Quimiorradioterapia versus quimiorradioterapia más cirugía para el cáncer esofágico
Resumen
Antecedentes
Ver Apéndice 4 para consultar el glosario de términos.
El resultado de los pacientes con cáncer esofágico generalmente es deficiente. Aunque el tratamiento multimodal es estándar, hay evidencia contradictoria con respecto al agregado de esofagectomía a la quimiorradioterapia.
Objetivos
Comparar la efectividad y la seguridad de la quimiorradioterapia más cirugía con las de la quimiorradioterapia sola en pacientes con carcinoma esofágico no metastásico.
Métodos de búsqueda
Se realizó una búsqueda electrónica de estudios relevantes, hasta febrero de 2017, en las bases de datos CENTRAL, MEDLINE y Embase utilizando términos médicos MeSH y palabras clave. Se realizaron búsquedas en cinco bases de datos en línea de ensayos clínicos, se buscaron manualmente actas de congresos y se revisaron las listas de referencias de los artículos recuperados.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados (ECA) que comparaban quimiorradioterapia más esofagectomía con quimiorradioterapia sola para el carcinoma esofágico localizado. Se excluyeron ECA que comparaban quimioterapia o radioterapia sola con esofagectomía.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los estudios, extrajeron los datos y evaluaron el riesgo de sesgo y la calidad de la evidencia, utilizando procedimientos metodológicos Cochrane estandarizados. El resultado primario fue la supervivencia general (SG), calculada con el cociente de riesgos instantáneos (CRI). Los resultados secundarios, calculados con el riesgo relativo (RR), fueron la supervivencia libre de progresión (SLP) local y distante, la calidad de vida (CdV), la mortalidad y la morbilidad relacionadas con el tratamiento, y la administración de procedimientos de rescate para la disfagia. Los datos se analizaron mediante un modelo de efectos aleatorizados en el software Review Manager 5.3.
Resultados principales
De 2667 referencias, se identificaron dos estudios aleatorizados, en seis informes, que incluyeron 431 participantes. Todos los participantes se clasificaron clínicamente como con carcinoma esofágico torácico al menos T3 o con nódulos positivos, de los cuales un 93% fue mediante histología de células escamosas. El riesgo de sesgo metodológico de los estudios incluidos fue de bajo a moderado.
La evidencia de alta calidad encontró que el agregado de esofagectomía tuvo poca o ninguna diferencia en la supervivencia general (CRI 0,99; IC del 95%: 0,79 a 1,24; P = 0,92; I² = 0%; dos ensayos). Ningún estudio informó la SLP, por lo tanto, la ausencia de recidiva locorregional se usó como un sustituto. La evidencia de calidad moderada indicó que el agregado de esofagectomía probablemente mejoró la ausencia de recidiva locorregional (CRI 0,55; IC del 95%: 0,39 a 0,76; p = 0,0004; I² = 0%; dos ensayos), aunque la evidencia de baja calidad indicó que puede aumentar el riesgo de mortalidad relacionada con el tratamiento (RR 5,11; IC del 95%: 1,74 a 15,02; P = 0,003;I² = 2%; dos ensayos).
Los otros resultados predeterminados (calidad de vida, toxicidad relacionada con el tratamiento y uso de procedimientos de rescate para la disfagia) fueron informados sólo por un estudio, que encontró evidencia de baja calidad de que el uso de esofagectomía se asoció con una CdV reducida a corto plazo (DM 0,93; IC del 95%: 0,24 a 1,62), y evidencia de baja calidad de que redujo la administración de procedimientos de rescate para la disfagia (CRI 0,52; IC del 95%: 0,36 a 0,75). Ningún estudio comparó la morbilidad relacionada con el tratamiento entre los grupos de tratamiento.
Conclusiones de los autores
Basado en la evidencia disponible, el agregado de esofagectomía a la quimiorradioterapia en el carcinoma escamocelular esofágico localmente avanzado, proporciona poca o ninguna diferencia en la supervivencia general y puede asociarse con una mayor mortalidad relacionada con el tratamiento. El agregado de la esofagectomía probablemente retarda la recidiva locorregional, sin embargo, esta variable de evaluación no se definió de forma adecuada en los estudios incluidos. No fue posible determinar si estos resultados pueden aplicarse al tratamiento de los adenocarcinomas, los tumores que incluyen el esófago distal y la unión gastroesofágica, y a los pacientes con una respuesta deficiente a la quimiorradiación.
PICO
Resumen en términos sencillos
Beneficios y efectos secundarios del agregado de cirugía a la quimiorradioterapia para el tratamiento del cáncer esofágico que pueden extraerse quirúrgicamente
Pregunta de la revisión
¿El agregado de cirugía a la quimiorradioterapia, mejora la supervivencia en los pacientes con cáncer esofágico resecable (cáncer que puede extraerse quirúrgicamente)?
Antecedentes
El cáncer del esófago (tubo muscular que se extiende desde la boca a través de la garganta hasta el estómago) es un trastorno letal. Por lo general, se trata con cirugía, radioterapia, quimioterapia o una combinación de estos tratamientos. No está claro si el agregado de cirugía después de la quimiorradioterapia (quimioterapia más radiación) aporta algún beneficio adicional para los pacientes con cáncer esofágico.
Características de los estudios
Se incluyeron dos estudios aleatorizados, en seis informes publicados, con 431 participantes con cáncer esofágico localmente avanzado. Se realizaron búsquedas de estudios en bases de datos biomédicas, registros de ensayos clínicos, actas de congresos y listas de referencias hasta el 7 de febrero de 2017.
Calidad de la evidencia
La calidad de la evidencia varió de muy baja a alta, según el resultado evaluado, debido a que los ensayos eran pequeños y en riesgo poco claro o alto de sesgo (un error sistemático o desviación de la verdad que afecta los resultados, favoreciendo un tratamiento sobre otro).
Resultados clave
Se encontró evidencia de que el agregado de cirugía redujo el riesgo de recidiva del cáncer en el sitio primario, aunque no mejoró la supervivencia general. Además, hubo más muertes relacionadas con el tratamiento en el grupo de participantes sometidos a cirugía.
