Topiramato para la profilaxis de la migraña episódica en adultos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. The study consisted of a 4‐week baseline period (possibly retrospective) and a prospective treatment period of 12 weeks Discontinuation rate: topiramate 30%, sodium valproate 22% Compliance (adherence) data: not available Rule for use of acute medication: during acute attacks, patients were allowed to use acetaminophen, NSAIDs, ergotamine, triptans, and opioids Methodological quality score: 3 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; migraine onset at least 6 months prior to study and before age 50; migraine frequency 4 to 10 attacks per month; attacks separated by 48 h pain‐free interval. Ages 18 to 65. Non‐pregnant, non‐lactating adequate contraception. Migraine prophylaxis withdrawn at least 1 month prior to study entry Exclusion: non‐migraine headaches; > 8 treatment days/month of ergots, NSAIDs, or triptans. No rule reported for exclusion of CDH Other exclusions: alcohol/drug dependence. Hemiplegic, basilar, or ophthalmoplegic migraine. Serious medical conditions Setting: single‐centre Country: Iran Intention‐to‐treat analysis of 56 patients. Of these, 9 had migraine with aura and 47 migraine without aura (ie, not stated that some had both). 44 females and 12 males included in the ITT analysis; mean age among ITT participants treated with topiramate 32.1 ± 10.2; mean age among ITT participants treated with sodium valproate 29.2 ± 9.6. 40 allocated to receive topiramate; 36 allocated to receive sodium valproate | |
| Interventions | Topiramate 50 mg/day versus sodium valproate 400 mg/day (12 weeks). Topiramate initiated with 25 mg/day for 1 week, thereafter 50 mg/day until study end. Dosing frequency not stated. Sodium valproate initiated with 200 mg/day for 1 week, thereafter 400 mg/day until study end. Dosing frequency not stated | |
| Outcomes | Headache frequency (4 weeks). Headache severity. Duration of episode. Weight. MIDAS at baseline and 8 weeks. HIT‐6 at baseline and 8 weeks. Responder rate Time point(s) considered in the review: last (third) month of double‐blind phase for frequency; entire double‐blind phase for MIDAS | |
| Notes | A migraine attack persisting longer than 72 hours was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration. Since it is unclear if the baseline was prospective, change scores from baseline were excluded from the analyses of this review. Complementary information requested by email (twice) and ordinary letter (once) but not provided by corresponding author Funders of the trial: Kermanshah University of Medical Sciences, Iran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Medication prescribed with preprinted medication code labels |
| Blinding of participants and personnel (performance bias) | High risk | Stated that both participants and clinicians were blinded by the use of preprinted medication code labels. However, there is no mention of equally appearing tablets. It is thus possible that standard medication was provided by third party according to allocation label |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | 20 randomised patients did not contribute to the ITT analysis: 8 AEs; 10 lack of efficacy (whereof 8 were allocated to topiramate); 2 moved |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, or analyses |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Retrospective baseline (presumably 1 month) followed by 8 weeks prospective treatment phase Discontinuation rate in double‐blind phase: topiramate 3%, propranolol 3% Compliance (adherence) data: not available Rule for use of acute medication: no mention Methodological quality score: 3 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐I; migraine onset at least 1 year prior to study and before age 50; migraine frequency 3 or more attacks per month during the 3 months prior to study entry; pain‐free interval of at least 48 h between attacks; concomitant migraine prophylactics withdrawn 1 month prior to study entry. Ages 18 to 65 Exclusion: no adequate rule reported for exclusion of CDH or overuse of acute medication. Other exclusions: pregnancy; breast feeding; general and neurologic diseases Setting: single‐centre Country: Iran Complete case analysis of 60 patients. Not reported how many had migraine with aura. 49 females and 11 males; mean age in topiramate group 31.7 ± 8; mean age in propranolol group 29.9 ± 9. 31 allocated to receive topiramate and 31 allocated to receive propranolol | |
| Interventions | Topiramate 50 mg/day versus propranolol 80 mg/day (8 weeks). Topiramate initiated with 25 mg/day for 1 week, thereafter 50 mg/day until study end. Dosing frequency not stated. Propranolol initiated with 40 mg/day for 1 week, thereafter 80 mg/day until study end. Dosing frequency not stated | |
| Outcomes | Migraine attack frequency per 4 weeks; attack duration (hours); headache intensity (VAS, not mentioned if at prespecified time points or maximum); AEs Time point(s) considered in the review: last (second) month of double‐blind phase | |
| Notes | Since the baseline was retrospective, change scores from baseline were excluded from the analyses of this review. Complementary information requested twice but not provided by corresponding author Funders of the trial: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised (1:1). Presumably sequence generation was by a lottery procedure (see below under 'Allocation concealment') |
| Allocation concealment (selection bias) | Unclear risk | After selection of 1 of 62 sealed envelopes, half of which contained medication codes for topiramate and the other half those for propranolol, a tear‐off label was removed, revealing the randomisation number. The medication code was removed from the envelope. It is not clear who delivered the study medication according to the medication code |
| Blinding of participants and personnel (performance bias) | Unclear risk | Both participants and clinicians were blinded. It is unclear how this was maintained |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 subject dropped out in each group (both due to AEs) and they were excluded from the data available for this review |
| Selective reporting (reporting bias) | Unclear risk | AEs of propranolol (not included in this review) are inadequately reported |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 28‐day baseline period. Duration of treatment: 8 weeks titration, then 18 weeks stable dosage, followed by open‐label extension Discontinuation rate: dropout reported as 47% for combined active treatment groups; 48% for placebo, but unclear how many patients completed the entire trial Compliance (adherence) data: only reported as percentage of patients achieving target dose Rule for use of acute medication: analgesics, ergot derivatives, triptans and opioids allowed Methodological quality score: 5 | |
| Participants | Inclusion: IHS migraine criteria; migraine frequency of 3 to 12 in 28‐day baseline phase; women practising adequate contraception or unable to bear children Exclusion: secondary headaches, daily headache, and analgesic overuse headache were all adequately excluded. Other exclusions: failure to respond to more than 2 previous migraine‐prophylactic regimens, migraine onset after age 50, continued use of various CNS‐active and other drugs, history of nephrolithiasis, previous exposure to topiramate, use of experimental drug or device within 30 days of screening Setting: multicentre Country: 52 North American clinical centres Intention‐to‐treat analysis of 468 patients. Patients both with and without aura recruited, but percentages not reported. 406 females and 62 males; age range 12 to 65. 117 received 50 mg/day dose, 120 received 100 mg/day dose, 117 received 200 mg/day dose and 114 received placebo | |
| Interventions | Topiramate 50 mg/day versus topiramate 100 mg/day versus topiramate 200 mg/day versus placebo (18 weeks). Dosage started at 25 mg/day and increased by 25 mg each week to reach assigned dose or maximum tolerated dose | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (50% reduction in frequency). Severity and duration of attacks. Month of onset of drug action. Quality of life (average maintenance AUC of MSQ and SF‐36 scores). AEs Time points considered in the review: through entire double‐blind phase (migraine frequency, response rate, AEs); week 8 to 26 of double‐blind phase (MSQ, SF‐36) | |
| Notes | Lowest allowable age was 12 years; hence some patients not adult. Headache frequency defined as the number of migraine periods per 28 days, where a migraine period is any occurrence of migraine headache that started, ended, or recurred with 24 hours. A migraine attack persisting into a second 24‐hour period was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration Funders of the trial: Johnson & Johnson Pharmaceutical Research and Development, LLC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule balanced by using permutated blocks of 4 and stratified by centre |
| Allocation concealment (selection bias) | Low risk | An interactive voice response system was used to assign randomisation numbers to patients |
| Blinding of participants and personnel (performance bias) | Low risk | Patients and clinicians were blinded to study medication. Study medication packaged and labelled according to a medication code schedule generated before the trial. Each bottle had a 2‐part, tear‐off label; study medication identification was concealed and could be revealed only in case of emergency |
| Blinding of outcome assessment (detection bias) | Unclear risk | Treatment assignments were not revealed to investigators or study monitors until all patients had completed therapy and the database had been finalised. Not clearly stated that blinding included the stage of analysis |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis excluded 15 patients who did not provide any post‐baseline data, out of 483 randomised |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Two months baseline period. Duration of treatment: 2 months Discontinuation rate: topiramate 20%, placebo 27%, levetiracetam 0% Compliance (adherence) data: "non‐compliance" reported as the most common reason for dropping out (6/7), but the extent to which patients took medications as prescribed is not reported Rule for use of acute medication: not reported Methodological quality score: 3 | |
| Participants | Inclusion: migraine without aura according to ICHD‐II; attack frequency not specified. Ages 18 to 49. Consent to additional neurophysiological tests Exclusion: no adequate rule reported for exclusion of secondary headaches, daily headache, or analgesic overuse headache. However, no included patient had CDH or other headache than migraine according to correspondence with the first author. Other exclusions: psychoactive drugs, general/neurological/psychiatric disorders Setting: single‐centre Country: Italy Intention‐to‐treat analysis of 39 patients. Patients with aura not recruited. 35 females and 10 males included; mean age 37.9 ± 12.4, age range 18 to 49. 15 received topiramate, 15 received placebo, and 15 received levetiracetam | |
| Interventions | Topiramate 100 mg/day (50 mg BID, presumably tablets) versus placebo versus levetiracetam (8 weeks). Dose escalation strategy not reported | |
| Outcomes | Migraine days per month. 50% or greater reduction in headache frequency. Flicker frequency dependent α‐rhythm phase synchronisation (phase synchronisation index). Mean iCNV amplitude and iCNV habituation index Time point(s) considered in the review: through entire treatment period (2 months) | |
| Notes | Levetiracetam arm of trial excluded as comparator from this review, since the intervention is experimental. Study was not principally designed to obtain clinical outcome data of the interventions Funders of the trial: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study was double‐blind, but methodological description is lacking |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Means (SD) for migraine frequency during treatment period not presented in publications but provided by first author after request. No safety data reported for placebo‐group |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 14‐day washout period then 28‐day baseline period. Duration of treatment: 8 weeks titration then 18 weeks maintenance Discontinuation rate: dropouts: 32% for topiramate 100 mg, 55% for topiramate 200 mg, 29% for propranolol, 31% for placebo Compliance (adherence) data: daily dose recorded; plasma concentration of topiramate recorded Rule for use of acute medication: aspirin, paracetamol, NSAIDs, ergot compounds, triptans, and opioids permitted Methodological quality score: 4 | |
| Participants | Inclusion: IHS migraine criteria, migraine onset more than 1 year prior to study, migraine frequency 3 to 12 per month during 28‐day baseline phase. Ages 12 to 65. No mixed or combination headaches included Exclusion: daily headache was adequately excluded; no information given on the exclusion of secondary headache and analgesic overuse headache. Other exclusions: failure to respond to more than 2 previous migraine‐prophylactic regimens, asthma, bradyarrhythmia, uncontrolled diabetes, contraindications to beta‐blockers Setting: multicentre Country: 13 countries in Europe, Asia, Australia, and Africa Intention‐to‐treat analysis of 568 patients. Patients both with and without aura recruited, but percentages not reported. 453 females and 115 males; age range 12 to 65. 139 received topiramate 100 mg/day, 143 received topiramate 200 mg/day, 143 received propranolol 160 mg/day, and 143 received placebo | |
| Interventions | Topiramate 100 mg/day versus topiramate 200 mg/day versus propranolol 160 mg/day versus placebo (18 weeks). Dosages started at 25 mg/day (topiramate) and 20 mg/day (propranolol) and increased by 25 mg (topiramate) or 20 mg (propranolol) each week to reach assigned dose or maximum tolerated dose | |
| Outcomes | Headache frequency per 28 days. Change in number of migraine days per month. Change in average monthly rate of rescue medication. Proportion of responders (50% reduction in frequency). Month of onset of drug action. Average duration Time point(s) considered in the review: through the core double‐blind phase | |
