 2 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
| 5.1 Day 7 | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.14] |
| 5.2 Day 28 | 1 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.53] |
| 6 Parasite clearance time Show forest plot | 8 | 446 | Mean Difference (IV, Fixed, 95% CI) | ‐8.82 [‐11.20, ‐6.45] |
| 6.1 Children | 7 | 420 | Mean Difference (IV, Fixed, 95% CI) | ‐9.03 [‐11.43, ‐6.63] |
| 6.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐15.56, 18.96] |
| 7 Proportion with parasite clearance Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.04] |
| 7.1 At 72 hours | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 7.2 At 7 days | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.70, 1.11] |
| 8 Fever clearance time (hours) Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Children | 8 | 457 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.55, ‐0.92] |
| 8.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐29.70 [‐54.14, ‐5.26] |
| 9 Need for blood transfusion Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Children | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.62, 2.59] |
| 9.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 10 Episodes of hypoglycaemia Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Children | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
| 10.2 Adults | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.64] |
| 11 Adverse events Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 QT prolongation | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.33, 7.19] |
| 11.2 Local skin reactions | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.50] |
| 11.3 Abscess | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.90] |
| 11.4 Urticarial rash | 1 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 11.5 Supraventricular tachycardia | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.59] |
| 11.6 Pruritus | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 11.7 Urinary tract infection | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.15, 81.36] |
| 11.8 Induration at injection site | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.44 [0.94, 253.49] |
| 11.9 Leg discomfort | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.16] |
| 11.10 Chest infection | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.53] |
| 11.11 GI bleeding | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adults | 2 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.09, 2.97] |
| 2 Need for blood transfusion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Adults | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.32] |
| 3 Episodes of hypoglycaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Spontaneous bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