Authors' conclusions
Summary of findings
| Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer | |||||
| Patient or population: nonmetastatic esophageal cancer | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants | Quality of the evidence | |
| Risk with chemoradiotherapy alone | Risk with chemoradiotherapy plus surgery | ||||
| Overall survival | 35.4% to 40.0% at 2 years | 34.0% to 39.9% at 2 years | HR 0.99 (95% CI 0.79 to 1.24) | 431 | ⊕⊕⊕⊕ |
| Freedom from locoregional relapse Follow‐up: median 4 to 6 years | 40.7% to 57.0% at 2 years | 64.3% to 66.4% at 2 years | HR 0.55 (95% CI 0.39 to 0.76) | 431 | ⊕⊕⊕⊝ |
| Quality of Life assessed with: Spitzer QoL index Scale: 0 to10 Follow‐up: 3 months | the mean Q0L score was 7.52 points in the chemoradiotherapy alone group | the mean QoL score in the chemoradiotherapy plus surgery group was 0.93 points worse (from ‐1.62 worse to ‐0.24 worse) | 165 (1 RCT) | ⊕⊝⊝⊝ | |
| Treatment‐related mortality Follow‐up: median 1 to 3 months | 1.9 per 100 | 9.5 per 100 (3.2 to 27.8) | RR 5.11 | 431 | ⊕⊕⊝⊝ |
| Use of salvage procedures for dysphagia Follow‐up: median 4 years | 46 per 100 | 24 per 100 | RR 0.52 (95% CI 0.36 to 0.75) | 259 (1 RCT) | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded one level due to risk of bias (detection bias as investigators were not blinded). | |||||
Background
Description of the condition
Esophageal cancer accounted for 16,980 new cancer cases and 15,590 cancer deaths in the United States alone in 2015 (Siegal 2015). The incidence rate is highest in Southern Africa and Eastern Asia (Torre 2016).
Esophageal cancer is usually classified histologically as squamous cell carcinoma (SCC) or adenocarcinoma. Squamous cell carcinoma has been increasing in certain Asian countries, such as Taiwan, and decreasing in Western countries, such as North America. Such trends are likely due to differences in the rates of alcohol consumption and tobacco use (Cook 2009; Lu 2010). Interestingly, incidence rates for adenocarcinoma have been increasing in Western countries, probably due to an increase in the prevalence of obesity (El‐Serag 2007; Post 2007). Another important risk factor may be chronic gastroesophageal (GE) reflux disease, which leads to Barrett's esophagus, a pre‐malignant condition associated with lower esophageal and GE junction adenocarcinoma.
Esophageal cancer remains an aggressive malignancy, despite current treatment modalities. The Surveillance, Epidemiology and End Results (SEER) registry demonstrated a statistically significant, but modest improvement in the five‐year relative overall survival (OS) from 5% between 1975 and 1977 to 18.5% from 2001 to 2007 (NCI 2011). Survival is dependent on the stage of disease, with five‐year relative OS of 37.3% for localized disease, 18.4% for regional disease, and 3.1% for metastatic disease. Unfortunately, more than half of the people presented with advanced (regional and metastatic) disease at diagnosis (NCI 2011).
Description of the intervention
The National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines established a standard of care for medically fit people with resectable disease (Lordick 2016). While surgery alone is appropriate for early‐stage disease (T1N0), combined modality therapy is offered to people with more advanced disease (T2 to T4 and/or node positive disease). Specifically, options include definitive chemoradiotherapy or trimodality treatment (preoperative chemoradiotherapy or chemotherapy followed by surgery; surgery followed by chemoradiotherapy). Treatment options depend on the tumor location, as well as histology.
Surgery alone
The type of approach for esophagectomy, such as transhiatal or thoracoabdominal, is dependent on the size, stage, and location of the primary tumor, surgeon's experience, and patient preference. Studies have demonstrated a five‐year survival rate of 20% with surgery alone (Altorki 2002; Bosset 1997; Hulscher 2002; Kelsen 1998; Orringer 1999). Survival postesophagectomy is not dependent on the type of surgical approaches or histology. Muller and colleagues reviewed the outcomes of various types of esophagectomy and did not find any significant differences in postesophagectomy survival (Muller 1990). Salazar and colleagues reported similar cumulative postoperative survival rates for people with SCC and adenocarcinoma (Salazar 1998).
Defintive chemoradiotherapy
The Radiation Therapy Oncology Group (RTOG) 85‐01 study demonstrated that concurrent chemoradiotherapy improved OS significantly in people with medically operable SCC when compared with radiotherapy alone (Cooper 1999). In this study, 121 people were randomized to receive four cycles of cisplatin plus 5‐fluorouracil with concurrent radiotherapy (50 Gy in 25 fractions), or radiotherapy (64 Gy in 32 fractions) alone. About 88% of the participants had SCC. People who received combined modality treatment had a significant improvement in five‐year OS (27% versus 0%) and median survival (14 months versus 9 months) compared with radiotherapy alone. The incidence of local failure (local recurrence or persistent disease) at one year was also lower in the combined modality arm (47% versus 65%). The results of this study have established definitive chemoradiotherapy as the standard of care for people with SCC who are not surgical candidates.
Trimodality treatment
Numerous phase II and III studies have compared preoperative chemoradiotherapy followed by surgery versus surgery alone. The use of pre‐ or perioperative chemotherapy and surgery has also been evaluated. One updated meta‐analysis of randomized studies, comparing the efficacy of preoperative chemoradiotherapy or chemotherapy followed by surgery with surgery alone, reported a significant survival benefit with preoperative treatment over surgery alone in people with resectable esophageal carcinoma (Sjoquist 2011). The hazard ratio (HR) for all‐cause mortality for preoperative chemoradiotherapy was 0.78 (95% confidence interval (CI) 0.70 to 0.88, P value < 0.0001), and for preoperative chemotherapy was 0.87 (95% CI 0.79 to 0.96, P = 0.005). Based on their findings, a clear advantage of preoperative chemoradiotherapy over chemotherapy could not be established. Moreover, this meta‐analysis did not directly address the question of definitive versus preoperative chemoradiotherapy.
The seminal randomized study that supported the use of surgery plus postoperative chemoradiotherapy is the US Intergroup 0116 study (Macdonald 2001). In this study, 556 people with resected adenocarcinoma of the stomach or GE junction were randomized to surgery alone or surgery plus postoperative chemoradiotherapy. Twenty per cent of the participants had a tumor at the esophagogastric junction. People who received postoperative chemoradiotherapy had significant improvement in three‐year survival (50% versus 41%), and median survival (36 months versus 27 months). A major criticism of this study was that 54% of the participants underwent less than a group 1 lymph node dissection (i.e. D1 dissection), raising the possibility that radiation may be compensating for inadequate surgery. Nevertheless, this study suggested that postoperative chemoradiotherapy was a reasonable option for people with GE junction adenocarcinoma. A subsequent randomized study from China investigated the role of perioperative chemoradiotherapy in locally advanced (Stage II to III) thoracic esophageal squamous cell carcinoma (Jin 2010). Two hundred and thirty‐eight participants were randomized into surgery alone (80 participants), preoperative chemoradiotherapy (80 participants), and postoperative chemoradiotherapy (78 participants). Progression‐free survival (PFS) and OS were improved with either preoperative (PFS: Chi² 6.81, P = 0.009; OS: Chi² 7.85, P = 0.005) or postoperative (PFS: Chi² 5.38, P = 0.02; OS: Chi² 5.33 P = 0.021) chemoradiotherapy, compared to surgery alone. There were no significant differences in PFS (Chi² 0.14, P = 0.71) or OS (Chi² 0.46, P = 0.50) between pre‐ and postoperative chemoradiotherapy.