| Notes | Lowest allowable age was 12 years; hence some patients not adult. Headache frequency defined as the number of migraine periods per 28 days, where a migraine period is any occurrence of migraine headache that started, ended, or recurred with 24 hours. A migraine attack persisting into a second 24‐hour period was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration Funders of the trial: Johnson & Johnson Pharmaceutical Research and Development, LLC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation, in equal proportions, to 1 of 4 treatment groups. Method not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants and clinicians were blinded. Method not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis excluded 7 patients who did not provide any post‐baseline data, out of 575 randomised |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 26‐week open‐label phase at target dose of topiramate 100 mg/day (allowing 50 to 200 mg/day), then randomising to 26‐week, double‐blind, parallel‐group phase of prolonged topiramate treatment at individualised dose or placebo Discontinuation rate in double‐blind phase: topiramate 18%, placebo 20% Compliance (adherence) data: not available Rule for use of acute medication: individuals with medication overuse not included; triptans, ergots, opiates, and other analgesics thereafter permitted Methodological quality score: 5 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; history of migraine at least 1 year; migraine frequency of ≥ 4 attacks/month. Ages 18 to 80 Exclusion: overuse of acute medication. Acceptable exclusion of secondary headaches. Other exclusions: prophylaxis in month preceding entry (3 months for flunarizine); prior poor response on > 2 prophylactics; pregnancy and breastfeeding Setting: 88 centres Country: 21 countries in Europe and the Middle East Intention‐to‐treat analysis of 507 patients. Patients both with and without aura recruited, but percentages not reported. 445/512 females and 67/512 males; mean age among allocated to continuing on topiramate 40.1 ± 10.6; mean age among allocated to switching to placebo 40.1 ± 10.7, age range 18 to 69. 255 allocated to continuing on topiramate and 259 allocated to switching to placebo | |
| Interventions | Topiramate 100 mg/day versus placebo (26 weeks). Topiramate target dose 100 mg/day (tablets 50 mg BID), individualised according to efficacy and tolerability between 50 and 200 mg/day; dose remaining stable 4 weeks prior to randomisation. Mean dose last month of double‐blind phase: 103 ± 37 mg/day. Matching placebo BID; topiramate meanwhile tapered out by 100 mg weekly. β‐blockers and amitriptyline were allowed in both groups for indications other than migraine | |
| Outcomes | Number of days with migraine headache per 28 days. Duration and severity of migraines. Number of days with acute medication. Patient satisfaction over double‐blind phase. Proportion of responders (50% reduction in frequency) not investigated. MIDAS. HIT‐6. SF‐12 Time point(s) considered in the review: last 4 weeks of the double‐blind phase, ie, weeks 17 to 26 | |
| Notes | CDH was not an exclusion criterion, but the migraine frequencies (migraine days per 28 days) during the last month of the open‐label phase (group continuing with topiramate: 4.9 ± 3.7 (data provided by Janssen‐Cilag); group that switched to placebo: 4.6 ± 4.0) confirm that the absolute majority had episodic migraine Funders of the trial: Janssen‐Cilag, EMEA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation, medication randomised in blocks of 4; blocks provided per study centre |
| Allocation concealment (selection bias) | Low risk | Subjects sequentially allocated to next available medication number within block at randomisation (= entry into double‐blind phase) |
| Blinding of participants and personnel (performance bias) | Low risk | Both clinicians and patients blinded. Medication provided by number. Use of identical appearing tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Mean monthly migraine frequency during the last month of the double‐blind phase only given in publication for the group that switched to placebo but provided by drug company for the group that continued with topiramate. Variance measures for change in MIDAS scores only roughly indicated in a graph (Fig. 4) |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 14 to 28‐day washout period then 28‐day baseline period. Duration of treatment: 4 weeks titration then 22 weeks maintenance followed by up to 2 weeks taper/exit phase Discontinuation rate: topiramate 43%, amitriptyline 44% Compliance (adherence) data: not available Rule for use of acute medication: use of acute headache medications including over‐the‐counter analgesics, NSAIDs, triptans, ergot derivatives, and dihydroergotamine mesylate, was permitted for symptomatic relief of headaches throughout the study, but was not to exceed 4 days per week Methodological quality score: 5 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐II, migraine onset at least 6 months prior to study, migraine frequency of 3 to 12 attacks/month during 3 months prior to screening and during baseline period. Ages 18 and above Exclusion: CDH during baseline, analgesic overuse (> 15 treatment days per month with abortive medication). Acceptable exclusion of secondary headaches. Other exclusions: failed > 2 adequate trials of migraine preventive medication, prior lack of efficacy for topiramate and/or amitriptyline, migraine onset after the age of 50 years, aura without headache only, history of cluster headache, progressive neurological disorder, condition more painful than migraine, contraindication for amitriptyline, unstable medical condition within the past 2 years, major psychiatric disorder within the past 6 months, drugs/alcohol abuse within the past 2 years, nephrolithiasis, active liver disease, liver function tests ≥ 2 times the upper limit of normal, pregnancy, lactation, inadequate contraception Setting: 32 centres Country: USA Intention‐to‐treat analysis of 331 patients. Patients both with and without aura recruited, but percentages not reported. 281 females and 50 males; mean age 38.8 ± 11.0, age range 18 to 70. 178 received topiramate and 169 received amitriptyline | |
| Interventions | Topiramate 100 mg/day versus amitriptyline 100 mg/day (26 weeks). Dosages started at 25 mg/day and increased by 25 mg each week to reach 50 mg BID (topiramate) and 100 mg at night with morning placebos (amitriptyline) or the maximum tolerated dose. A stable dose of at least 50 mg/day was required | |
| Outcomes | Mean 28‐day rate of migraine episodes defined as the period from the onset to the cessation of painful migraine symptoms, not to exceed 24 h. Response rates (≥ 25%, ≥ 50%, ≥ 75%, or 100% reduction) on 28‐day migraine and headache days (migraine and non‐migrainous headache). Mean 28‐day rate of days with headache, abortive medication use, migraine duration, migraine severity, severity of migraine‐associated symptoms, frequency of migraine‐associated vomiting. Severity of functional disability (MIDAS, MSQ, Q‐LES‐Q‐SF). Mean change in weight and BMI Time point(s) considered in the review: through entire treatment period (26 weeks) | |
| Notes | If painful migraine symptoms lasted > 24 hours, this was considered a new and distinct migraine episode. Such a definition runs the risk of confounding reduction in headache frequency with reduction in attack duration Funders of the trial: Ortho‐McNeil Janssen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 5‐digit subject numbers and 4‐digit medication code numbers. Randomisation in permuted blocks of 4 by site |
| Allocation concealment (selection bias) | Low risk | Numbers were assigned as subjects qualified for participation; assigned medication code was retained for duration of study |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double‐dummy. Capsules of identical appearance. Topiramate: 2 active capsules BID + 2 placebo capsules evening. Amitriptyline: 2 placebo capsules morning + 4 active capsules evening. Treatment assigned by medication code |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many participants in the topiramate group contributed to the endpoint ≥ 50% reduction in headache frequency. Complementary data requested twice but not provided by corresponding author |
| Selective reporting (reporting bias) | Unclear risk | Within‐group variance measures lacking for changes in least squares mean of migraine frequencies and MSQ scores. Complementary data requested twice but not provided by corresponding author |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 4‐week baseline period. Duration of treatment: 6 weeks titration then 8 weeks stable dosage Discontinuation rate: dropouts: 6 of 15 in topiramate group; 4 of 15 in placebo group Compliance (adherence) data: compliance data reported as number of patients reaching target dose (11 of 15) Rule for use of acute medication: acute medication permitted; allowed types not specified Methodological quality score: 3 | |
| Participants | Inclusion: IHS migraine criteria, migraine onset before age 50, migraine for more than 1 year, migraine frequency 2 to 8 per month, negative pregnancy test Exclusion: daily headaches and analgesic abuse headaches were adequately excluded. Other exclusions: pregnancy or lactation; substance‐related disorder in 3 months prior to study; Axis I disorders; other relevant medical conditions; history of renal calculi; participant in any other clinical trial within 30 days of study onset Setting: not reported (appears to be single‐centre) Country: USA 30 patients recruited and analysed; various analyses undertaken. Patients with and without aura recruited but percentages not reported. 29 females and 1 male; age range 30 to 62. 15 received topiramate and 15 received placebo | |
| Interventions | Topiramate 200 mg/day versus placebo (14 weeks). Dosage started at 25 mg/day and increased by 25 mg each week to reach target dose | |
| Outcomes | Number of migraine attacks per 28 days in entire double‐blind period. Number of migraine attacks per 28 days in last 10 weeks of study. Proportion of responders (50% reduction in frequency). Severity and disability scores Time point(s) considered in the review: through entire double‐blind period | |
| Notes | Funders of the trial: Ortho‐McNeil Pharmaceutical | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants and clinician were blinded, and placebo was used. No more information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Data available only from abstract and poster presentation |
| Selective reporting (reporting bias) | High risk | Data available only from abstract and poster presentation |
| Methods | Prospective, randomised, double‐blind, triple cross‐over trial. Study duration 23 weeks (although stated 20 weeks); 4 weeks prospective baseline followed by 4 weeks with first intervention, 1‐week washout, cross‐over to 4 weeks with second intervention, 1‐week washout, cross‐over to 4 weeks with third intervention, 1‐week washout, and finally cross‐over to 4 weeks with fourth intervention. Each subject received all treatments in a specified order Discontinuation rate: topiramate 7%, lamotrigine 7%, topiramate placebo 7%, lamotrigine placebo 7% Compliance (adherence) data: not available Rule for use of acute medication: patients were allowed to take tablets with a combination of paracetamol and diclofenac potassium (supplied to them) at their choice Methodological quality score: 2 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐I; migraine frequency of 4 to 10 attacks/month, history of migraine at least 1 year, debut of migraine before age 50, at least 48 h pain free interval between attacks. Ages 18 to 65 Exclusion: headaches other than migraines. > 8 days/month of NSAIDs, ergots, or triptans. Paracetamol overuse and CDH not mentioned as exclusion criteria. Other exclusions: migraine prophylactic drug (or drug with such potential) last month, antipsychotic/antidepressant drug last 3 months, alcohol, or other drug dependence, nephrolithiasis, participated in earlier study of lamotrigine or topiramate, used lamotrigine or topiramate 2 weeks or longer, used experimental drug last month Setting: single‐centre Country: India Intention‐to‐treat analysis of 57 patients. 32% (19/60) of included patients had migraine with aura. 47 females and 13 males; mean age 29.4 ± 7.7 years (range 16 to 48). 57 received topiramate, 57 received lamotrigine, 57 received topiramate placebo, and 57 received lamotrigine placebo | |
| Interventions | Topiramate 50 mg/day versus topiramate placebo versus lamotrigine 50 mg/day versus lamotrigine placebo (4 weeks). Topiramate and lamotrigine were given as 25 mg tablet BID (stable dosage), and placebos as 1 tablet BID (stable dosage) | |
| Outcomes | Change in migraine frequency per 28 days compared to baseline. Proportion of responders (50% reduction in frequency). Responder rate migraine intensity. Attack frequency. Attack duration. Intensity on VAS. Phonophobia. Photophobia. Rescue medication use. Response to rescue medication. Aura frequency. "Reports of AEs communicated historically during visits, as transcribed on headache diaries" Time point(s) considered in the review: through entire treatment period (4 weeks) | |
| Notes | Lamotrigine (and lamotrigine placebo) data excluded as comparator from this review, since the intervention is experimental. Unclear if any participants had CDH. Shorter treatment periods (1 month) than recommended (3 months) by IHS for evaluation of efficacy in clinical trials. Since 2 potentially active drugs were used, there is an obvious risk of carry‐over effect (analysis for order effects lacking) Funders of the trial: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule allocating participants to 1 of 4 treatment arms: LTG – LPLAC‐TOP‐TPLAC or LPLAC‐LTG‐TPLAC‐TOP or TOP‐TPLAC‐LTG‐LPLAC or TPLAC‐TOP‐LPLAC‐LTG |
| Allocation concealment (selection bias) | Low risk | Preprinted medication code labels. Sealed envelopes containing the code labels with a tear‐off label concealing the randomisation number were provided to the investigator |
| Blinding of participants and personnel (performance bias) | High risk | Patients and clinicians were blinded. Placebo was identical in appearance and packaging to active drug, but since the lamotrigine and topiramate tablets were different in appearance, 2 different placebos were used. For effective blinding a double‐dummy design would be required. Study medication was packaged and labelled according to a medication code schedule generated before the trial. Each package had all 4 medications numbered according to the phase of the trial. Each bottle had a 2‐part, tear‐off label; study medication identification was concealed and could be revealed only in case of emergency |
| Blinding of outcome assessment (detection bias) | Unclear risk | Investigators were blinded but not clearly stated that this included the stage of analysis |
| Incomplete outcome data (attrition bias) | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Total study duration up to 266 days; consisting of a pretreatment phase of up to 70 days (screening/washout period, followed by a 28 to 35‐day baseline period), a 26‐week double‐blind phase (6‐week titration followed by 20‐week maintenance), and 1‐week taper/exit phase Discontinuation rate: topiramate 37%, placebo 44% Compliance (adherence) data: not available Rule for use of acute medication: subjects were permitted to take acute headache medication as indicated. The type and method of acute headache medication use was as consistent as possible with that used by the subject prior to enrolment Methodological quality score: 5 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; migraine frequency of 9 to 14 days/month; history of migraine at least 1 year; onset of migraine before age 50. Ages 18 to 65. Other inclusion criteria: good health, capable of taking oral medication, no risk of pregnancy Exclusion: < 15 total headache days/month; had used a combination of acute headache medications for any reason for > 4 days/week on a regular basis during the 3 months before baseline period. Secondary headaches were adequately excluded. Other exclusions: previously failed > 2 adequate trials of migraine prophylactic drugs; use of migraine prophylactic drugs in the 6 weeks before baseline period; previously discontinued topiramate therapy due to lack of efficacy or AE; exclusively migraine aura without headache; other equally painful condition; cluster headache; basilar or hemiplegic migraine; progressive neurological disorder other than migraine; malignancy; significant medical history or medical condition of neurological, cardiovascular, hepatic, or renal disease; nephrolithiasis; unstable medical condition that may have impaired participation in the study or necessitate the use of drugs not permitted; abnormal renal, liver, or blood tests (specified); suicidality and/or psychiatric disease; drug or alcohol abuse within the past 2 years and positive urine drug screen Setting: 87 centres Country: not reported (all authors from USA) Intention‐to‐treat analysis of 330 "efficacy‐evaluable" (EE) patients. Patients both with and without aura recruited, but percentages not reported. 294 females and 36 males; mean age topiramate group 39.6 ± 10.6; mean age placebo group 40.9 ± 11.2; age range not reported. 188 received topiramate and 197 received placebo | |
