Inmunoterapia oral y sublingual para la alergia al huevo
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010638.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 20 abril 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Tabaquismo
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Protocol draft: OR, MGC
Develop a search strategy: OR, MGC
Search for trials: OR, MAT
Obtain copies of trials: OR, MAT
Select which trials to include: OR, MGC
Extract data from trials: OR, MGC
Enter data into RevMan: OR, MGC, SZ
Carry out the analysis: OR, MGC, SZ
Interpret the analysis: OR, MGC, SZ
Draft the final review: OR, MAT, MGC, SZ
Update the review: OR, MGC, SZ, MAT
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden.
to OR
-
Istituto Giannina Gaslini, Genoa, Italy.
External sources
-
No sources of support supplied
Declarations of interest
Olga Romantsik declares she has no competing conflicts of interest.
Maria Grazia Calevo declares she has no competing conflicts of interest.
Maria Angela Tosca declares she has no competing conflicts of interest.
Simona Zappettini declares she has no competing conflicts of interest.
Acknowledgements
We thank Dr Rita Banzi for her precious advice.
We thank Lindsay Stead for her guidance in the search for trials.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Apr 20 | Oral and sublingual immunotherapy for egg allergy | Review | Olga Romantsik, Maria Angela Tosca, Simona Zappettini, Maria Grazia Calevo | |
| 2014 Nov 18 | Oral and sublingual immunotherapy for egg allergy | Review | Olga Romantsik, Matteo Bruschettini, Maria Angela Tosca, Simona Zappettini, Ornella Della Casa Alberighi, Maria Grazia Calevo | |
| 2013 Jul 05 | Oral and sublingual immunotherapy for egg allergy | Protocol | Olga Romantsik, Matteo Bruschettini, Maria Angela Tosca, Ornella Della Casa Alberighi, Maria Grazia Calevo | |
Differences between protocol and review
We relocated two outcomes from secondary to primary outcomes because adverse events are an important consideration during oral immunotherapy. These outcomes are: number of participants with serious adverse events and number of participants with mild‐to‐severe adverse events.
Furthermore, a new secondary outcome was added: "medication used due to adverse events". This is a relevant outcome due to numbers of adverse events present during oral immunotherapy.
The "satisfaction" outcome was removed because of unclear definition.
The review objectives were rephrased to make them more clear.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Oral;
- Desensitization, Immunologic [adverse effects, *methods];
- Egg Hypersensitivity [*therapy];
- Egg Proteins, Dietary [*administration & dosage, immunology];
- Epinephrine [therapeutic use];
- Immunoglobulin E [immunology];
- Randomized Controlled Trials as Topic;
- Sublingual Immunotherapy [adverse effects, methods];
Medical Subject Headings Check Words
Adolescent; Child; Child, Preschool; Humans; Infant;
PICO
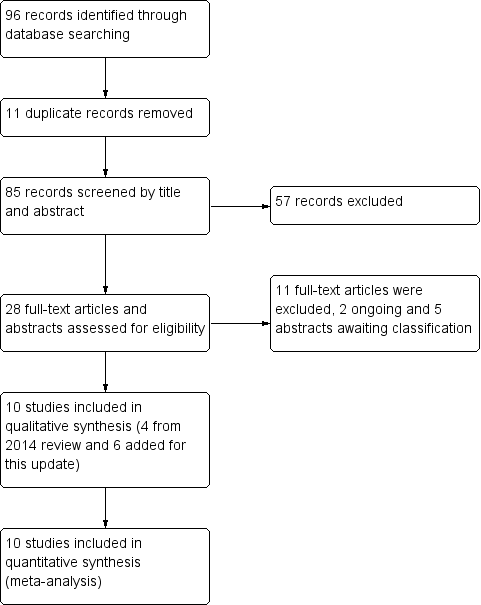
Study flow diagram.
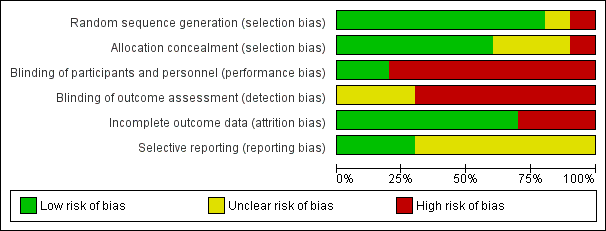
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 1 Increase in the amount of egg that can be tolerated.

Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 2 Complete recovery.

Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 3 Number of participants with serious adverse events.

Comparison 1 Oral and sublingual immunotherapy versus no therapy for egg allergy, Outcome 4 Number of participants with mild‐to‐severe adverse events.
| Oral and sublingual immunotherapy versus no therapy for egg allergy | ||||||
| Population: children with egg allergy Setting: hospitals Comparison: placebo or egg‐free diet. (Three studies used placebo and seven studies used an egg avoidance diet as controls) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral immunotherapy versus no therapy for egg allergy | |||||
| Increase in the amount of egg that could be tolerated | Study population | RR 7.48 | 410 | ⊕⊕⊝⊝ | 82% of children in the oral immunotherapy group could ingest a partial serving of egg (1 g to 7.5 g) compared to 10% of the control group. | |
| 102 per 1000 | 763 per 1000 | |||||
| Complete recovery | Study population | RR 4.25 | 439 | ⊕⊕⊝⊝ | 45% of children receiving oral immunotherapy were able to tolerate a full serving of egg compared to 10% of the control group. | |
| 100 per 1000 | 425 per 1000 | |||||
| Numbers of children with serious adverse events | See comment | See comment | Not estimable | 439 | ⊕⊕⊝⊝ | All 10 trials reported numbers of children with serious adverse events (SAEs): SAEs requiring epinephrine/adrenaline occurred in 21/249 (8.4%) of children in the oral immunotherapy group and none in the control group. Because adverse events were classified differently among the studies, it is difficult to comment on whether they were under‐ or over‐estimated. Surprisingly, only one study showed a high level of SAEs (Vazquez‐Ortiz 2014): 13 children required epinephrine/adrenaline administration 18 times, and one grade 5 reaction occurred. |
| Number of children with mild‐to‐severe adverse events | Study population | RR 8.35 | 439 | ⊕⊕⊝⊝ | Mild‐to‐severe adverse events were frequent; 75% of children presented mild‐to‐severe adverse events during oral immunotherapy treatment versus 6.8% of children in the control group. | |
| 68 per 1000 | 568 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The assumed risk is the risk of the control arm 1 Downgraded one level because of risk of bias: all studies were assessed at high or unclear risk of bias in at least one domain. | ||||||
| Study name | Oral immunotherapy | Control/placebo | Oral immunotherapy versus control/placebo | |||||
| Pre‐treatment | Post‐treatment | P value | Pre‐treatment | Post‐treatment | P value | P value pre‐treatment | P value post‐treatment | |
| 10.5 (2.5 to 26.0) | not available | not available | 13.0 (7.5 to 20.0) | not available | not available | not significant | P = 0.02 | |
| 11.0 (6.5 to 18) | 9.2 | P = 0.05 | 9.0 (6.0 to 16.0) | 10.0 | not available | not significant | P = 0.88 | |
| 10.0 (7.0 to 15.0) | 5.0 (4.0 to 13.0) | not available | 9.25 (5.5 to 15.0) | 10.0 (5.5 to 15.0) | not available | P = 0.976 | P = 0.007 | |
| 6.0 (3.0 to 11.0) | 5.0 (3.0 to 8.0) | P = 0.001 | 6.0 (3.0 to 12.0) | 5.5 (0 to 13.0) | P = 0.45 | P = 0.2 | P = 0.16 | |
| 8.74 (4 to 16) | not available | P < 0.001 | 9.68 (3.0 to 16.0) | not available | not available | not available | not available | |
| 5.5 (2.5 to 7.0) | 3.5 (1.0 to 5.0) | P < 0.01 | 4.0 (1.0 to 7.0) | 6.0 (1.0 to 8.0) | P = NS | not available | not available | |
| 7.0 (5.0 to 10.0) | not available | not available | 7.0 (4.0 to 12.0) | not available | not available | not available | P < 0.05 | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Increase in the amount of egg that can be tolerated Show forest plot | 9 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.48 [4.91, 11.38] |
| 2 Complete recovery Show forest plot | 10 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [2.77, 6.53] |
| 3 Number of participants with serious adverse events Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Number of participants with mild‐to‐severe adverse events Show forest plot | 10 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.35 [5.31, 13.12] |