Compared to trimodality treatment, the role of definitive chemoradiotherapy alone is uncertain. This is particularly so, given the high local failure rate with chemoradiotherapy, the inability to predict a pathologic complete response even with repeat imaging or endoscopy (or both), and lack of data for non‐surgical management of people with adenocarcinoma.
Why it is important to do this review
The benefits of adding surgery to chemoradiotherapy when compared to chemoradiotherapy alone for nonmetastatic esophageal cancer are unclear. Definitive chemoradiotherapy alone has been shown to provide a five‐year OS in up to 27% of people with SCC (Cooper 1999). This result is similar to that achieved with preoperative chemoradiotherapy followed by surgery alone (O'Reilly 1995; Urba 2001; Walsh 1996). There was also no clear consensus from the NCCN guidelines on whether a trimodality approach was preferred over chemoradiotherapy alone, in people with resectable disease (www.nccn.org). We were unable to locate any systematic reviews or meta‐analyses that specifically addressed the efficacy of a trimodality approach when compared to chemoradiotherapy alone.
However, we did find several narrative reviews that addressed the management of people with locally advanced esophageal cancer (Ku 2009; Mariette 2007; Wolf 2011). Most authors concluded that definitive chemoradiotherapy has become a reasonable treatment option, especially for people with SCC. Performing surgery on people who respond to initial chemoradiotherapy may improve local control, but may not clearly impact OS. While the overall conclusion was similar in these studies, they lacked explicit methodology in their review, which limited interpretation of the data and valid conclusions.
Hence, we conducted a systematic review and meta‐analysis to compare the efficacy and safety of surgery plus chemoradiotherapy with chemoradiotherapy alone in people with nonmetastatic esophageal cancer.
Objectives
To compare the effectiveness and safety of chemoradiotherapy plus surgery with that of chemoradiotherapy alone in people with nonmetastatic esophageal carcinoma, in terms of overall survival (OS), progression‐free survival (PFS), quality–of‐life (QoL), treatment‐related mortality and morbidity, and the use of salvage procedures for dysphagia.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomized studies in this review. The nature of the intervention made it difficult for blinding to be part of the study design, and therefore, was not a requirement for study inclusion. We included published and unpublished studies, full articles, and abstracts satisfying the criteria listed below, without any language restriction.
Types of participants
People with nonmetastatic carcinoma (stage I to III) of the esophagus, who had been treated with curative intent.
Types of interventions
The control arm of the study was chemoradiotherapy alone. The intervention arm was the group that underwent chemoradiotherapy plus surgery. Treatment had to be given with curative intent. The timing of the chemotherapy and radiotherapy could be sequential or concomitant; surgery may have been performed pre‐ or post‐chemoradiotherapy.
Types of outcome measures
Primary outcomes
The primary outcome was overall survival (OS) — time from randomization to death from any cause.
Secondary outcomes
-
Local progression‐free survival (PFS) — time from randomization to disease progression at initially treated site by radiotherapy, or death;
-
Distant PFS — time from randomization to disease progression at sites not treated by radiotherapy, or death;
-
Quality of life (QoL) — measured using a validated scale;
-
Treatment‐related mortality;
-
Treatment‐related toxicity (both acute and chronic). Toxicity resulting from treatment is typically classified as acute (that which occurs within 90 days of treatment) or chronic (that which occurs after 90 days of treatment);
-
Use of salvage procedures for dysphagia.
Search methods for identification of studies
We sought papers in all languages and carried out translations if necessary.
Electronic searches
We performed the search for studies with the assistance of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group. The electronic search strategy searched the following databases from its date of inception to 7 February 2017:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1) in the Cochrane Library (searched 7 February 2017; Appendix 2);
-
MEDLINE OVIDSP (1966 to 7 February 2017; Appendix 3);
-
Embase OVIDSP (1988 to 7 February 2017; Appendix 4).
We used a search strategy to identify randomized controlled studies performed in humans. We used MeSH headings, subject headings, and additional free‐text words.
Unpublished and grey literature
We identified prospective and ongoing studies by searching the prospective study registers (February 2017):
-
International Standard Randomized Controlled Trial Number Registry (www.controlled‐trials.com);
-
US National Institutes of Health (www.clinicaltrials.gov);
-
U.S. National Cancer Institute (www.cancer.gov/clinicaltrials/search);
-
International Clinical Trials Registry Platform (www.who.int/trialsearch);
-
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
Searching other resources
Handsearching
We handsearched the citation lists of included studies, key textbooks, and previous systematic reviews, and contacted experts in the field to identify further reports of studies. We handsearched reports of conferences in the following sources:
-
annual meeting of the American Society of Clinical Oncology (2006 to 2016);
-
annual meeting of the American Society for Therapeutic Radiology and Oncology (2006 to 2016);
-
annual meeting of the European Society of Medical Oncology (2006 to 2016);
-
annual Gastrointestinal Cancers Symposium (2006 to 2016).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts obtained by electronic searches to a reference management database (Microsoft Excel) and removed duplicates. Four review authors (BAV, YYS, CNL, JCST) independently reviewed the remaining titles and abstracts. They excluded studies that clearly did not meet the inclusion criteria. We obtained full‐text articles of the remaining articles, and four review authors independently determined the eligibility of the retrieved papers.
We resolved disagreements by consensus, or by consulting a fifth review author (JJL) if necessary. We documented reasons for exclusion during this process. We did not blind the review authors to the source of the document for article selection or data extraction.
Data extraction and management
Four review authors (BAV, YYS, CNL, JCST) independently extracted data on characteristics of participants and interventions, risk of bias, duration of follow‐up, outcomes and deviations from the protocol to a data abstraction form especially designed for the review. We resolved differences between review authors by discussion or by consulting with a fifth review author if necessary.
We extracted the following participant data: age, gender, performance status, clinical pretreatment staging (American Joint Committee on Cancer), location of primary tumor (upper, middle, or lower third, or GE junction), histopathological subtype (SCC versus adenocarcinoma), and pathological staging (if available). If documented, we noted modalities used for pretreatment clinical staging, such as barium studies, endoscopy, endoscopic ultrasound, computed tomography, and positron emission tomography.