| Interventions | Topiramate 100 mg/day versus placebo (26 weeks). Topiramate initiated with a single 25 mg tablet in the evening day 1 to 7, then increased each week by a single 25 mg tablet/day until a total dosage of 100 mg/day (two 25 mg tablets BID). The titration was adjusted at the discretion of the investigator on the basis of subject tolerability. Subjects must have maintained a dose of at least 3 tablets/day beginning at day 42 and throughout the study period. The mean dose used during maintenance period was 89.5 ± 14.2 mg/day. Placebo initiated with a single tablet in the evening day 1 to 7, then increased each week by a single tablet per day until a total dosage of 2 tablets BID. The titration was adjusted at the discretion of the investigator on the basis of subject tolerability. Subjects must have maintained a dose of at least 3 tablets/day beginning at day 42 and throughout the study period | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (≥ 50% and ≥ 75% reduction in headache days and migraine days). ≥ 15 headache days per 28‐day period (CDH) at month 6. CDH during the last 28‐day period of the double‐blind phase for those subjects that had completed at least 28 days of the double‐blind phase. Time to first reporting of CDH. CDH of which at least half of days with migraine headache. Time to first reporting of CDH of which at least half of days with migraine headache. 28‐day rate of headache days. 28‐day rate of acute medication days. Change in the 28‐day frequency of nausea, photophobia, and phonophobia. MSQ. MIDAS Time point(s) considered in the review: through the 26‐week double‐blind phase | |
| Notes | Funders of the trial: Ortho‐McNeil Janssen Scientific Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were assigned to either of the 2 treatment groups based on a computer‐generated predetermined randomisation schedule prepared by the sponsor before the study. Randomisation sequences were generated for each site |
| Allocation concealment (selection bias) | Low risk | Medication code numbers were preprinted on study medication labels and assigned as subjects qualified for the study and were randomised to treatment. Sealed envelopes containing the study medication identification (ie, active or placebo) were provided to the investigator and kept in a limited access area |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and clinicians were blinded. The double‐blind study medication tablets were identical in appearance and packaged in identically appearing bottles |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Efficacy only reported for the subgroup (EE) of ITT participants who completed at least 28 days of the double‐blind phase |
| Selective reporting (reporting bias) | High risk | ≥ 50% and ≥ 75% reduction in headache days and migraine days were collected but only reported as "higher in the topiramate group compared with the placebo treatment group". For MSQ and MIDAS results, the authors refer to www.clinicaltrials.gov (study identifier: NCT00212810). More than 5 years after study completion, no results from this study have yet been posted there. Corresponding author requested twice about the numbers of subjects with 50% or greater reduction in 28‐day migraine day frequency in both groups without providing data |
| Methods | Prospective, randomised, open, parallel‐group trial. Total study duration 13 months; 1‐month baseline period and 12‐month treatment period Discontinuation rate: topiramate 12%, flunarizine 22% Compliance (adherence) data: not available Rule for use of acute medication: subjects were permitted to take acute headache medication as indicated. Allowed rescue drugs included aspirin, acetaminophen, oral NSAIDs, ergot derivatives, triptans and opioids Methodological quality score: 2 | |
| Participants | Inclusion: migraine with aura, migraine without aura, and/or chronic migraine according to ICHD‐II; at least 2 attacks/month that produce disability lasting 3 or more days per month despite the use of acute treatment; history of migraine at least 1 year. Ages 18 to 65 Exclusion: other primary headache including TTH (confirmed by corresponding author); overuse of analgesics, triptans, or other specific agents for the acute treatment of migraine, including simple analgesics > 15 days/month and combined analgesics > 10 days/month. Secondary headaches were adequately excluded. Other exclusions: use of migraine prophylactic medications in the month before trial entry or flunarizine in the 3 months before trial entry. Prior poor or no efficacy of > 2 migraine prophylactic medications. History of depressive illness, extrapyramidal disorders, chronic obstructive pulmonary disease, bronchospasm, asthma, heart failure, sinus bradycardia, second‐degree atrioventricular block, hypotension or peripheral vascular disease, serious diseases (diabetes, serious hepatic, renal, cardiovascular, respiratory, or malignant illness). Pregnancy, lactation, or childbearing potential without adequate contraception. History of allergy to flunarizine or topiramate Setting: single‐centre Country: China Total number of randomised participants: 150, of which 50 assigned to topiramate (44 contributed to results), 50 to flunarizine (39 contributed to results), and 50 to topiramate + flunarizine. No information on proportion with migraine with aura. Among topiramate completers 30 were females and 14 males; among flunarizine completers 29 were females and 10 males; mean age topiramate completers 42.2 ± 12.4 (range 21 to 65); mean age flunarizine completers 43.2 ± 13.9 (range 20 to 64) | |
| Interventions | Topiramate 100 mg/day versus flunarizine 5 mg/day versus a combination of both (12 months). Topiramate (presumably tablets) were initiated at 25 mg/day and thereafter increased weekly by 25 mg until reaching target a dose of 100 mg/day. If there was any significant AE, patients were instructed to decrease to the previously tolerated dose. Mean topiramate dose was 62.5 ± 24.4 mg/day. Flunarizine (presumably capsules) was given in a 5 mg/day dose | |
| Outcomes | The primary efficacy parameter was the reduction in mean monthly migraine frequency of at least 50% as compared with baseline. Secondary efficacy parameters were mean monthly frequency of attacks, accumulated monthly migraine duration, and severity of head pain. Weight. Other AEs Time point(s) considered in the review: third month of treatment phase | |
| Notes | According to the publication, only patients who had chronic migraine were to be included, but the corresponding author reports that only a small minority (7/126 completers; 6%) had chronic migraine according to ICHD‐II Funders of the trial: National Natural Science Foundation, Science and Technology Item of Guandong Province of China, and Natural Science Foundation of Guandong Province of China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method for random sequence generation except for resulting 1:1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Subjects who discontinued prematurely (6 in topiramate group whereof 5 due to AEs and 1 lost to follow‐up; 9 in flunarizine group whereof 8 due to lack of efficacy and 3 lost to follow‐up) were excluded from efficacy analyses |
| Selective reporting (reporting bias) | Unclear risk | In Tables 1 and 2, migraine frequency data are mislabelled. Corresponding author confirmed it should be attacks (not days) per month in Table 1 and absolute means (not change) in Table 2. AEs (not included in this review) are inadequately reported |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. One month baseline period. Duration of treatment: 4 weeks titration, then 12 weeks stable dosage Discontinuation rate: dropout 35% for active treatment group, 40% for placebo Compliance (adherence) data: no description of how compliance was assessed Rule for use of acute medication: NSAID and triptan use monitored; unclear as to whether other drugs were permitted Methodological quality score: 4 | |
| Participants | Inclusion: IHS migraine criteria, migraine frequency of 2 to 6 per month Exclusion: renal pathology, women taking oral contraceptives, women with the possibility of becoming pregnant during the study period, commencement of any migraine prophylactic medication in the 2 months prior to the trial Setting: single‐centre Country: Italy Complete case analysis of 72 patients. Percentages of patients with aura: 23% in active treatment group, 16% in placebo. 39 females and 33 males; age range 20 to 60. 35 received 100 mg/day topiramate, 37 received placebo | |
| Interventions | Topiramate 100 mg/day versus placebo (16 weeks). Dosage started at 25 mg/day then increased by 25 mg each week until 100 mg dose reached | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (50% reduction in frequency). Severity and duration of attacks, consumption of rescue medications, days of disability Time point(s) considered in the review: last 4 weeks of the double‐blind phase | |
| Notes | Funders of the trial: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (ratio 1:1) in balanced blocks of 2 using a computer‐generated random number scheme |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study was double‐blinded, and placebo was used. No more information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Analysis of responder rate appears to consider complete cases only, and safety data are only presented for a vaguely defined subgroup of participants |
| Selective reporting (reporting bias) | Unclear risk | Standard deviations lacking for migraine frequency during treatment phase |
| Methods | Prospective, randomised, double‐blind, double‐cross‐over trial. 1‐month baseline period. Duration of treatment: 1 week titration, followed by 7 weeks stable dose of first drug. 2 months washout, then 1‐week titration, followed by 7 weeks stable dose of second drug Discontinuation rate: no dropouts were recorded Compliance (adherence) data: compliance was reported as good, but no details or results of compliance measurement are given Rule for use of acute medication: unspecified analgesics allowed, but not more than once per day. No other details provided Methodological quality score: 3 | |
| Participants | Inclusion: IHS migraine criteria, migraine for at least 6 months prior to trial, migraine frequency 3 or more per month in the 3 months prior to trial Exclusion: no clear details given on the exclusion of secondary headache, daily headache, or analgesic overuse headache. Other exclusions: concurrent medical treatment; concurrent serious medical problems; other neurological disease; lactating or pregnant Setting: single neurology clinic Country: Iran Complete case analysis of 64 patients. Patients with and without aura recruited, but percentages not reported. 36 males and 28 females; age range 14 to 57 years | |
| Interventions | Topiramate 50 mg/day versus sodium valproate 400 mg/day (8 weeks); repeat in cross‐over phase. Topiramate dose started at 25 mg/day and was incremented to 50 mg/day; sodium valproate was started at 200 mg/day and incremented to 400 mg/day | |
| Outcomes | Headache frequency per month; migraine intensity; migraine duration Time point(s) considered in the review: last (second) month of stable dosage treatment phase | |
| Notes | Study appears to use doses of both topiramate and valproate that are lower than normal clinical doses Funders of the trial: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients and clinicians were blinded, but method description is lacking |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts are acknowledged |
| Selective reporting (reporting bias) | High risk | Only 2 types of AEs are reported for topiramate |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 28‐day baseline period. Duration of treatment: 8 weeks titration, then 18 weeks stable dosage Discontinuation rate: dropout reported as 47% for combined active treatment groups; 41% for placebo. It is unclear how many patients contributed to efficacy data but in fact discontinued the study early Compliance (adherence) data: compliance data reported only as percentage of patients achieving target dose Rule for use of acute medication: analgesics, ergot derivatives, triptans, and opioids allowed Methodological quality score: 5 | |
| Participants | Inclusion: IHS migraine criteria; migraine frequency of 3 to 12 in 28‐day baseline phase; women practicing adequate contraception or unable to bear children Exclusion: secondary headaches, daily headache, and analgesic overuse headache were all adequately excluded. Other exclusions: failure to respond to more than 2 previous migraine‐prophylactic regimens, migraine onset after age 50, continued use of various CNS‐active and other drugs, history of nephrolithiasis, previous exposure to topiramate, use of experimental drug or device within 30 days of screening Setting: multicentre Country: USA Intention‐to‐treat analysis of 469 patients. Patients both with and without aura recruited, but percentages not reported. 416 females and 53 males; age range 12 to 65. 117 received 50 mg/day dose, 125 received 100 mg/day dose, 112 received 200 mg/day dose, and 115 received placebo | |
| Interventions | Topiramate 50 mg/day versus topiramate 100 mg/day versus topiramate 200 mg/day versus placebo (18 weeks). Dosage started at 25 mg/day and increased by 25 mg each week to reach assigned dose or maximum tolerated dose | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (50% reduction in frequency). Number of days requiring rescue medication. Time to onset of drug action. Quality of life (average maintenance AUC of MSQ and SF‐36 scores). AEs Time point(s) considered in the review: through entire double‐blind phase (migraine frequency, response rate, AEs); week 8 to 26 of double‐blind phase (MSQ, SF‐36) | |
| Notes | Lowest allowable age was 12 years; hence some patients not adult. Headache frequency defined as the number of migraine periods per 28 days, where a migraine period is any occurrence of migraine headache that started, ended, or recurred with 24 hours. A migraine attack persisting into a second 24‐hour period was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration Funders of the trial: Johnson & Johnson Pharmaceutical Research and Development, LLC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in permutation blocks of 4 stratified by centre |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing study drug information were provided to investigators in case such information was required on unblinding a patient |
| Blinding of participants and personnel (performance bias) | Low risk | Patients and clinicians were blinded to study medication with preprinted medication code labels. Placebo was identical in appearance and packaging to active drug |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis excluded 18 patients who did not provide any post‐baseline data, out of 487 randomised |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Study duration up to 7 months; up to 1 month screening/washout, 1 month prospective baseline, and 5 months double‐blind phase (2 months titration and 3 months maintenance) Discontinuation rate: topiramate 36%, placebo 18% Compliance (adherence) data: not available Rule for use of acute medication: use of acute medications was allowed for the symptomatic relief of breakthrough migraine pain Methodological quality score: 3 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐I; average migraine frequency of 3 to 8 migraine episodes/month for 3 months before screening; history of migraine at least 1 year; migraine onset before age 50. Ages 18 to 65 Exclusion: > 15 headache days/month during the 3 months before screening, during screening, or during the prospective baseline period; overused acute migraine treatment (eg, triptan use on > 8 days/month); transformed migraine. Secondary headaches acceptably excluded. Other exclusions: previously failed to respond to topiramate therapy; preventive medication within 2 weeks of the start of baseline period; cluster headache; basilar, ophthalmoplegic, or hemiplegic migraine; migraine aura exclusively (without headache); previously failure to respond to > 2 adequately dosed migraine preventive medications; receipt of injected corticosteroids, local anaesthetics, or botulinum toxin within 60 days before screening; risk of pregnancy; lactation; serum alanine and/or aspartate aminotransferase levels > 2 times the upper limit of the normal range; active liver disease Setting: 27 centres Country: USA Intention‐to‐treat analysis of 211 patients. 75 subjects (36%) in ITT group had migraine with aura. 181 females and 30 males; mean age 40.5 ± 11.1; age range 18 to 64. 140 received topiramate 73 received placebo | |
| Interventions | Topiramate 200 mg/day versus placebo (20 weeks). Topiramate (presumably tablet) 25 mg/day for the first week, followed by weekly increases of 25 mg to a maximum of 100 mg BID or the maximum tolerated dose at week 8. Mean dosage during maintenance period: 161 ± 53 mg/day. Placebo (tablet?) 1/day for the first week, followed by weekly increases of 1 to a maximum of 4 BID or the maximum tolerated dose at week 8 | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (those with ≥ 50%, ≥ 75%, or 100% reduction in monthly migraine frequency). Safety assessments included measurement of vital signs, physical examinations, clinical laboratory test, and evaluation of AEs Time point(s) considered in the review: through the 20‐week double‐blind phase | |
| Notes | Funders of the trial: Ortho‐McNeil Neurologics Inc., Titusville, New Jersey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in publication except for 2:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients and clinicians were blinded. No description of method except for the use of placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Data on mean migraine frequencies during the double‐blind period lacking (only changes in least squares means without variance measures given in publication). Supplementary information requested twice from corresponding author, but no reply |
| Methods | Prospective, randomised, double‐blind, parallel trial. Total duration: 20 weeks. 4‐week baseline period, 8 weeks titration, 8‐week maintenance period Discontinuation rate: dropout 16% for active treatment; 10% for placebo Compliance (adherence) data: no compliance data reported Rule for use of acute medication: abortive medications permitted (no further specification) Methodological quality score: 3 | |
| Participants | Inclusion: IHS migraine criteria; migraine onset at least 1 year prior to trial; 2 or more attacks per month for previous 12 months; adequate contraception for women; negative pregnancy test 72 hours prior to trial Exclusion: secondary headaches, daily headaches, and analgesic overuse headaches were adequately excluded. Other exclusions: substance‐related disorders, psychiatric disorder, carbonic anhydrase inhibitors, other experimental interventions, history of renal calculi, diagnosis of multiple sclerosis, other contraindications Setting: single neurology clinic Country: USA 40 migraine patients participated; numbers with and without aura not reported. 39 females and 1 male; allowed age range 18 to 65 years | |
| Interventions | Topiramate versus placebo (16 weeks). Dosage titrated and maintained at 200 mg/day or maximum tolerated dose. Mean actual dose 125 mg/day | |
| Outcomes | Number of migraine attacks per 28 days. Migraine severity (3‐point scale). Change in body weight Time point(s) considered in the review: through entire double‐blind phase | |
| Notes | Unusual feature of trial: concomitant migraine prophylactics were allowed if patients had been on stable dose for 3 months prior to start of trial, and no changes in dose took place during trial Funders of the trial: Ortho‐McNeil Pharmaceutical, Raritan, New Jersey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information except 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients and clinicians were blinded. No description of method except for the use of placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
| Methods | Prospective, randomised, open, parallel‐group trial. Total study duration 10 to 12 months: 1 to 3 months baseline, 3 months treatment period, and 6 months additional follow‐up Discontinuation rate: topiramate 32%, relaxation 13% Compliance (adherence data on file provided by corresponding author): 77% in topiramate group (defined as using the drug > 2 months in accordance with the prescription and measured using self reports), 87% in relaxation group (defined as participating in 6 or more sessions at the clinic plus verbal confirmation of practice at home) Rule for use of acute medication: medication overuse headache was an exclusion criterion. No restrictions were thereafter placed on the use of concomitant acute medication. Acute medication use (doses/month) was documented during the whole treatment period Methodological quality score: 3 | |
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; 2 to 8 migraine attacks/month; history of migraine at least 1 year; onset of migraine before 50 years of age. Ages 18 to 65 Exclusion: medication overuse headache according to ICHD‐II. Other secondary headaches adequately excluded. CDH not an exclusion criterion (1 patient had chronic migraine). Other exclusions: interval headaches not distinguishable from migraine; regular exercise (once or more per week during the 12 weeks prior to the study); earlier regular practice of relaxation; pregnancy; breastfeeding; use of daily migraine prophylaxis in the 12 weeks prior to the study; inability to understand Swedish; use of antipsychotic or antidepressive medication in the 12 weeks prior to the study; drug or alcohol abuse; topiramate intolerance Setting: single‐centre Country: Sweden Intention‐to‐treat analysis of 91 patients; 7 had migraine with aura only, 44 had migraine without aura only, and 40 had both migraine with and without aura. 82 females and 9 males; mean age 44.3 ± 10.6; allowed age range 18 to 65 years. 31 received topiramate, 30 received relaxation and 30 received exercise | |
| Interventions | Topiramate 200 mg/day versus relaxation versus exercise (36 weeks). Topiramate tablets started by 25 mg at night and thereafter increased weekly by 25 mg in dialogue with a neurologist until reaching the highest tolerable dose with a maximum of 100 mg BID. Participants in relaxation arm had to attend a scheduled individual appointment for relaxation with a registered physiotherapist once a week. The relaxation programme (Larsson 2002) is based on relaxation, breathing, and stress‐management techniques and includes a series of 6 exercises, each of which is based on the one before. Each relaxation exercise lasted for between 5 and 20 minutes, and verbal and written information was given before the introduction of a new relaxation exercise. After each session there was an opportunity for the participant to discuss their progress with the physiotherapist. If they were absent, they were contacted and informed about how to continue on their own. Between the scheduled sessions, the participants practised at home every day with a compact disc | |
| Outcomes | Migraine attack frequency per 28 days. Proportion of responders (≥ 50% and 25% to 49% reduction in migraine attack frequency). Migraine days per 28 days. Mean pain intensity (VAS 0 to 100). Acute medication use (doses per 28 days). Quality of life (MSQoL, 0 to 100 points). Level of physical activity (MET‐minutes/week). Sedentary hours/day. Oxygen uptake. AEs Time point(s) considered in the review: third month of treatment | |
| Notes | Exercise arm of trial excluded as comparator from this review, since the intervention is experimental Funders of the trial: The Swedish Research Council; The Gothenburg Research and Development Council; Praktikertjänst Inc, Stockholm, Sweden; The Minnesfonden at the Swedish Association of Registered Physiotherapists; The Renée Eander Fund; The Neurological Research Foundation; The Olle Engkvist Byggmästare Foundation; GlaxoSmithKline; AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation procedure was conducted by an independent person (separate from clinician and patient) according to a lottery procedure. Six pieces of paper, 2 for each group (n = 3), were folded twice and put into an opaque envelope. One piece of paper was taken each time a patient entered the study. After 6 participants had been included, the procedure started again |
| Allocation concealment (selection bias) | High risk | High number of withdrawals in topiramate arm ("refusal to start") suggests treatment selection bias by the subjects (predetermined treatment preference). Given the open nature of the study this may have influenced outcome reporting |
| Blinding of participants and personnel (performance bias) | High risk | As this study compared pharmacological and non‐pharmacological treatments, blindness to treatment was not possible to achieve |
| Blinding of outcome assessment (detection bias) | Low risk | The completed assessment forms were encoded and returned to the study secretary in sealed envelopes. The evaluator was effectively blinded |
| Incomplete outcome data (attrition bias) | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
Abbreviations: AE = adverse event; AUC = area under the curve; BID = twice (two times) a day; BMI = body mass index; CDH = chronic daily headache; CNS = central nervous system; h = hour; HIT‐6 = Headache Impact Test; ICHD‐I/ICHD‐II = International Classification of Headache Disorders, 1st/2nd Edition; iCNV = initial contingent negative variation; IHS = International Headache Society; ITT = intention‐to‐treat; MET = metabolic equivalents; MIDAS = Migraine Disability Assessment; MSQ = Migraine‐Specific Questionnaire; MSQoL = Migraine‐Specific Quality of Life Questionnaire; NSAIDs = non‐steroidal anti‐inflammatory drugs; Q‐LES‐Q‐SF = Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form; SD = standard deviation; SF‐12 = Medical Outcomes Study 12‐item Short‐Form Health Survey; SF‐36 = Medical Outcomes Study 36‐item Short‐Form Health Survey; TTH = tension‐type headache; VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Reports data on chronic migraine only | |
| Serious flaws including selective outcome reporting and concerns about data integrity | |
| Comparator not used prophylactically but taken early in each attack during premonitory symptoms | |
| Poor reporting with many details lacking and important data conflicting in tables, graphs, and text | |
| Basic science paper | |
| No control group | |
| Combined analysis of data from 2 included trials (Edwards 2000 and Storey 2001) | |
| Conference abstract only | |
| Not randomised or pseudo‐randomised | |
| Highly selected and small sample of women with vertigo. Potential effect of inadequate randomisation procedure cannot be weighted (patient characteristics are missing). Inclusion of patients with CDH. Lack of estimates of variance | |
| Conference abstract only | |
| Not controlled trial (brief information sheet) | |
| All participants had depression. Since the comparator (amitriptyline) is an antidepressant, the results are not valid for migraineurs without psychiatric morbidity | |
| Not randomised or pseudo‐randomised | |
| No treatment arm in which topiramate alone was given | |
| No treatment arm in which topiramate alone was given | |
| No control group | |
| Meta‐analysis of data on adverse drug reactions. Of 6 migraine studies analysed, 5 are included in this review (Brandes 2004; Diener 2004; Mei 2004; Silberstein 2004; Silberstein 2006), while the 6th is excluded (Silvestrini 2003) | |
| Comparator is experimental (subcutaneous histamine) | |
| Comparator is experimental (zonisamide) | |
| Review article | |
| Post hoc analysis of Diener 2007 (included) | |
| Data obtained retrospectively by using MIDAS, which is not designed to measure migraine attack frequency. Means and variance thus lacking for migraine frequency, as are responder rates | |
| Conference abstract only | |
| Review article | |
| Reports data on chronic migraine only |
Abbreviations: CDH = chronic daily headache; MIDAS = Migraine Disability Assessment
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 9 | 1793 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.59, ‐0.80] |
| Analysis 1.1 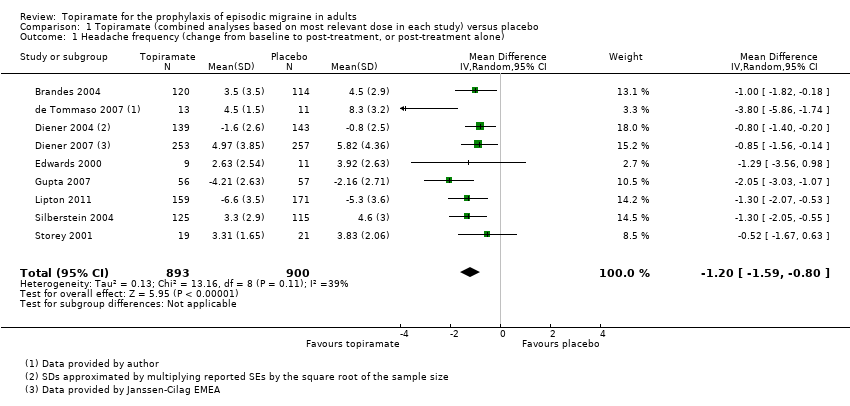 Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone). | ||||
| 2 ORs for responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | 1246 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [2.10, 4.82] |
| Analysis 1.2  Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 2 ORs for responders (patients with ≥ 50% reduction in headache frequency). | ||||
| 3 RRs for responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.57, 2.60] |
| Analysis 1.3  Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 3 RRs for responders (patients with ≥ 50% reduction in headache frequency). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone). | ||||
| 1.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 576 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.95, 0.04] |
| 1.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 6 | 1620 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.58, ‐0.71] |