We extracted the following data on types of intervention:
-
radiotherapy: the total dose and dose fractionation, treatment target volume, beam arrangement, beam energy, modality (photons, electrons, or both), treatment planning (two‐dimensional, three‐dimensional), treatment delivery (conventional, intensity modulated, or brachytherapy), and compliance to the recommended protocol;
-
chemotherapy: chemotherapeutic agents, biologics, schedule, route of administration, dose intensity, and compliance to the recommended protocol.
The type of surgery was to have a curative intent, and consist of esophagectomy to resect all gross and microscopic disease. We documented the type of surgery (transhiatal or transthoracic, two‐ or three‐staged resection).
We extracted these data for outcomes:
-
For time‐to‐event (OS and PFS) data, we extracted the log of the hazard ratio (log (HR)) and its standard error; if these were not reported, we attempted to estimate the log (HR) and its standard error using the methods from Parmar (Parmar 1998).
-
For dichotomous outcomes (e.g. adverse events or deaths, if it was not possible to use an HR), we extracted the number of participants in each treatment arm who experienced the outcome of interest, and the number of participants originally randomized, in order to estimate a risk ratio (RR).
-
For continuous outcomes (e.g. QoL measures), we extracted the final value and standard deviation of the outcome of interest, and the number of participants assessed at the end point in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard deviation.
We extracted both unadjusted and adjusted statistics. We used the data extracted in an intention‐to‐treat analysis, in which participants were analyzed in the groups to which they were assigned. We noted the time points at which outcomes were reported.
Assessment of risk of bias in included studies
Four review authors (BAV, YYS, CNL, JCST) independently used the 'Risk of bias' tool to assess the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They resolved disputes by consensus, and consulted with a fifth author (LLJ) if necessary. We assessed the risk of bias according to the following domains:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete outcome data;
-
selective outcome reporting;
-
other bias.
We graded each potential source of bias as high, low, or unclear risk, and provided a quote from the study report, together with a justification for our judgement, in the 'Risk of bias' table. We summarized the 'Risk of bias' judgements across different studies for each of the domains listed in a 'Risk of bias' summary table (Figure 1). We interpreted the results of meta‐analyses with respect to the risk of overall bias.
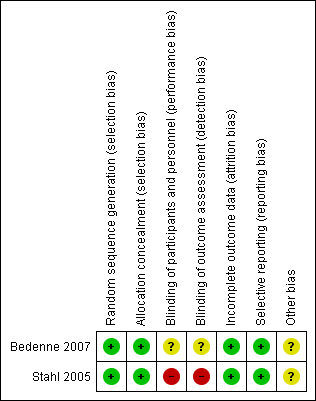
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol, and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review (Vellayappan 2013).
Measures of treatment effect
We used the following measures of the effect of treatment:
-
for time‐to‐event data, we used hazard ratio (HR), if possible;
-
for dichotomous outcomes, we used the risk ratio (RR);
-
for continuous outcomes, we used the mean difference (MD) between treatment arms.
Unit of analysis issues
The unit of analysis was the individual participant. We did not find any cross‐over or cluster‐randomized studies.
Dealing with missing data
We were not able to contact the study corresponding authors to obtain missing numerical outcome data. We did not impute any missing data.
Assessment of heterogeneity
We assessed heterogeneity between studies by visually inspecting the forest plots (L'Abbé 1987), estimating the percentage heterogeneity between studies that could not be attributed to sampling variation (Higgins 2003), formally applying a statistical test of significance of the heterogeneity (Deeks 2001), and if possible, by conducting a subgroup analysis.
Assessment of reporting biases
Given that we only included two studies, we did not perform a funnel plot assessment.
Data synthesis
If sufficient studies were available, we had planned to pool their data in a meta‐analysis. For time‐to‐event data, we pooled HRs using the generic inverse variance function in RevMan 5 (RevMan 2014). For dichotomous outcomes, we calculated a pooled RR. For continuous outcomes, we calculated a pooled mean difference between the two arms at the end of follow‐up, if all studies measured the outcome on the same scale. If different scales were used, used a standardized mean difference.
If any studies had multiple treatment groups, we divided the 'shared' comparison group into the number of treatment groups, and treated comparisons between each treatment group and the split comparison group as independent comparisons.
We used a random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986). If possible, we synthesized studies making different comparisons using the methods of Bucher (Bucher 1997).
Subgroup analysis and investigation of heterogeneity
We had planned to perform subgroup analysis, grouping the studies by:
-
concomitant versus sequential chemoradiotherapy;
-
use of targeted therapies (e.g. cetuximab, transtuzumab, and bevacizumab) versus none;
-
type of chemotherapy used (5‐fluorouracil‐based versus cisplatin‐based versus others);
-
histological subtype (SCC versus adenocarcinoma);
-
type of surgery (transhiatal versus transthoracic versus two‐ or three‐stage resections);
-
type of radiation delivery techniques (intensity modulated versus conventional versus brachytherapy);
-
sequencing of intervention in the experimental arm (chemoradiotherapy followed by surgery versus surgery followed by chemoradiotherapy).
We considered factors such as age, clinical staging of esophageal cancer, radiation dose, length of follow‐up, and adjusted and unadjusted analysis in interpretation of heterogeneity.
Sensitivity analysis
We had planned to perform sensitivity analysis for the following:
-
exclusion of studies at high risk of bias;
-
using a fixed‐effect model in place of a random‐effects model.
Results
Description of studies
Results of the search
We identified 2665 records from the search results, and two records from other sources. Once we removed duplicates, we screened 2532 titles and abstracts, excluding 2523 that were obviously not eligible. We obtained the full‐text copy of nine reports and included two randomized controlled studies (Bedenne 2007; Stahl 2005), in six reports (Bedenne 2007; Bonnetain 2006; Burtin 2008; Crehange 2007; Stahl 2005; Vincent 2015), which included 431 participants (Figure 2).
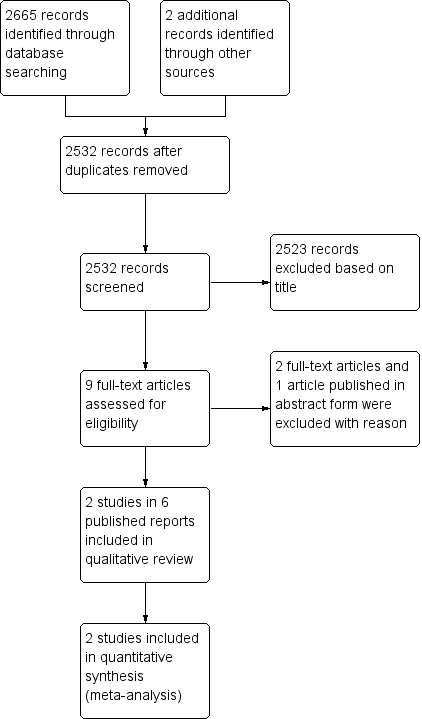
PRISMA flow diagram.