| 1.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 804 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.53, ‐0.36] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 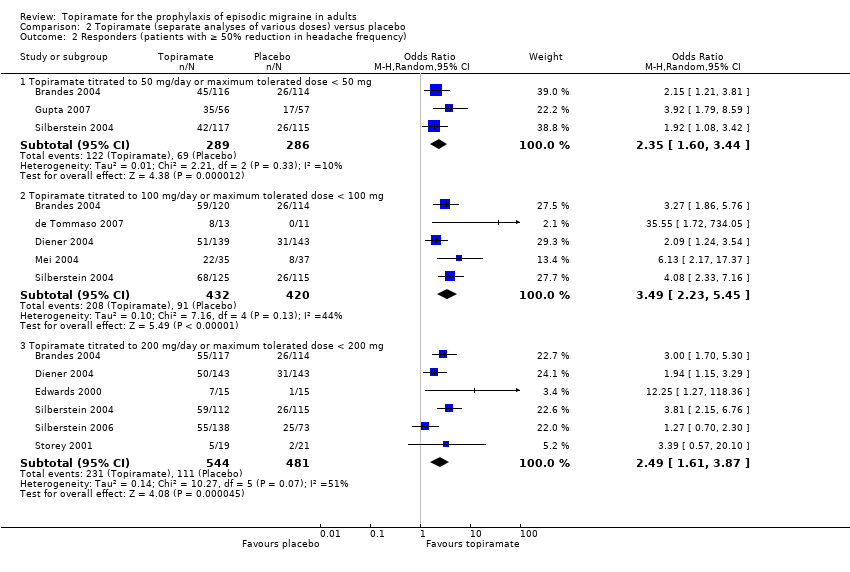 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency). | ||||
| 2.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [1.60, 3.44] |
| 2.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 852 | Odds Ratio (M‐H, Random, 95% CI) | 3.49 [2.23, 5.45] |
| 2.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 6 | 1025 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [1.61, 3.87] |
| 3 Any adverse event Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3 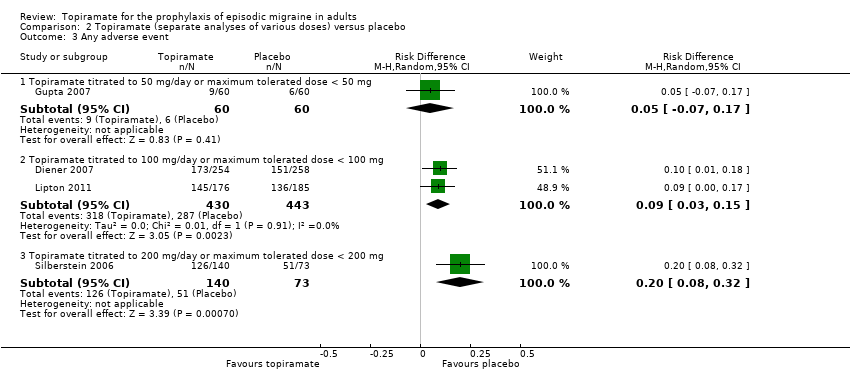 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 3 Any adverse event. | ||||
| 3.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.07, 0.17] |
| 3.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 873 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.03, 0.15] |
| 3.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 1 | 213 | Risk Difference (M‐H, Random, 95% CI) | 0.20 [0.08, 0.32] |
| 4 Anorexia Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 4 Anorexia. | ||||
| 4.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.07] |
| 4.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 4.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.05, 0.12] |
| 5 Fatigue Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 5 Fatigue. | ||||
| 5.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.07, 0.15] |
| 5.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 5.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.04, 0.13] |
| 6 Memory problems Show forest plot | 5 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6 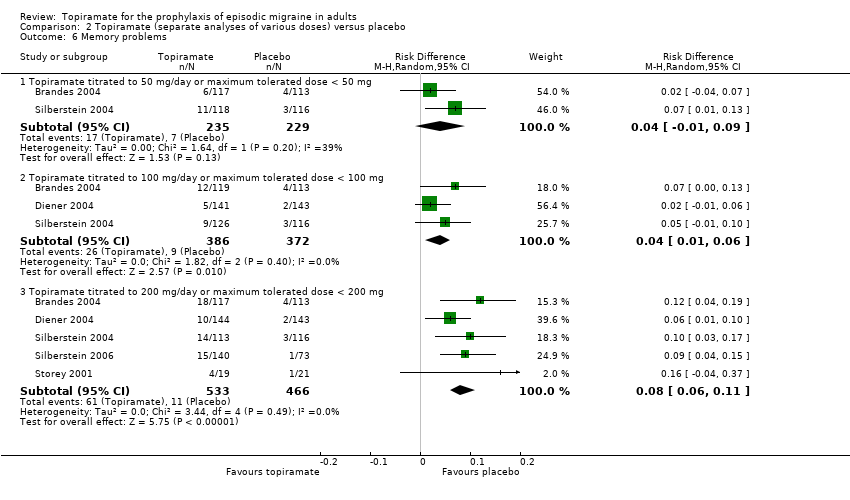 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 6 Memory problems. | ||||
| 6.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 6.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 3 | 758 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 6.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.06, 0.11] |
| 7 Nausea Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.7 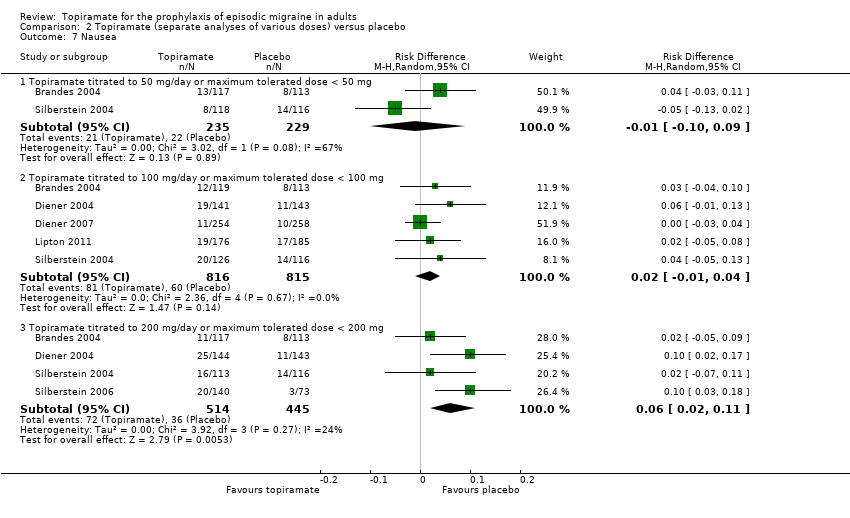 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 7 Nausea. | ||||
| 7.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 7.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.04] |
| 7.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 8 Paresthesia Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.8 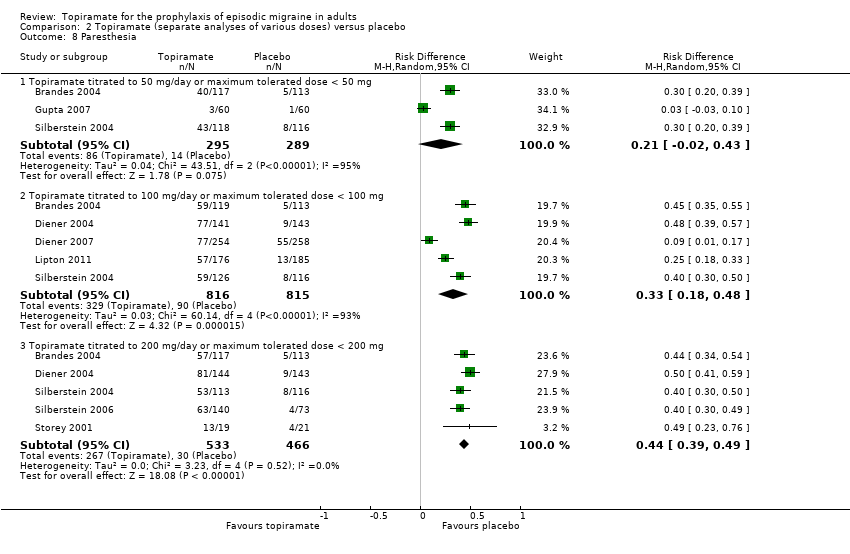 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 8 Paresthesia. | ||||
| 8.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [‐0.02, 0.43] |
| 8.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.33 [0.18, 0.48] |
| 8.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [0.39, 0.49] |
| 9 Taste disturbance Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 9 Taste disturbance. | ||||
| 9.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.21] |
| 9.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1623 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.12] |
| 9.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 786 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.09, 0.19] |
| 10 Weight loss Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 10 Weight loss. | ||||
| 10.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 10.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 4 | 1270 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 10.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.07, 0.12] |
| 11 MSQ‐role function restrictive Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.11 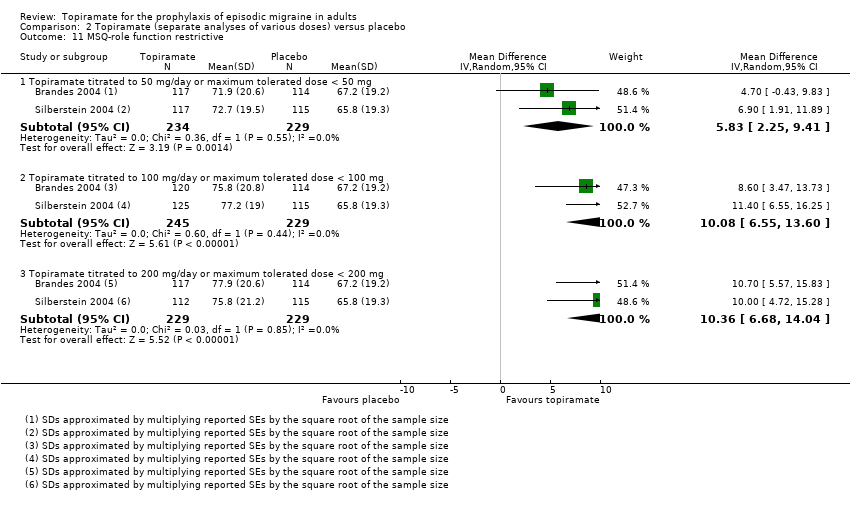 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 11 MSQ‐role function restrictive. | ||||
| 11.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.83 [2.25, 9.41] |
| 11.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.08 [6.55, 13.60] |
| 11.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 10.36 [6.68, 14.04] |
| 12 MSQ‐role function prevention Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.12 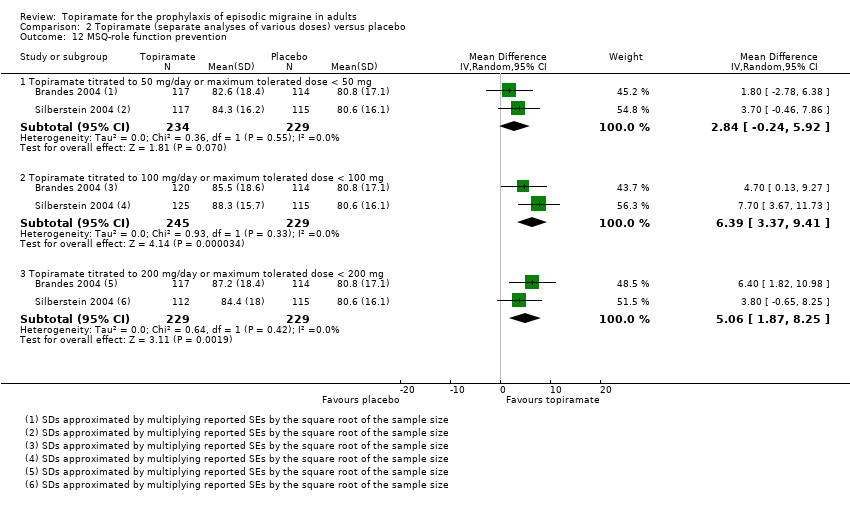 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 12 MSQ‐role function prevention. | ||||
| 12.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.84 [‐0.24, 5.92] |
| 12.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.39 [3.37, 9.41] |
| 12.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.06 [1.87, 8.25] |
| 13 MSQ‐emotional function Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.13  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 13 MSQ‐emotional function. | ||||
| 13.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.58 [0.61, 8.54] |
| 13.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.22 [6.31, 14.14] |
| 13.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.45 [4.38, 12.52] |
| 14 SF‐36 role physical Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.14 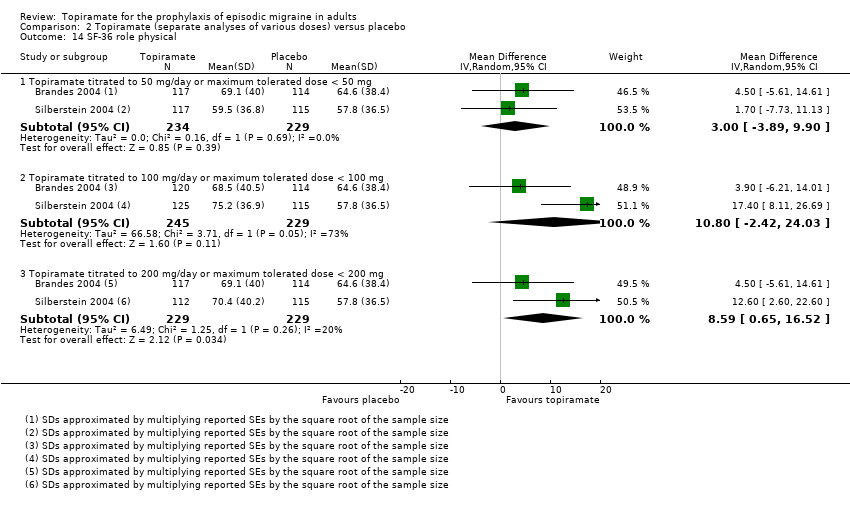 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 14 SF‐36 role physical. | ||||
| 14.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐3.89, 9.90] |
| 14.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.80 [‐2.42, 24.03] |
| 14.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.59 [0.65, 16.52] |
| 15 SF‐36 vitality Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.15  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 15 SF‐36 vitality. | ||||
| 15.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.08 [‐4.68, 8.84] |
| 15.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.48 [‐7.77, 16.73] |
| 15.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐4.52, 7.24] |
| 16 SF‐36 physical functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.16 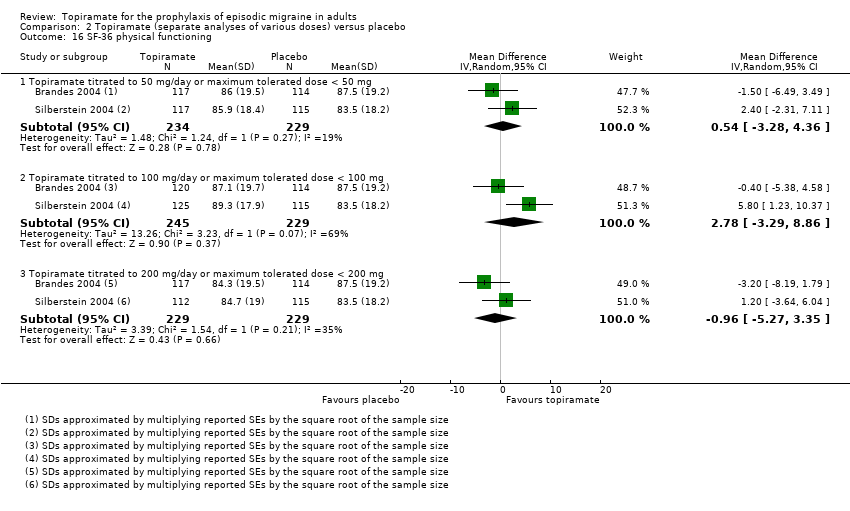 Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 16 SF‐36 physical functioning. | ||||
| 16.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐3.28, 4.36] |
| 16.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.78 [‐3.29, 8.86] |
| 16.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐5.27, 3.35] |
| 17 SF‐36 bodily pain Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.17  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 17 SF‐36 bodily pain. | ||||
| 17.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.35 [0.04, 8.66] |
| 17.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.35 [‐1.29, 14.00] |
| 17.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.12 [‐1.25, 11.49] |
| 18 SF‐36 general health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.18  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 18 SF‐36 general health. | ||||
| 18.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐2.18, 5.08] |
| 18.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.18 [‐1.21, 9.57] |
| 18.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐1.00, 6.15] |
| 19 SF‐36 social functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.19  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 19 SF‐36 social functioning. | ||||
| 19.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.00 [‐1.92, 5.92] |
| 19.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 3.13 [‐3.73, 9.99] |
| 19.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐2.07, 5.96] |
| 20 SF‐36 role emotional Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.20  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 20 SF‐36 role emotional. | ||||
| 20.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐4.56, 9.16] |
| 20.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.64 [‐3.39, 12.68] |
| 20.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.75 [‐4.99, 10.48] |
| 21 SF‐36 mental health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.21  Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 21 SF‐36 mental health. | ||||
| 21.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐4.59, 6.98] |
| 21.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐5.65, 10.81] |
| 21.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.57 [‐4.21, 7.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Topiramate direct dose comparisons, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone). | ||||
| 1.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.32, ‐0.10] |
| 1.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.53, ‐0.40] |
| 1.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.55, 0.61] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Topiramate direct dose comparisons, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency). | ||||
| 2.1 Topiramate 100 mg versus 50 mg | 2 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.25, 2.60] |
| 2.2 Topiramate 200 mg versus 50 mg | 2 | 462 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [1.15, 2.41] |
| 2.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.24] |
| 3 MSQ‐role function restrictive Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Topiramate direct dose comparisons, Outcome 3 MSQ‐role function restrictive. | ||||
| 3.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 4.22 [0.65, 7.80] |
| 3.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.55 [0.82, 8.28] |
| 3.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐3.37, 3.99] |
| 4 MSQ‐role function prevention Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Topiramate direct dose comparisons, Outcome 4 MSQ‐role function prevention. | ||||
| 4.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 3.54 [0.48, 6.60] |
| 4.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐2.13, 6.69] |
| 4.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐6.67, 4.30] |
| 5 MSQ‐emotional function Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Topiramate direct dose comparisons, Outcome 5 MSQ‐emotional function. | ||||
| 5.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 5.62 [1.67, 9.58] |
| 5.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐0.21, 8.02] |
| 5.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐5.80, 2.34] |
| 6 SF‐36 role physical Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.6 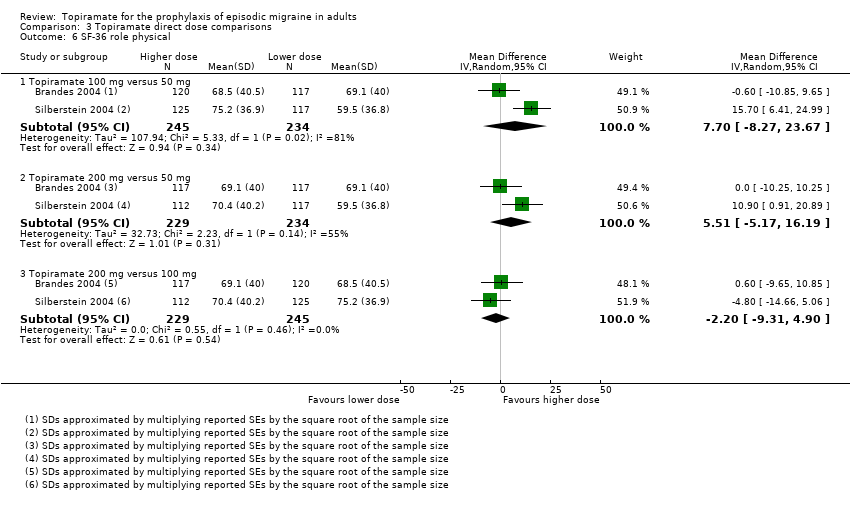 Comparison 3 Topiramate direct dose comparisons, Outcome 6 SF‐36 role physical. | ||||
| 6.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 7.70 [‐8.27, 23.67] |
| 6.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.51 [‐5.17, 16.19] |
| 6.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐9.31, 4.90] |
| 7 SF‐36 vitality Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Topiramate direct dose comparisons, Outcome 7 SF‐36 vitality. | ||||
| 7.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐3.05, 7.92] |
| 7.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐4.64, 3.39] |
| 7.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.01 [‐9.38, 3.35] |
| 8 SF‐36 physical functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Topiramate direct dose comparisons, Outcome 8 SF‐36 physical functioning. | ||||
| 8.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.35 [‐1.02, 5.72] |
| 8.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐4.92, 2.04] |
| 8.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐7.18, ‐0.32] |
| 9 SF‐36 bodily pain Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.9  Comparison 3 Topiramate direct dose comparisons, Outcome 9 SF‐36 bodily pain. | ||||
| 9.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.13 [‐1.83, 6.08] |
| 9.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐3.27, 4.96] |
| 9.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐5.23, 2.91] |
| 10 SF‐36 general health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.10 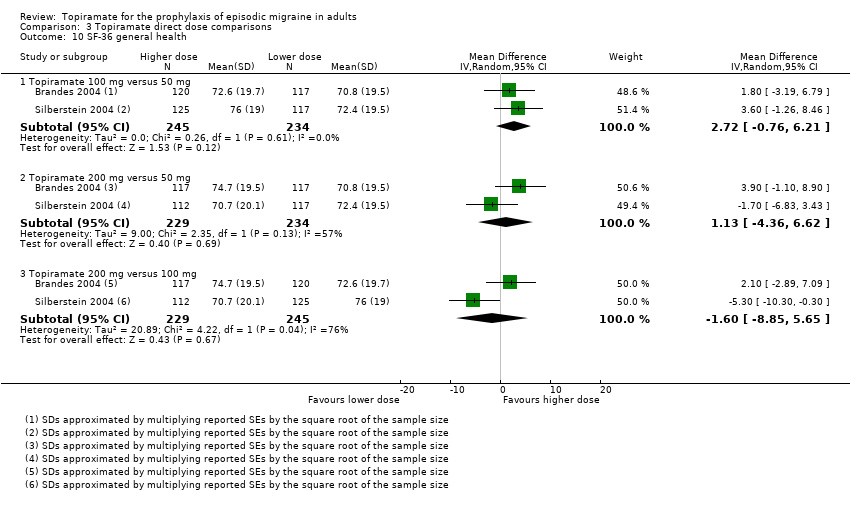 Comparison 3 Topiramate direct dose comparisons, Outcome 10 SF‐36 general health. | ||||
| 10.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.72 [‐0.76, 6.21] |
| 10.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐4.36, 6.62] |
| 10.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.85, 5.65] |
| 11 SF‐36 social functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.11 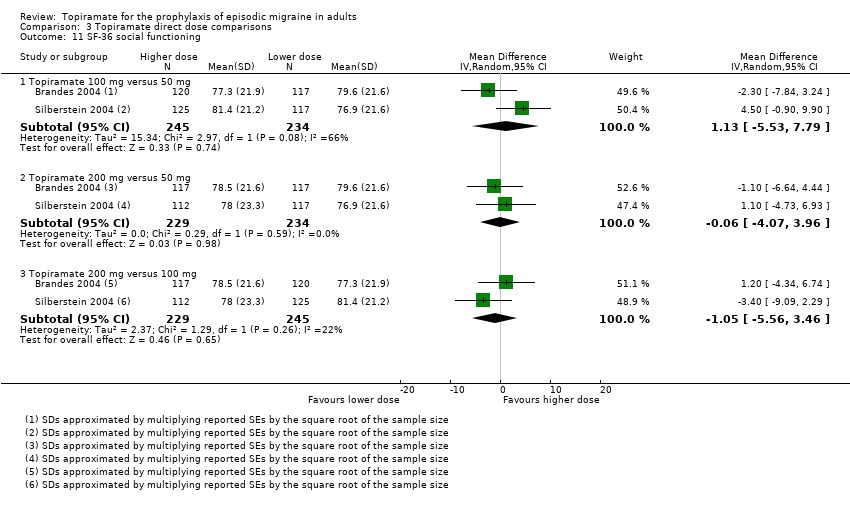 Comparison 3 Topiramate direct dose comparisons, Outcome 11 SF‐36 social functioning. | ||||
| 11.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐5.53, 7.79] |
| 11.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐4.07, 3.96] |
| 11.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐5.56, 3.46] |
| 12 SF‐36 role emotional Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.12 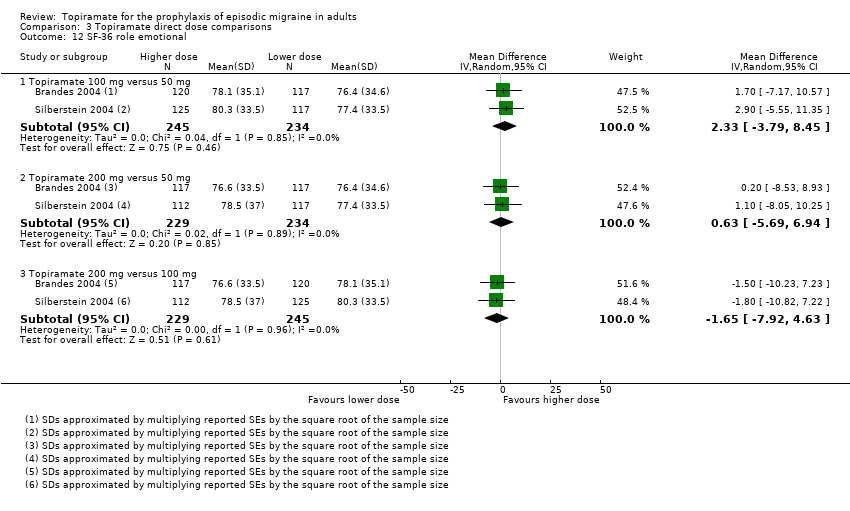 Comparison 3 Topiramate direct dose comparisons, Outcome 12 SF‐36 role emotional. | ||||
| 12.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐3.79, 8.45] |
| 12.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐5.69, 6.94] |
| 12.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐7.92, 4.63] |
| 13 SF‐36 mental health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.13  Comparison 3 Topiramate direct dose comparisons, Outcome 13 SF‐36 mental health. | ||||
| 13.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐1.87, 4.49] |
| 13.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.87, 3.67] |
| 13.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐4.10, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1 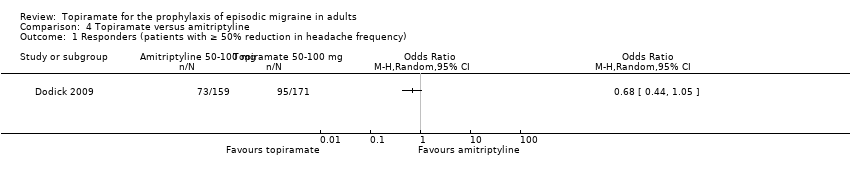 Comparison 4 Topiramate versus amitriptyline, Outcome 1 Responders (patients with ≥ 50% reduction in headache frequency). | ||||
| 2 MIDAS score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Topiramate versus amitriptyline, Outcome 2 MIDAS score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (post‐treatment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Topiramate versus flunarizine, Outcome 1 Headache frequency (post‐treatment). | ||||
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Topiramate versus flunarizine, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 2 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.61, 0.34] |
| Analysis 6.1  Comparison 6 Topiramate versus propranolol, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone). | ||||
| 1.1 Topiramate 50 mg versus propranolol 80 mg | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.15, 0.41] |
| 1.2 Topiramate 100 mg versus propranolol 160 mg | 1 | 282 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.60, 0.60] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Topiramate versus propranolol, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (post‐treatment) Show forest plot | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| Analysis 7.1  Comparison 7 Topiramate versus sodium valproate, Outcome 1 Headache frequency (post‐treatment). | ||||
| 2 MIDAS score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Topiramate versus sodium valproate, Outcome 2 MIDAS score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 Topiramate versus relaxation, Outcome 1 Headache frequency (change from baseline to post‐treatment). | ||||
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 Topiramate versus relaxation, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency). | ||||
| 3 Change from baseline in MSQoL Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 Topiramate versus relaxation, Outcome 3 Change from baseline in MSQoL. | ||||