Included studies
Both included studies were published as full journal articles (Bedenne 2007; Stahl 2005). Sample size in the included studies ranged from 172 to 259. Of the 431 included participants, only 29 had adenocarcinoma histology; the remainder had squamous cell cancer.
Bedenne 2007 included participants with operable T3N0 thoracic esophageal cancer. There was no restriction for histology or tumor location. All registered participants (N = 444) received induction chemoradiation, however, only participants who showed objective response were randomized (N = 259).
Chemotherapy consisted of fluorouracil 800 mg/m² and cisplatin 15 mg/m², given on days one to five, every three weeks. All participants received two cycles prior to randomization; the non‐surgical arm received three more cycles, together with radiation.
Radiotherapy was given by conventional fraction or split‐course, until January 1999, when the split‐course arm was discontinued due to inferior results. Radiotherapy volumes included the gross tumor and lymph nodes, with a 3 cm superior‐inferior margin, and 2 cm axial margin. Split‐course radiotherapy was delivered in daily fractions of 3 Gy, in two sequences of five days each (15 Gy each, two weeks apart) prior to randomization, and one sequence after (total 45 Gy). Conventional radiotherapy was delivered to 46 Gy (2 Gy per fraction, five fractions per week) prior to randomization, and 20 Gy after (total 66 Gy).
Surgery was performed between days 50 and 60 in the surgical arm, however, no particular type of surgery was recommended.
The primary outcome was overall survival; secondary outcomes included duration of hospital stay, quality of life, type of recurrence, and procedures against dysphagia. The primary and selected secondary outcomes were reported in Bedenne 2007. Quality of life outcomes were reported in Bonnetain 2006. The results of split‐course versus conventionally‐fractionation were reported in Crehange 2007. Outcome of the registered, but non‐randomized, participants were published by Vincent 2015
Stahl 2005 included locally advanced (T3 to T4, and/or node positive) squamous cell cancers of the upper or mid‐thoracic esophagus. Participants were randomized to induction chemotherapy followed by chemoradiotherapy and surgery versus induction chemotherapy followed by chemoradiotherapy alone. Induction chemotherapy, in both arms, consisted of 3 cycles of bolus flurouracil 500 mg/m², leucovorin 300 mg/m², etoposide 100 mg/m², and cisplatin 30 mg/m² on days one to three, every three weeks.
The surgical arm received chemoradiotherapy consisting of cisplatin 50 mg/m² on days two to eight, and etoposide 80 mg/m² on days three to five with 40 Gy of radiation (2 Gy per fraction, over four weeks). The volume of irradiation, included the gross tumor with 5 cm superior‐inferior margin and 2 cm axial margin, as well as the supraclavicular, infraclavicular, and lower cervical nodal regions. This was followed by esophagectomy, two weeks later, involving a right thoracic and abdominal approach (e.g. Ivor‐Lewis procedure) and excision of para‐esophageal, paracardial, left gastric, and celiac lymph nodes (two‐field lymphadenectomy).
Participants in the non‐surgical arm were treated with chemoradiotherapy involving the same chemotherapeutic agents and radiation volumes (to 40 Gy). This was followed by a sequential radiation boost. For T4 or obstructing T3 tumors, the gross tumor with a 2 cm superior‐inferior margin and 1 cm axial margin (CTV boost) was treated with 50 Gy, followed by a hyperfractionated boost to 65 Gy (1.5 Gy twice a day, six hours apart, over one week). Non‐obstructing tumors were treated with 60 Gy (CTV boost), followed by two fractions of high‐dose rate brachytherapy boost (4 Gy to 5 mm depth).
The primary outcome was overall survival. Secondary outcomes, although not stated explicitly, were assumed to be local progression‐free survival, and treatment‐related mortality.
We extracted time‐to‐event data for OS (primary outcome) from both studies. However, secondary outcomes were inconsistently reported, therefore, were only combined where adequate information was available (Effects of interventions).
Excluded studies
We excluded ISRCTN 89052791 after review of the study protocol, as the study arms were not in line with our inclusion criteria. This is an ongoing randomized controlled study in esophageal squamous cell cancer, between induction chemotherapy followed by chemoradiotherapy versus surgery.
We excluded Nomura 2015, which is available only in abstract form. This study is a secondary analysis from two randomised controlled studies. The authors compared neoadjuvant chemotherapy followed by surgery to definitive chemoradiotherapy. This study was excluded as randomization was not performed between chemoradiotherapy plus surgery versus chemoradiotherapy alone.
We excluded Wang 2007, as a process of randomization was not described. Participants were prospectively assigned to neo‐adjuvant chemoradiotherapy, followed by surgery or definitive chemoradiotherapy, followed by consolidation chemotherapy in a non‐randomized fashion.
Risk of bias in included studies
We have summarized the risk of bias of the included studies under Characteristics of included studies and Figure 1.
Allocation
The risk of bias in random sequence generation was low in both studies. Bedenne 2007 used a minimization algorithm, whereas Stahl 2005 used computer‐generated randomization. The risk of bias from allocation concealment was also low in both studies, as they were performed centrally.
Blinding
Blinding of participants and personnel was only reported by Stahl 2005. This was an unblinded study, and risk of bias from lack of blinding was high for subjective outcomes, such such as local progression‐free survival and treatment‐related mortality, and low for objective outcomes, such as overall survival. Bedenne 2007 did not describe blinding, so the risk of bias is unclear. Similar to the other study, it is expected that risk of bias would be low for objective outcomes (OS) and high for subjective outcomes (such as quality of life, treatment‐related morbidity, and salvage procedures for dysphagia).
Incomplete outcome data
The risk of bias from incomplete outcome data was low for both studies. Neither of the included studies had missing data. Both studies followed an intention‐to‐treat principle in the analysis of survival data.
Selective reporting
Risk of bias from selective reporting was low in both studies. Stahl 2005 reported on the pre‐specified primary outcome (OS), and Bedenne 2007 reported on the pre‐specified primary outcome (OS), and secondary outcomes (duration of hospital stay, quality of life, type of recurrence, and procedures against dysphagia).
Other potential sources of bias
We did not find any other potential sources of bias in the included studies. Although a funnel plot was initially planned to examine possible publication bias, due to the small number of included studies, a funnel plot analysis was deemed not useful and therefore, not performed.
Effects of interventions
Overall survival
Overall survival was reported in both the included studies (N = 431; Bedenne 2007; Stahl 2005). We used HRs as published, or estimated indirectly from published data, for the calculation of summary statistics. The data appeared homogenous (Chi² = 0.18; P = 0.67; I² = 0%). Pooled data showed little or no difference with the addition of esophagectomy (HR 0.99, 95% confidence interval (CI) 0.79 to 1.24; Analysis 1.1).