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
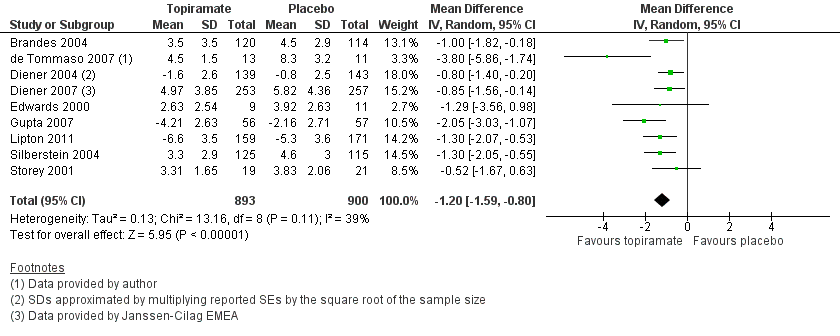
Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Forest plot of comparison: 2 Topiramate (separate analyses of various doses) versus placebo, outcome: 2.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.3 RRs for responders (patients with ≥ 50% reduction in headache frequency).

Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 2 ORs for responders (patients with ≥ 50% reduction in headache frequency).
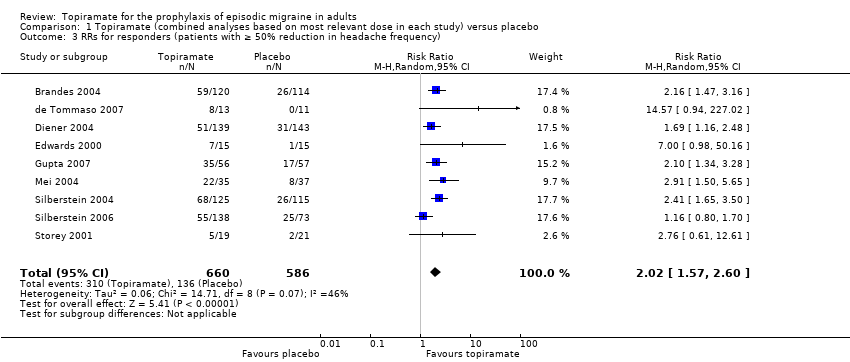
Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 3 RRs for responders (patients with ≥ 50% reduction in headache frequency).

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
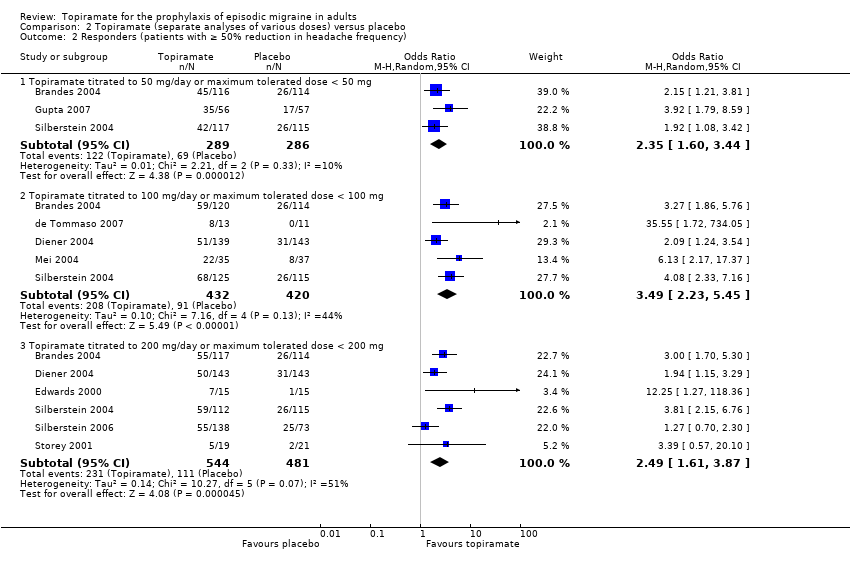
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
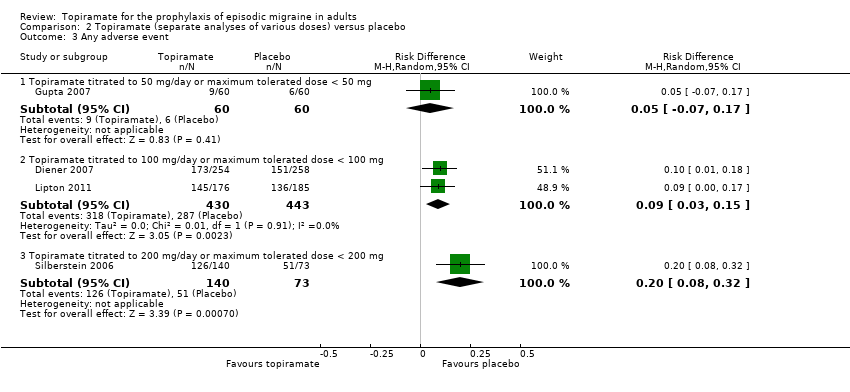
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 3 Any adverse event.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 4 Anorexia.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 5 Fatigue.
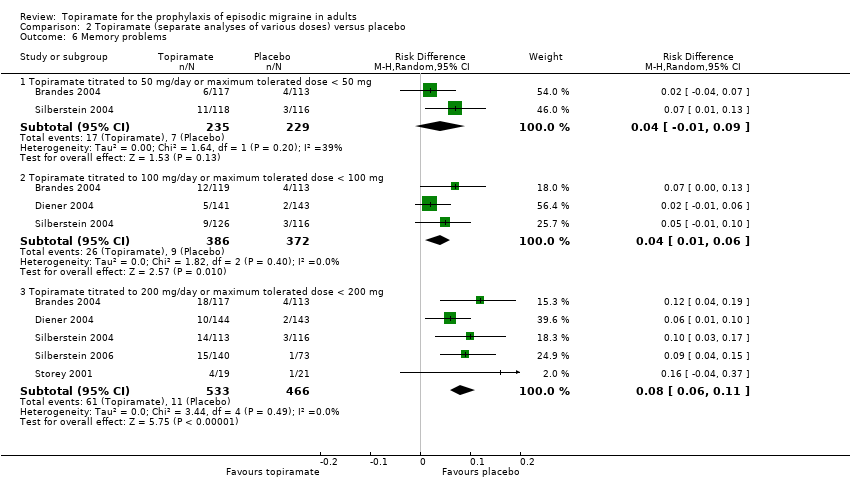
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 6 Memory problems.
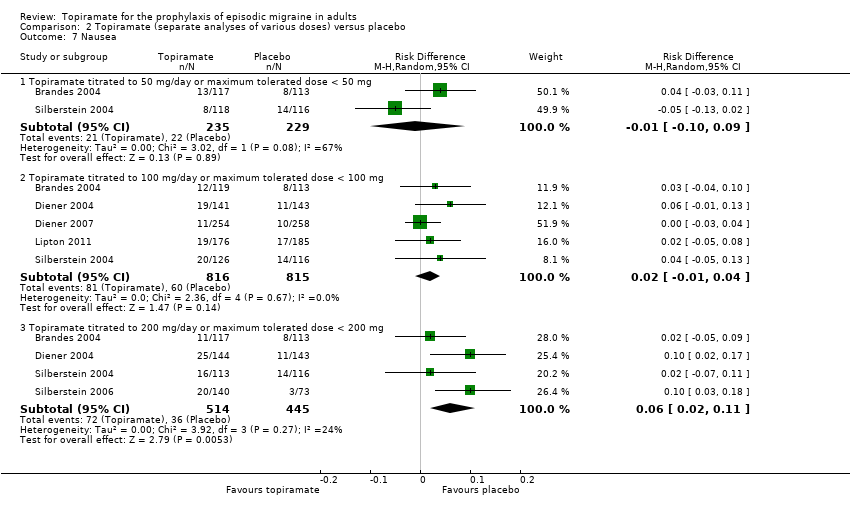
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 7 Nausea.
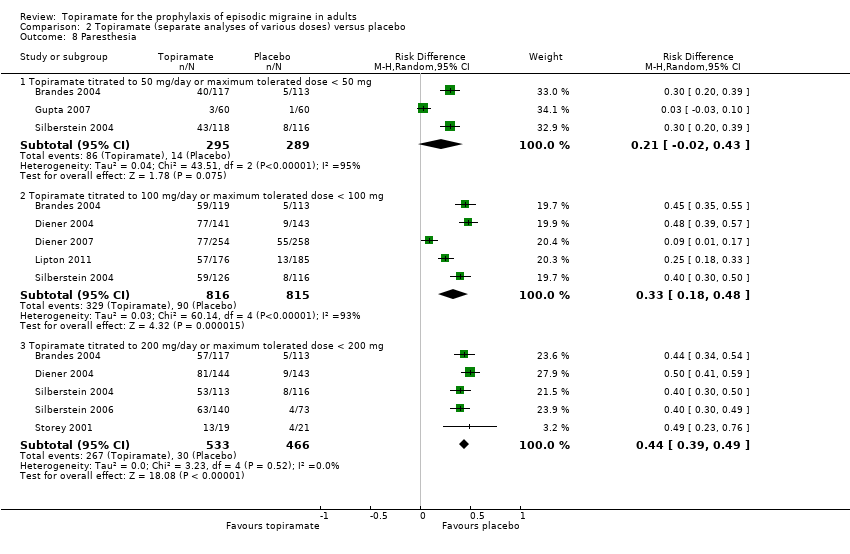
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 8 Paresthesia.
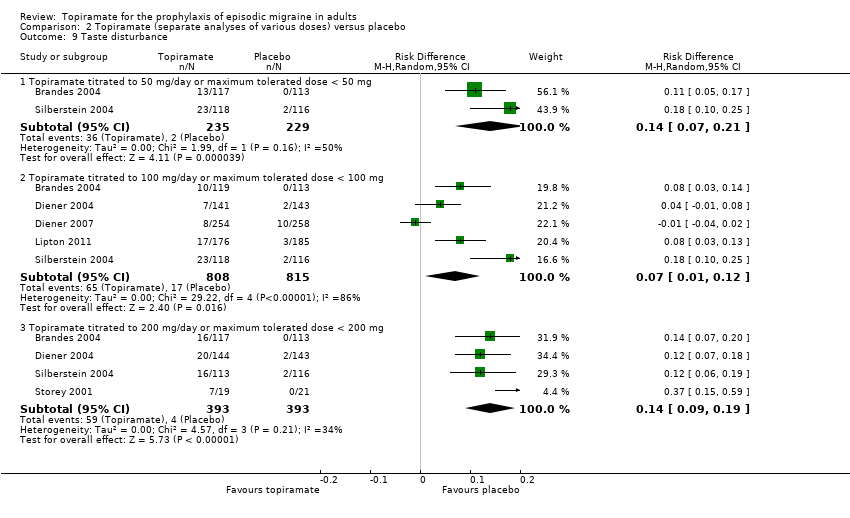
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 9 Taste disturbance.
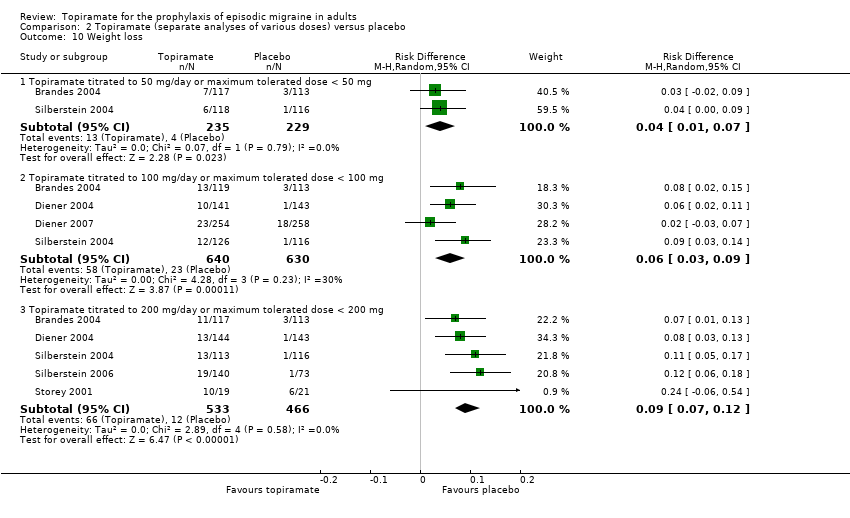
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 10 Weight loss.
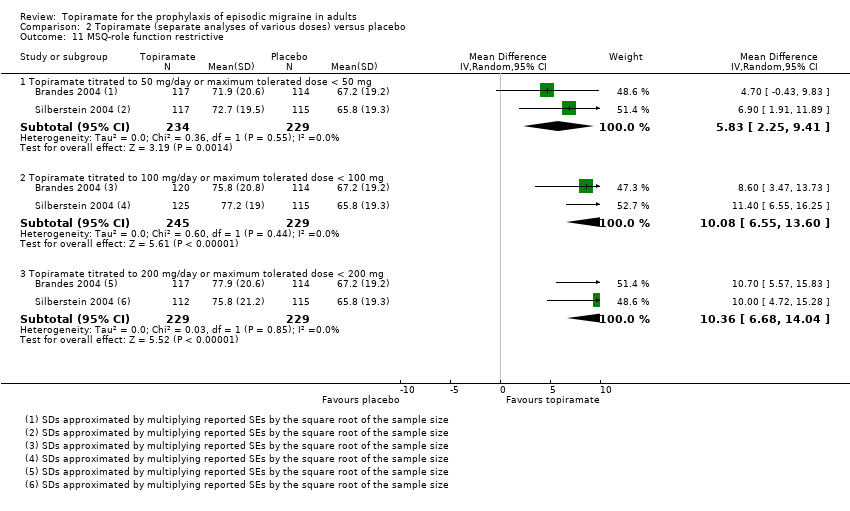
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 11 MSQ‐role function restrictive.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 12 MSQ‐role function prevention.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 13 MSQ‐emotional function.
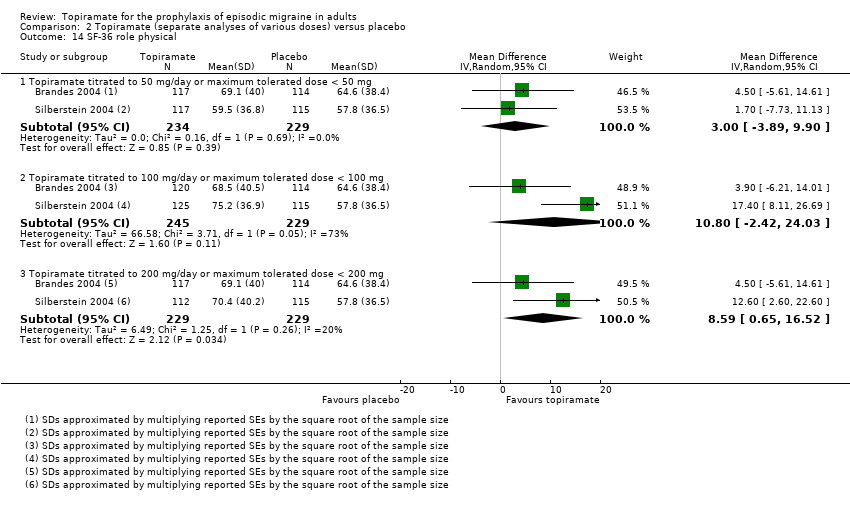
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 14 SF‐36 role physical.
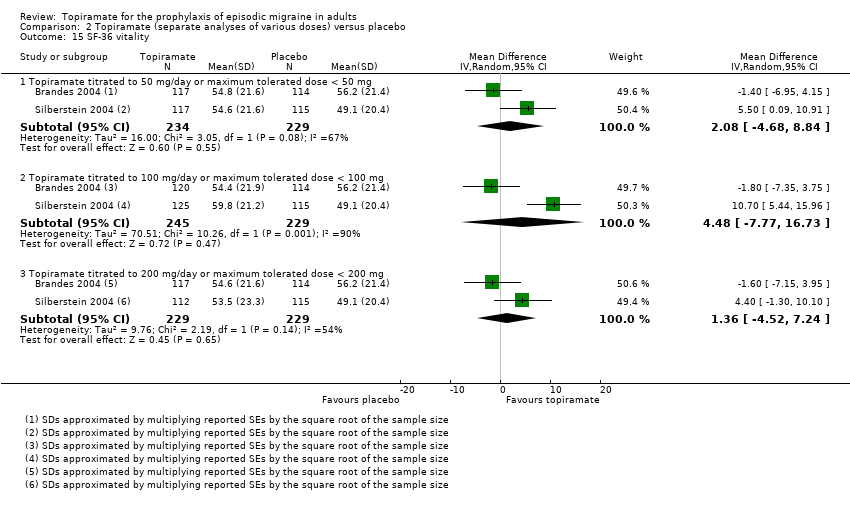
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 15 SF‐36 vitality.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 16 SF‐36 physical functioning.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 17 SF‐36 bodily pain.
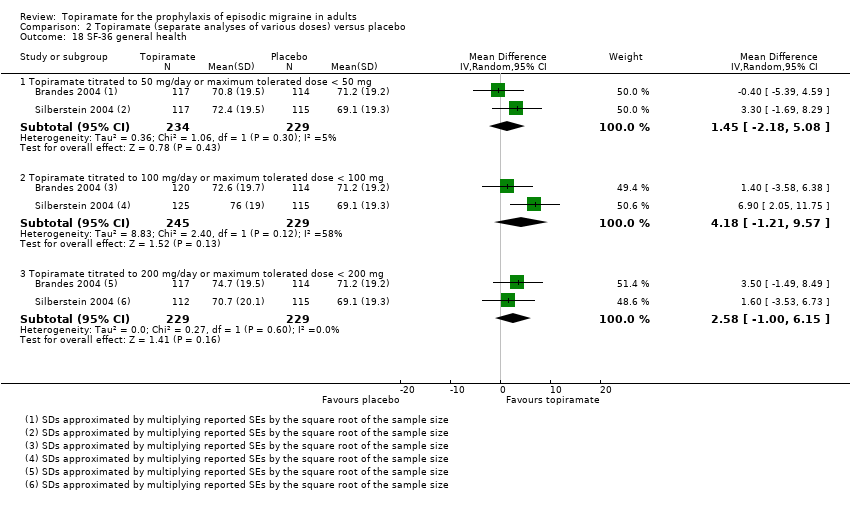
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 18 SF‐36 general health.
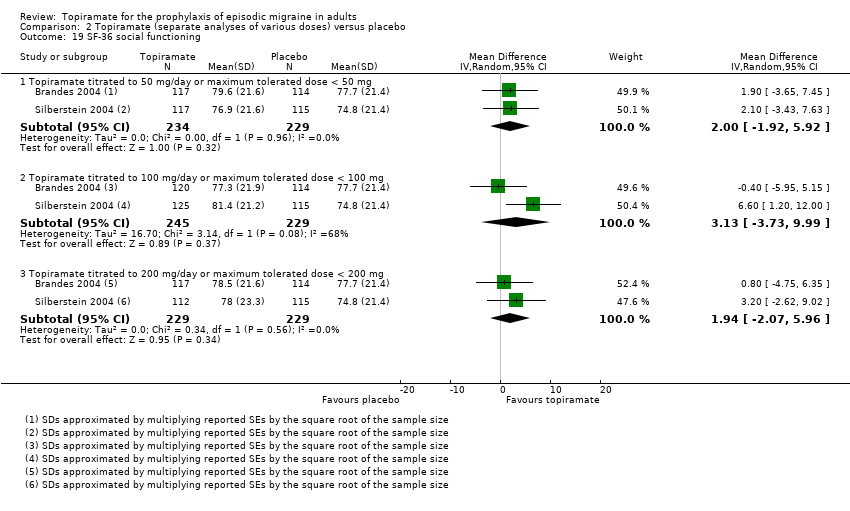
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 19 SF‐36 social functioning.
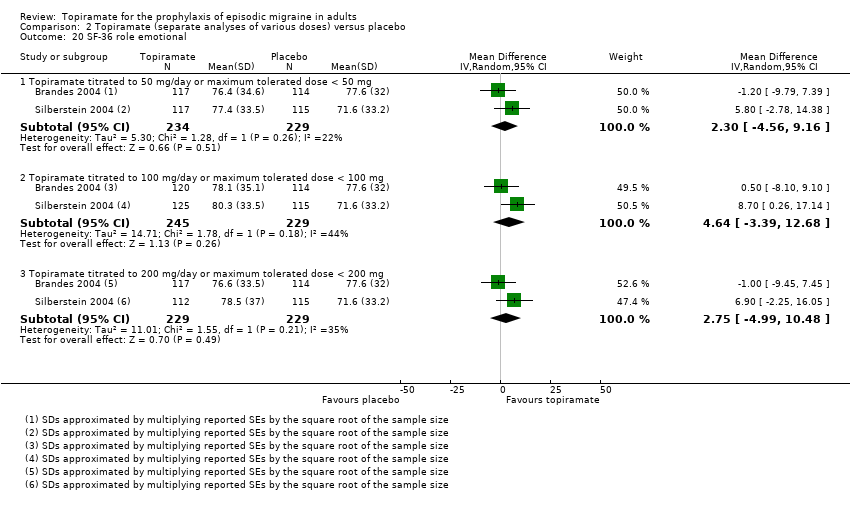
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 20 SF‐36 role emotional.

Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 21 SF‐36 mental health.

Comparison 3 Topiramate direct dose comparisons, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).

Comparison 3 Topiramate direct dose comparisons, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).

Comparison 3 Topiramate direct dose comparisons, Outcome 3 MSQ‐role function restrictive.

Comparison 3 Topiramate direct dose comparisons, Outcome 4 MSQ‐role function prevention.

Comparison 3 Topiramate direct dose comparisons, Outcome 5 MSQ‐emotional function.

Comparison 3 Topiramate direct dose comparisons, Outcome 6 SF‐36 role physical.

Comparison 3 Topiramate direct dose comparisons, Outcome 7 SF‐36 vitality.

Comparison 3 Topiramate direct dose comparisons, Outcome 8 SF‐36 physical functioning.
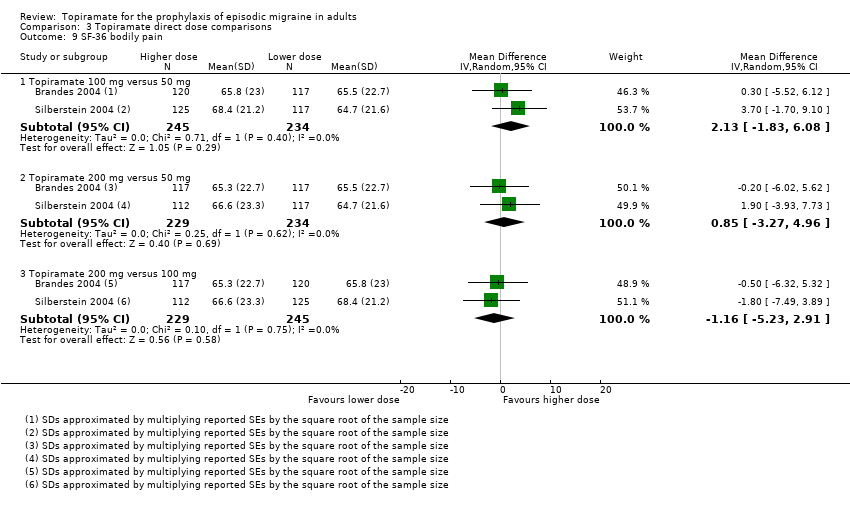
Comparison 3 Topiramate direct dose comparisons, Outcome 9 SF‐36 bodily pain.

Comparison 3 Topiramate direct dose comparisons, Outcome 10 SF‐36 general health.
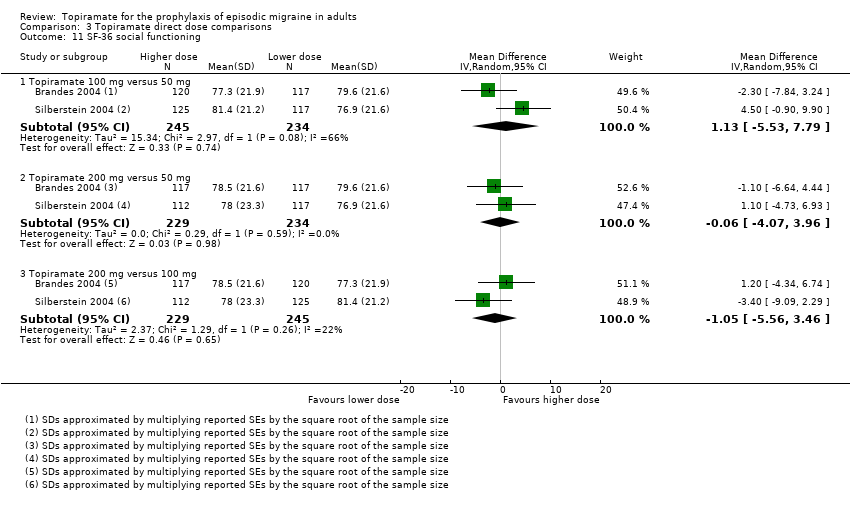
Comparison 3 Topiramate direct dose comparisons, Outcome 11 SF‐36 social functioning.

Comparison 3 Topiramate direct dose comparisons, Outcome 12 SF‐36 role emotional.

Comparison 3 Topiramate direct dose comparisons, Outcome 13 SF‐36 mental health.

Comparison 4 Topiramate versus amitriptyline, Outcome 1 Responders (patients with ≥ 50% reduction in headache frequency).
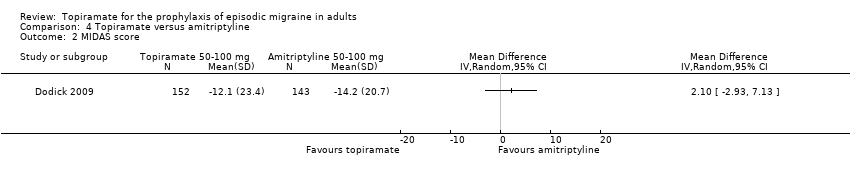
Comparison 4 Topiramate versus amitriptyline, Outcome 2 MIDAS score.
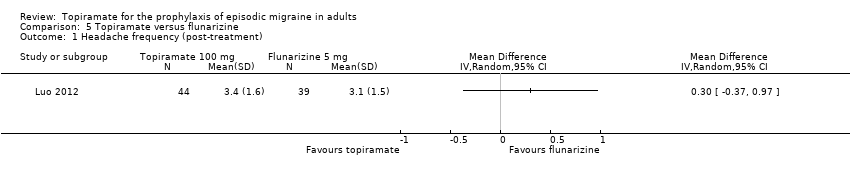
Comparison 5 Topiramate versus flunarizine, Outcome 1 Headache frequency (post‐treatment).

Comparison 5 Topiramate versus flunarizine, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).

Comparison 6 Topiramate versus propranolol, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
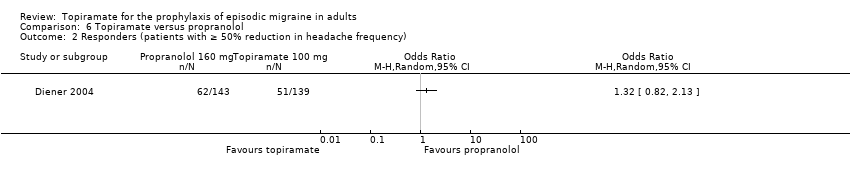
Comparison 6 Topiramate versus propranolol, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).

Comparison 7 Topiramate versus sodium valproate, Outcome 1 Headache frequency (post‐treatment).
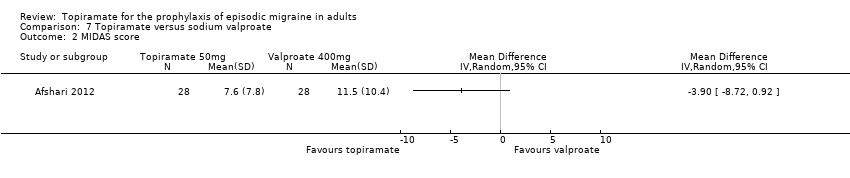
Comparison 7 Topiramate versus sodium valproate, Outcome 2 MIDAS score.

Comparison 8 Topiramate versus relaxation, Outcome 1 Headache frequency (change from baseline to post‐treatment).

Comparison 8 Topiramate versus relaxation, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
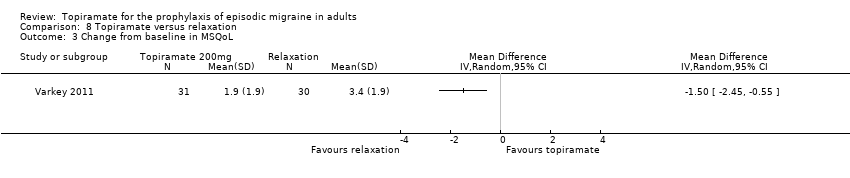
Comparison 8 Topiramate versus relaxation, Outcome 3 Change from baseline in MSQoL.
| Type of AE | 50 mg/day | 100 mg/day | 200 mg/day |
| Any AE | NNH not defined* | 11 (7 to 33) | 5 (3 to 12) |
| Anorexia | NNH not defined* | 17 (10 to 50) | 12 (8 to 20) |
| Fatigue | NNH not defined* | 25 (17 to 100) | 12 (8 to 25) |
| Memory problems | NNH not defined* | 25 (17 to 100) | 12 (9 to 17) |
| Nausea | NNH not defined* | NNH not defined* | 17 (9 to 50) |
| Paresthesia | NNH not defined* | 3 (2 to 6) | 2 (2 to 3) |
| Taste disturbance | 7 (5 to 14) | 14 (8 to 100) | 7 (5 to 11) |
| Weight loss | 25 (14 to 100) | 17 (11 to 33) | 11 (8 to 14) |
| Percentage of patients in active group withdrawing because of AEs | Afshari 2012: 5%; Ashtari 2008: 3%; Brandes 2004: 17%; Gupta 2007: 2%; Silberstein 2004: 17% | Brandes 2004: 26%; de Tommaso 2007: 8%; Diener 2004: 28%; Dodick 2009: 20%; Lipton 2011: 12%; Mei 2004: 29%; Silberstein 2004: 19% | Brandes 2004: 21%; Diener 2004: 44%; Edwards 2000: 27%; Silberstein 2004: 32%; Silberstein 2006: 15%; Storey 2001: 11%; Varkey 2011: 12% |
| * The 95% CI of the difference in AE rates between treatment and placebo arms (the risk difference, RD) crosses zero. Abbreviations: AE = adverse event; CI = confidence interval; NNH = number needed to harm | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 9 | 1793 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.59, ‐0.80] |
| 2 ORs for responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | 1246 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [2.10, 4.82] |
| 3 RRs for responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.57, 2.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 576 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.95, 0.04] |
| 1.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 6 | 1620 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.58, ‐0.71] |
| 1.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 804 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.53, ‐0.36] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [1.60, 3.44] |
| 2.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 852 | Odds Ratio (M‐H, Random, 95% CI) | 3.49 [2.23, 5.45] |
| 2.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 6 | 1025 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [1.61, 3.87] |
| 3 Any adverse event Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.07, 0.17] |
| 3.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 873 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.03, 0.15] |
| 3.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 1 | 213 | Risk Difference (M‐H, Random, 95% CI) | 0.20 [0.08, 0.32] |
| 4 Anorexia Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.07] |
| 4.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 4.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.05, 0.12] |
| 5 Fatigue Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.07, 0.15] |
| 5.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 5.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.04, 0.13] |
| 6 Memory problems Show forest plot | 5 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 6.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 3 | 758 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 6.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.06, 0.11] |
| 7 Nausea Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 7.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.04] |
| 7.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 8 Paresthesia Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [‐0.02, 0.43] |
| 8.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.33 [0.18, 0.48] |
| 8.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [0.39, 0.49] |
| 9 Taste disturbance Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.21] |
| 9.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1623 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.12] |
| 9.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 786 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.09, 0.19] |
| 10 Weight loss Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 10.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 4 | 1270 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 10.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.07, 0.12] |
| 11 MSQ‐role function restrictive Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.83 [2.25, 9.41] |
| 11.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.08 [6.55, 13.60] |
| 11.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 10.36 [6.68, 14.04] |
| 12 MSQ‐role function prevention Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.84 [‐0.24, 5.92] |
| 12.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.39 [3.37, 9.41] |
| 12.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.06 [1.87, 8.25] |
| 13 MSQ‐emotional function Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.58 [0.61, 8.54] |
| 13.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.22 [6.31, 14.14] |
| 13.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.45 [4.38, 12.52] |
| 14 SF‐36 role physical Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐3.89, 9.90] |
| 14.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.80 [‐2.42, 24.03] |
| 14.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.59 [0.65, 16.52] |
| 15 SF‐36 vitality Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.08 [‐4.68, 8.84] |
| 15.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.48 [‐7.77, 16.73] |
| 15.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐4.52, 7.24] |
| 16 SF‐36 physical functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐3.28, 4.36] |
| 16.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.78 [‐3.29, 8.86] |
| 16.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐5.27, 3.35] |
| 17 SF‐36 bodily pain Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.35 [0.04, 8.66] |
| 17.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.35 [‐1.29, 14.00] |
| 17.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.12 [‐1.25, 11.49] |
| 18 SF‐36 general health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐2.18, 5.08] |
| 18.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.18 [‐1.21, 9.57] |
| 18.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐1.00, 6.15] |
| 19 SF‐36 social functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.00 [‐1.92, 5.92] |
| 19.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 3.13 [‐3.73, 9.99] |
| 19.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐2.07, 5.96] |
| 20 SF‐36 role emotional Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐4.56, 9.16] |
| 20.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.64 [‐3.39, 12.68] |
| 20.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.75 [‐4.99, 10.48] |
| 21 SF‐36 mental health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐4.59, 6.98] |
| 21.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐5.65, 10.81] |
| 21.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.57 [‐4.21, 7.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.32, ‐0.10] |
| 1.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.53, ‐0.40] |
| 1.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.55, 0.61] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Topiramate 100 mg versus 50 mg | 2 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.25, 2.60] |
| 2.2 Topiramate 200 mg versus 50 mg | 2 | 462 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [1.15, 2.41] |
| 2.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.24] |
| 3 MSQ‐role function restrictive Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 4.22 [0.65, 7.80] |
| 3.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.55 [0.82, 8.28] |
| 3.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐3.37, 3.99] |
| 4 MSQ‐role function prevention Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 3.54 [0.48, 6.60] |
| 4.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐2.13, 6.69] |
| 4.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐6.67, 4.30] |
| 5 MSQ‐emotional function Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 5.62 [1.67, 9.58] |
| 5.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐0.21, 8.02] |
| 5.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐5.80, 2.34] |
| 6 SF‐36 role physical Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 7.70 [‐8.27, 23.67] |
| 6.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.51 [‐5.17, 16.19] |
| 6.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐9.31, 4.90] |
| 7 SF‐36 vitality Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐3.05, 7.92] |
| 7.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐4.64, 3.39] |
| 7.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.01 [‐9.38, 3.35] |
| 8 SF‐36 physical functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.35 [‐1.02, 5.72] |
| 8.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐4.92, 2.04] |
| 8.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐7.18, ‐0.32] |
| 9 SF‐36 bodily pain Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.13 [‐1.83, 6.08] |
| 9.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐3.27, 4.96] |
| 9.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐5.23, 2.91] |
| 10 SF‐36 general health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.72 [‐0.76, 6.21] |
| 10.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐4.36, 6.62] |
| 10.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.85, 5.65] |
| 11 SF‐36 social functioning Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐5.53, 7.79] |
| 11.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐4.07, 3.96] |
| 11.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐5.56, 3.46] |
| 12 SF‐36 role emotional Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐3.79, 8.45] |
| 12.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐5.69, 6.94] |
| 12.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐7.92, 4.63] |
| 13 SF‐36 mental health Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐1.87, 4.49] |
| 13.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.87, 3.67] |
| 13.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐4.10, 2.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 MIDAS score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (post‐treatment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) Show forest plot | 2 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.61, 0.34] |
| 1.1 Topiramate 50 mg versus propranolol 80 mg | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.15, 0.41] |
| 1.2 Topiramate 100 mg versus propranolol 160 mg | 1 | 282 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.60, 0.60] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (post‐treatment) Show forest plot | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| 2 MIDAS score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Headache frequency (change from baseline to post‐treatment) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Change from baseline in MSQoL Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