Progression‐free survival (PFS)
Local PFS
We had defined local progression‐free survival as a secondary outcome in the initial protocol, but neither of the studies reported these data. Bedenne 2007 reported higher locoregional relapse without esophagectomy (HR 1.63, 95% CI 1.04 to 2.55, P = 0.03). Stahl 2005 reported freedom from local progression favouring esophagectomy (HR 2.1, 95% CI 1.3 to 3.5, P = 0.003).
We pooled the data from the two studies (N = 431) and found an improved freedom from locoregional relapse favouring esophagectomy (HR 0.55, 95% CI 0.39 to 0.76; Analysis 1.2). The data appeared homogenous (Chi² = 0.5; P = 0.48; I² = 0%).
Distant PFS
This outcome was reported by neither study, and could not be included in a summary statistic calculation.
Bedenne 2007 reported a two‐year metastatic probability of 39.1% (SE 5.3) for trimodality therapy and 29% (SE 4.7; P = 0.24) for chemoradiation alone.
Quality of life
Quality of life (QoL) was only reported by the FFCD 9102 study (Bedenne 2007). As such, a pooled estimate could not be estimated. In this study, QoL was assessed using the Spitzer QoL index (scored 0 to10), where a higher score indicated a worsening of quality of life. At three‐month follow‐up, only 165 participants had reported QoL scores. Mean difference was worse with trimodality therapy (MD ‐0.93, 95% CI ‐1.62 to ‐0.24; Analysis 1.3). However, subsequent follow‐up did not show any difference between treatment groups (P = 0.26).
Treatment‐related mortality
Death due to acute treatment toxicities in both arms were analyzed. This may be classified as grade 5 toxicity, according to the Common Toxicity Criteria for Adverse Events (CTCAE 2006). Perioperative mortality (within 90 days of surgery) was also analyzed under this outcome.
Both studies reported this outcome,allowing us to obtain a pooled estimate (N = 431; Bedenne 2007; Stahl 2005). The pooled estimate for treatment‐related mortality favoured chemoradiation alone (RR 5.11, 95% CI 1.74 to 15.02; P = 0.003; Analysis 1.4). The data appeared homogenous (Chi² = 1.02; P = 0.31; I² = 2%).
Treatment‐related toxicity (acute or chronic)
Toxicity was reported according to the organ systems, and was to be graded according to the intensity of these symptoms. We considered grade 3 and grade 4 toxicities to be severe, and grouped them together. Grade 1 and grade 2 toxicities, if reported, were considered mild (Cox 1995). Acute and chronic treatment‐related toxicities were analyzed separately.
Treatment‐related toxicity was not reported uniformly, and we were unable to combine data in a meta‐analysis. Stahl 2005 reported acute toxicity after induction chemotherapy, prior to starting chemoradiation. The data on acute toxicity for the individual arms were not presented, and we assumed them to be equal, since identical induction chemotherapy was used for both arms. Bedenne 2007 only presented the incidence of acute toxicity (Grade 3/4) for the chemoradiation arm, as a per‐protocol analysis, and made no comparison with surgery.
Use of salvage procedures for dysphagia
This outcome was measured quantitatively to objectively determine the difference between the two groups. Procedures may have included balloon dilation, endoscopic stent insertion, laser debulking of tumor, or tube insertion for enteral nutrition. The outcome was only reported by Bedenne 2007. A higher proportion of participants undergoing chemoradiotherapy alone required salvage procedures, either dilation or stent placement, for dysphagia (46.2% versus 24%; P < 0.001), with a RR of 0.52 (95% CI 0.36 to 0.75; Analysis 1.5).
Discussion
Summary of main results
Moderate‐quality evidence found that the addition of esophagectomy to chemoradiotherapy probably improved freedom from locoregional relapse. However, low‐quality evidence found there may be increased treatment‐related mortality, and high‐quality evidence found little or no improvement in overall survival (OS). The impact of esophagectomy on quality of life (QoL), treatment‐related morbidity, and use of salvage procedures for dysphagia was only reported by one study, and hence, remains undetermined.
Overall completeness and applicability of evidence
Despite our systematic and extensive search, we only found two eligible studies to include in the meta‐analysis. From the standpoint of the individual studies, both were only powered to show equivalence, and therefore, any difference in survival may have been deemed not significant. A meta‐analysis provides the ideal statistical tool to increase the power of these comparisons.
We judged that the included studies provided sufficient evidence to draw reliable conclusions for overall survival, freedom from locoregional relapse, and treatment‐related mortality. The other outcomes of interest, which were determined a priori, were only reported by one of the two studies (Bedenne 2007).
Stage and location of the disease
Multimodal treatment is generally considered necessary for advanced esophageal cancers. No restrictions were placed on stage during our selection of studies. However, it is important to note that these studies included only locally advanced, resectable, cancers and therefore, the applicability should be restricted to this group i.e. T3 to T4, node positive, or both. Stahl 2005 used both endoscopic ultrasound and computed tomography (CT), whereas Bedenne 2007 relied solely on CT. With regards to T classification, it is possible that a portion of participants were incorrectly‐staged, as CT alone has been shown to be a poor assessor for depth of tumor infiltration (Kim 2009). It remains unclear if the inclusion of a minority of participants with potentially Stages I or II disease would have changed our findings. Stahl 2005 only included participants with upper and middle esophageal tumors, whereas Bedenne 2007 included all thoracic esophageal tumors. It is unclear how many participants with distal esophageal cancers were included in the latter study, although most squamous cell carcinomas occur in the proximal two‐thirds of the esophagus. As such, these results may not be applicable to distal esophageal and gastroesophageal junction tumors.
Effect of histology
There were no restrictions imposed during the search for the studies. However, Stahl 2005 included only squamous cell carcinoma (SCC) participants and Bedenne 2007 included participants with both SCC and a minority with adenocarcinoma. Overall, 93% of the included participants had squamous cell carcinoma (SCC). It is widely regarded that SCC and adenocarcinoma are considered two separate disease entities, with individual treatment strategies. Therefore, these results should not be applied to people with adenocarcinomas.
Responders versus non‐responders
For ethical reasons, Bedenne 2007 only randomized participants who responded to induction chemoradiation. In a recent publication by Vincent and colleagues, the non‐responding participants were reported to have much poorer outcomes; however, the addition of salvage surgery in these participants improved OS (hazard ratio (HR) 0.39, 95% confidence interval (CI) 0.25 to 0.61, P < 0.0001; Vincent 2015). Stahl 2005 randomized all participants, regardless of their response to induction multi‐agent chemotherapy. However, subgroup analysis corroborated the findings that participants who responsed to induction therapy fared better. These results should not be applied to people who do not respond to induction chemoradiation, i.e. those who had progressive or residual primary tumors. Salvage surgery remains a strong consideration for such people.
Chemotherapy and radiotherapy dose and design
A landmark practice changing study (CROSS) published impressive results for the use of neoadjuvant chemoradiotherapy prior to surgery (Van Hagen 2012). Many centers have adopted this regimen of weekly carboplatin and paclitaxel with a reduced dose of radiotherapy (41.4 Gy in 23 fractions) prior to surgery. However, for people who are treated with chemoradiotherapy alone, the standard of care remains 50 Gy with platinum and flurouracil‐based chemotherapy, based on the INT 0123 study (Minsky 2002). This study was closed early due to mortalities in the dose‐escalated arm (64 Gy).
Stahl 2005 used different radiotherapy regimens in both intervention groups. Participants undergoing surgery had 40 Gy of external beam radiotherapy, whereas participants who received chemoradiation alone, received a total dose of 65 Gy, or more. Notably, all participants in the chemoradiation arm received a coned‐down boost, either with hyper‐fractionated external beam radiotherapy (70%) or high‐dose rate brachytherapy (30%). Bedenne 2007 allowed for both conventionally fractioned radiotherapy and split‐course radiotherapy. However, the split‐course strategy was disallowed midway due to an increased number of deaths. Like Stahl 2005, participants in the surgical arm received chemoradiation (46 Gy), while participants treated with chemoradiation alone received an additional 20 Gy (total 66 Gy). Based on our findings, the addition of surgery did not confer a survival benefit compared to high‐dose chemoradiotherapy alone (more than 65 Gy). However, it remains unclear if surgery may have conferred a survival advantage compared to standard dose chemoradiotherapy alone (50 Gy).
Considering that all participants in the Stahl 2005 study received induction multi‐agent chemotherapy, which in itself has been shown to reduce mortality by 13% (HR 0.87, 95% CI 0.79 to 0.96; Sjoquist 2011), these results may not be applicable to people treated without induction therapy.
Quality of the evidence
We assessed the quality of the evidence for all reported outcomes, including those whose results could not be pooled.
We determined the quality of evidence using the guideline development tool developed by the GRADE Working Group (GRADEpro 2015; Ryan 2016)
Overall survival (two studies): high‐quality evidence. Absence of blinding is unlikely to have influenced this objective outcome.
Freedom from locoregional relapse (two studies): moderate‐quality evidence. Downgraded one level due to risk of bias from the absence of blinding.
Treatment‐related mortality (two studies): low‐quality evidence. Downgraded one level due to risk of bias from the absence of blinding and imprecision (large confidence interval).
Quality of life (one study): very low‐quality evidence. Downgraded two levels due to risk of bias (absence of blinding and loss to follow‐up), downgraded one level due to imprecision (small effect size with overlapping confidence interval) and publication bias.
Use of salvage procedures (one study): low‐quality evidence. Downgraded one level due to risk of bias from absence of blinding, imprecision and publication bias.
Quality of evidence for treatment‐related toxicity could not be judged as it was reported by neither study.
Please refer to summary of findings Table for the main comparison.
Potential biases in the review process
The strengths of this review are that it addresses a clinically relevant and pragmatic question. In addition, this is the first published quantitative review for this specific question
A limitation of this review was that we used published results rather than individually updated patient data (IPD). Although these results may overestimate the benefits of additional upfront surgery, it is unlikely that an IPD meta‐analysis would alter the conclusions. This stands to reason, as the effects of upfront surgery on locoregional control and treatment‐related mortality are likely to remain significant, whereas the effects on OS are likely to remain non‐significant.
We did not perform a funnel‐plot analysis, as we only identified two studies. Although publication bias may exist, it is unlikely that a large unpublished randomized controlled study exists that would alter our findings. For the same reasons of limited studies, sensitivity and subgroup analysis (as stated in the protocol) were not performed, as they would not have been meaningful.
We had not specified, a priori, a subgroup analysis of outcomes between responders and non‐responders to induction treatment. Bedenne 2007 did not randomize participants who failed to respond to induction chemoradiotherapy, and Stahl 2005 did not provide sufficient information on non‐responders. As such, a quantitative subgroup analysis could not be performed.
As mentioned above, both studies were in concordance that non‐responding participants had inferior survival outcomes. Vincent 2015 suggested that the survival of non‐responders from the FFCD 9102 study who underwent upfront surgery, was comparable to that of responders having surgery in the randomized arms (median survival 17.3 to 17.7 months). This corroborated with information provided by Stahl’s commentary, where a salvage esophagectomy improved the survival in non‐responders.
Another limitation of this study was the inability to summarize the data on local and distant progression‐free survival using the Kaplan‐Meier method, as stated in the protocol. Stahl 2005 reported two‐year freedom from local progression, whereas Bedenne 2007 reported two‐year recurrence probability and locoregional relapses. Deviating from the protocol, we combined the available data to formulate a hazard ratio for freedom from locoregional relapse. Although not stated a priori, this provided a close and reliable estimate of local progression‐free survival.
A further limitation was the heterogeneity in reporting outcomes, such as distant progression‐free survival, treatment‐related morbidity, use of salvage procedures, and QoL. We thought these outcomes were clinically relevant, so we included them in our protocol. However, only Bedenne 2007 reported on them, so we were unable to perform quantitative analyses.
Agreements and disagreements with other studies or reviews
Best 2016 performed a similar systematic review comparing the benefits and harms of non‐surgical treatment with surgical treatment approaches for esophageal cancer. Besides including randomized studies of chemoradiotherapy, with or without esophagectomy, Best 2016 also included randomized studies that compared radiotherapy or chemoradiotherapy with esophagectomy alone. Best 2016 performed a subgroup analysis that pooled the overall survival results of Bedenne 2007 and Stahl 2005. It showed that there was no survival benefit when esophagectomy was added to chemo‐radiotherapy, similar to the findings of this review.
We acknowledge that while there was some overlap between this review and the review by Best 2016, there are important differences as well. First, our review's question was more specific, as we were only interested in knowing if there was any clinical benefit for people who had undergone chemoradiotherapy for esophageal cancer if esophagectomy was added, whereas Best 2016 was more interested in comparing the effects of a non‐surgical approach with a surgical approach for treatment of esophageal cancer. The definitions of non‐surgical and surgical approaches in Best 2016 review were extremely varied. Non‐surgical approaches could include radiotherapy alone or chemoradiotherapy treatment, and surgical approaches included esophagectomy alone or esophagectomy plus chemoradiotherapy. Second, we examined the effects of adding esophagectomy to chemoradiotherapy more thoroughly than Best 2016, who only pooled the effects of adding esophagectomy for overall survival. Besides overall survival, we also pooled the effects of additional esophagectomy on freedom from locoregional relapse, and treatment‐related mortality.
In addition, prospective non‐randomized studies corroborated our findings. Wang 2007 conducted a prospective non‐randomized study comprising 50 participants with esophageal cancer, 67% of whom had adenocarcinoma histology, and comparing carboplatin and paclitaxel and radiotherapy to 45 Gy, followed by esophagectomy versus carboplatin and paclitaxel and radiotherapy to 50.4 Gy, followed by consolidation chemotherapy alone. The survival outcomes were not difference between the two groups (three‐year survival 60% in each group). A single institution retrospective series similarly found no difference in survival outcomes with the addition of surgery (Rawat 2013).
Both of our included studies were conducted in the 1990’s, therefore one has to re‐examine the effect of increased treatment‐related mortality with esophagectomy, which may have negated any potential survival advantage (Finks 2011; Jafari 2013). Could there be a potential survival benefit with improved modern surgical techniques and post‐operative care? As such, the applicability of these results to modern day treatment techniques (surgery, radiotherapy) may be questioned.
Practice guidelines from NCCN (Version 3.2015) are in line with our findings. These guidelines recommend surveillance in people with SCC undergoing definitive chemoradiation, unless there is evidence of persistent local disease, for which salvage esophagectomy should be undertaken. Similarly, ESMO guidelines recommend either chemoradiation with planned surgery, or close surveillance with salvage surgery, for people with locally advanced SCC (Stahl 2013).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
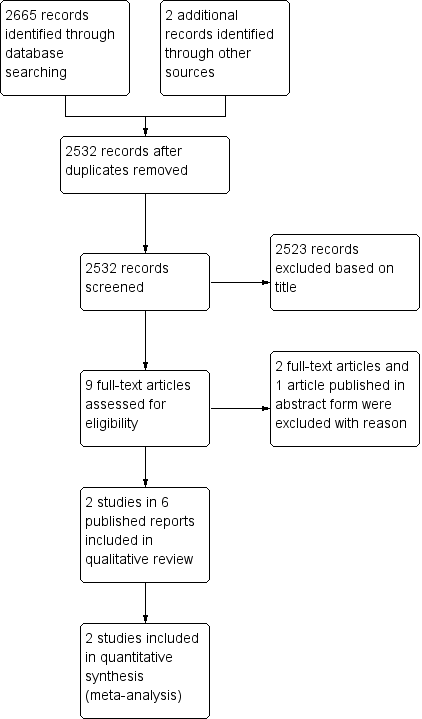
PRISMA flow diagram.
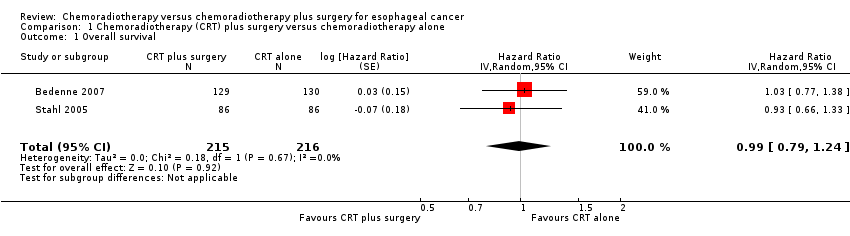
Comparison 1 Chemoradiotherapy (CRT) plus surgery versus chemoradiotherapy alone, Outcome 1 Overall survival.

Comparison 1 Chemoradiotherapy (CRT) plus surgery versus chemoradiotherapy alone, Outcome 2 Freedom from locoregional relapse.
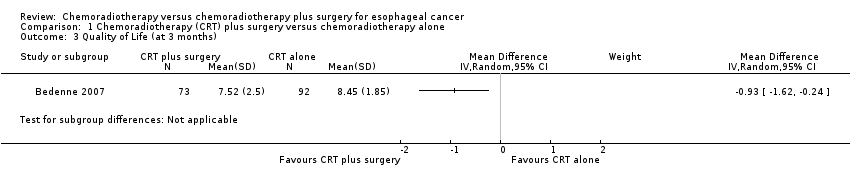
Comparison 1 Chemoradiotherapy (CRT) plus surgery versus chemoradiotherapy alone, Outcome 3 Quality of Life (at 3 months).

Comparison 1 Chemoradiotherapy (CRT) plus surgery versus chemoradiotherapy alone, Outcome 4 Treatment‐related mortality.

Comparison 1 Chemoradiotherapy (CRT) plus surgery versus chemoradiotherapy alone, Outcome 5 Use of salvage procedures for dysphagia.
| Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer | |||||
| Patient or population: nonmetastatic esophageal cancer | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants | Quality of the evidence | |
| Risk with chemoradiotherapy alone | Risk with chemoradiotherapy plus surgery | ||||
| Overall survival | 35.4% to 40.0% at 2 years | 34.0% to 39.9% at 2 years | HR 0.99 (95% CI 0.79 to 1.24) | 431 | ⊕⊕⊕⊕ |
| Freedom from locoregional relapse Follow‐up: median 4 to 6 years | 40.7% to 57.0% at 2 years | 64.3% to 66.4% at 2 years | HR 0.55 (95% CI 0.39 to 0.76) | 431 | ⊕⊕⊕⊝ |
| Quality of Life assessed with: Spitzer QoL index Scale: 0 to10 Follow‐up: 3 months | the mean Q0L score was 7.52 points in the chemoradiotherapy alone group | the mean QoL score in the chemoradiotherapy plus surgery group was 0.93 points worse (from ‐1.62 worse to ‐0.24 worse) | 165 (1 RCT) | ⊕⊝⊝⊝ | |
| Treatment‐related mortality Follow‐up: median 1 to 3 months | 1.9 per 100 | 9.5 per 100 (3.2 to 27.8) | RR 5.11 | 431 | ⊕⊕⊝⊝ |
| Use of salvage procedures for dysphagia Follow‐up: median 4 years | 46 per 100 | 24 per 100 | RR 0.52 (95% CI 0.36 to 0.75) | 259 (1 RCT) | ⊕⊕⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded one level due to risk of bias (detection bias as investigators were not blinded). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 431 | Hazard Ratio (Random, 95% CI) | 0.99 [0.79, 1.24] |
| 2 Freedom from locoregional relapse Show forest plot | 2 | 431 | Hazard Ratio (Random, 95% CI) | 0.55 [0.39, 0.76] |
| 3 Quality of Life (at 3 months) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Treatment‐related mortality Show forest plot | 2 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 5.11 [1.74, 15.02] |
| 5 Use of salvage procedures for dysphagia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |

