Piridoxal 5 fosfato para la discinesia tardía inducida por neurolépticos
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: Randomised Blinding: Double Duration: 9 weeks Setting: Inpatients in Be'er Sheva Mental Health Centre, Israel. Design: Cross‐over study, divided into two phases of 4 weeks each, with 1 week wash‐out period. | |
| Participants | Diagnosis: Schizophrenia or schizoaffective disorder. N = 15. Age: 28 ‐ 71 years. Sex: 4M,11F. History: Mean chlorpromazine equivalent of 490 mg/day. Included were patients who fulfilled diagnostic criteria for tardive dyskinesia; stable on antipsychotic medication for at least one month; Excluded were patients on vitamin treatment, concurrent medical/neurological disorder and those with substance or alcohol abuse. | |
| Interventions | 1. Vitamin B6, increased by 100 mg/week from 100 mg/day to 400 mg/day in twice daily divided doses (n = 8). 2. Placebo (n = 7). | |
| Outcomes | Global: Clinical efficacy: reduction in ESRS scores from baseline by 4 weeks Adverse effects other than tardive dyskinesia ‐ by 4 weeks Average time to discontinuation of P5P: in days ‐ by 4 weeks Average endpoint dose of P5P: in mg ‐ by 4 weeks Deterioration in tardive dyskinesia symptoms: ESRS ‐ by 4 weeks Average endpoint tardive dyskinesia scores: ESRS ‐ by 4 weeks | |
| Notes | The authors did not mention the specific diagnostic instrument used in confirming the diagnoses of participants included in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details were provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described by authors. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The study design was double blind, with crossover and placebo control." No further details were provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The raters were kept blind to the results". The specific method by which blinding was achieved was not described. |
| Incomplete outcome data (attrition bias) | Low risk | No attrition among participants was reported. |
| Selective reporting (reporting bias) | High risk | Not all outcomes were accounted for e.g. the specific number of participants who showed none, minimal, moderate or marked improvement in their tardive dyskinesia symptoms from both arms of the study were not reported. |
| Other bias | Unclear risk | The authors did not give details as to what extent raters in the study were independent. It was not mentioned if any funding was received for the study. The specific diagnostic criteria used for inclusion of participants in the study was not mentioned. |
| Methods | Allocation: Randomised Blinding: Double Setting: Inpatients at Be'er Sheva Mental Health centre, Israel. Duration: 26 weeks Design: Cross‐over study, divided into two phases of 12 weeks each with 2 weeks wash‐out period. | |
| Participants | Diagnosis: All participants met DSM‐IV criteria for Schizophrenia (n = 34) or Schizoaffective disorder (n =16). N = 50 Age: Mean ± SD = 47 ± 11 years, Range = 20 ‐ 66 years Sex: 28 Males, 22 Females History: Diagnosis of tardive dyskinesia; exposure to neuroleptics; stable psychotropic regimen for at least 1 month; duration of symptoms of at least 1 year; mean antipsychotic dose = 396.7 ± 280.4 mg/day in Chlorpromazine equivalents. Excluded: Concurrent medical/neurologic illness; pregnant/lactating mothers; patients on any vitamin supplements; substance/alcohol abuse. | |
| Interventions | 1. Vitamin B6 (n = 28), 600 mg twice daily (Total ‐ 1200 mg/day) 2. Placebo (n = 22), two tablets twice daily | |
| Outcomes | Clinical efficacy: ESRS ‐ reduction in ESRS score by 12 weeks Adverse effects other than tardive dyskinesia Average endpoint dose of P5P: in mg ‐ by 12 weeks Average time to discontinuation of P5P: in days ‐ by 12 weeks. Deterioration in tardive dyskinesia symptoms: ESRS ‐ by 12 weeks Average endpoint tardive dyskinesia score: ESRS ‐ by 12 weeks. | |
| Notes | The study was supported by a clinical trials grant from the Stanley Medical Research Institute, Bethesda, Md. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further descriptions. |
| Allocation concealment (selection bias) | Unclear risk | Specific method of allocation concealment not described. " After breaking the code following database lock......" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further details was provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The plasma levels of Vitamin B6 were not reported to the raters, in order to keep them 'blind' to the patients' drug assignment." The specific method by which blinding was achieved was not described. The extent to which the raters were independent was not mentioned by the authors. |
| Incomplete outcome data (attrition bias) | Low risk | All participants randomised at the beginning of the study were accounted for. |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting in the study. |
| Other bias | Low risk | Funded by Stanley Medical Research Institute. |
| Methods | Allocation: Randomised. Blinding: Double blind Setting: Inpatients at the Mental Health centre in Beer Sheva, Israel. Duration: 9 weeks Design: Cross‐over study, divided into two phases of 4 weeks each, with 1 week wash‐out period. | |
| Participants | Diagnosis: Schizophrenia, schizoaffective disorder or Schizophreniform disorder (diagnosis based on ICD‐10) N: 15 Age: Range = 28 ‐ 71 years, Mean ± SD = 50.0 ± 14.2 years,. Gender: 4 males, 11 females. History: History of tardive dyskinesia for at least 1 year; Duratio of illness ± SD = 18.6 ± 13.13 years with a range of 2 to 42 years. No change in the pharmacotherapeutic treatment in the month prior to inclusion; have no other significant organic diseases on physical examination. Excluded: patients with psychotic disorders caused by psychoactive drugs or by other organic disorders; patients with known lack of vitamins, eating disorders, malabsorption disorders, or known hypersensitivity to vitamin B6; Pregnant or lactating women and patients being treated with penicillamine, isoniazid and combined oral contraceptives. | |
| Interventions | 1. Vitamin B6: n = 8, dose = maximum of 400 mg/day 2. Placebo: n = 7 | |
| Outcomes | Average endpoint dose of P5P: in mg ‐ by 4 weeks Average endpoint psychiatric symptoms score: PANSS ‐ by 4 weeks. | |
| Notes | The original study was written in Hebrew and then translated into English. Other data such as endpoint tardive dyskinesia scores were reported on an unscaled graph and were therefore not usable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description. |
| Allocation concealment (selection bias) | Unclear risk | No specific method of allocation was described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further details were provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "patients and investigators were blinded to the results of pyridoxal phosphate assessment". The specific method by which this was achieved was not stated. The level to which raters were independent is unknown. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were accounted for at the end of the trial. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting was found. |
| Other bias | Unclear risk | The authors did not state if there was any source of funding. |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
ICD‐10: International Statistical Classification of Diseases and Related Health Problems, 10th Revision
ESRS: Extrapyramidal Symptom Rating Scale
mg: milligrams
PANSS: Positive and Negative Symptoms Scale
P5P: Pyridoxal 5 Phosphate
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: randomised Participants: schizophrenia Intervention: folic acid, vitamin B12 and Pyridoxine. Outcomes: unusable ‐ measured outcomes were the effects of interventions on homocysteine level, cognitive deficits and psychopathology, not tardive dyskinesia. | |
| Allocation: randomised Participants: schizophrenia/schizoaffective disorders Intervention: vitamin B6 versus placebo Outcomes: unusable ‐ incomplete study data (report is an abstract presented in a conference). Full text not available from authors. | |
| Allocation: randomised Participants: patients with tardive dyskinesia. Specific diagnoses not stated. Intervention: vitamin B6 versus placebo Outcomes: unusable data ‐ incomplete study data. Full text not available from authors when requested. | |
| Allocation: randomised Participants: schizophrenia/schizoaffective disorders Intervention: Vitamin B6 versus placebo Outcome: unusable data were reported. Study was published in a book chapter. Full text of study not obtainable from the authors. | |
| Allocation: randomised Participants: diagnoses not specified Intervention: vitamin B6 versus other drugs Outcome: No useable data from this report as the study data were incomplete (only the proportion of participants in the treatment arm who experienced a significant reduction in scores for the measured outcomes were reported). Full text of report not obtainable from the authors. | |
| Allocation: randomised Participants: first episode psychosis, not specific diagnosis of schizophrenia or schizoaffective disorder. | |
| Allocation: randomised Participants: Mixed diagnosis e.g. mood disorder, schizophrenia, epilepsy, dementia etc. Although patients with schizophrenia comprised 53.7% of the total diagnoses, the specific number of patients with schizophrenia allocated to each arm of treatment is unknown, making the study data unusable. | |
| Allocation: randomised Participants: patients with organophosphorous insecticide poisoning, not schizophrenia. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Safety and efficacy of avastrem (Pyridoxal 5' ‐phosphate) in the treatment of tardive dyskinesia |
| Methods | Randomised controlled, double blind trial |
| Participants | Not stated |
| Interventions | Vitamin B6 versus placebo |
| Outcomes | Tardive dyskinesia |
| Starting date | May 2009 |
| Contact information | http://www.clinicaltrials.gov |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||
| 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.97 [2.87, 139.19] | ||||||||||
| Analysis 1.1  Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline. | ||||||||||||||
| 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate Show forest plot | Other data | No numeric data | ||||||||||||
| Analysis 1.2
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate. | ||||||||||||||
| 3 Global: 2. Other adverse effects than tardive dyskinesia Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.20, 78.59] | ||||||||||
| Analysis 1.3  Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 3 Global: 2. Other adverse effects than tardive dyskinesia. | ||||||||||||||
| 4 Global: 3. Discontinuation of Vitamin B6 Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.72 [0.51, 149.75] | ||||||||||
| Analysis 1.4  Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 4 Global: 3. Discontinuation of Vitamin B6. | ||||||||||||||
| 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.14] | ||||||||||
| Analysis 1.5 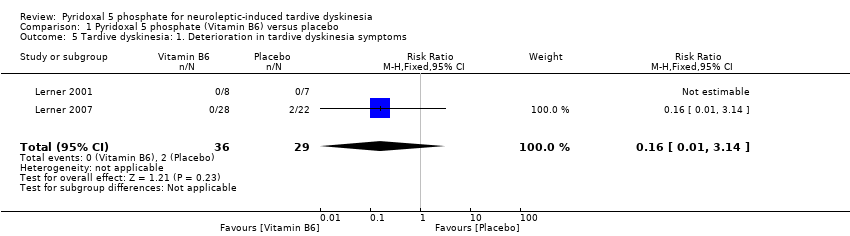 Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms. | ||||||||||||||
| 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐6.36, ‐1.79] | ||||||||||
| Analysis 1.6 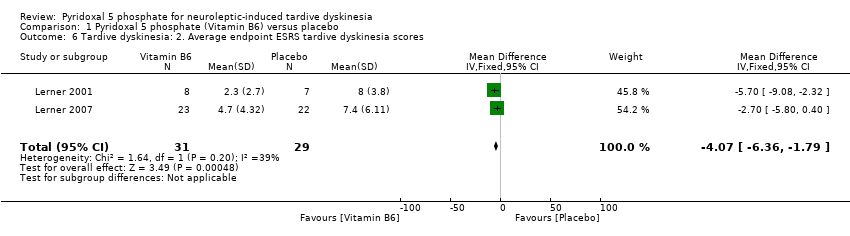 Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores. | ||||||||||||||
| 7 General Mental State: Average endpoint positive psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐4.80, 1.80] | ||||||||||
| Analysis 1.7 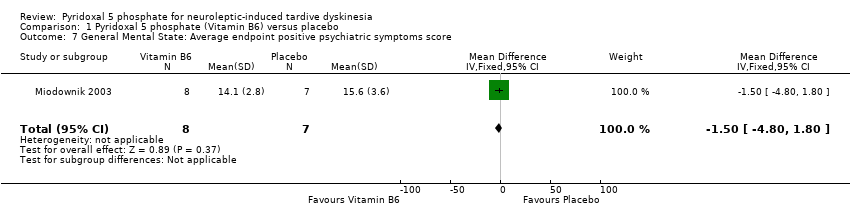 Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 7 General Mental State: Average endpoint positive psychiatric symptoms score. | ||||||||||||||
| 8 General mental state: Average endpoint negative psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.92, 3.72] | ||||||||||
| Analysis 1.8 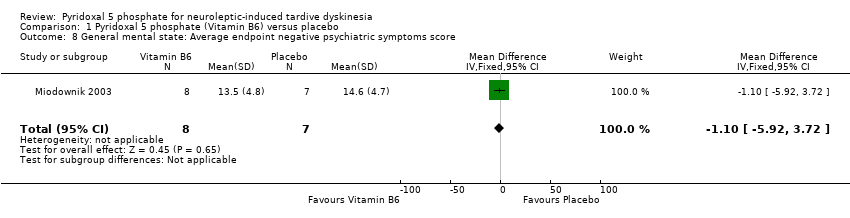 Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 8 General mental state: Average endpoint negative psychiatric symptoms score. | ||||||||||||||
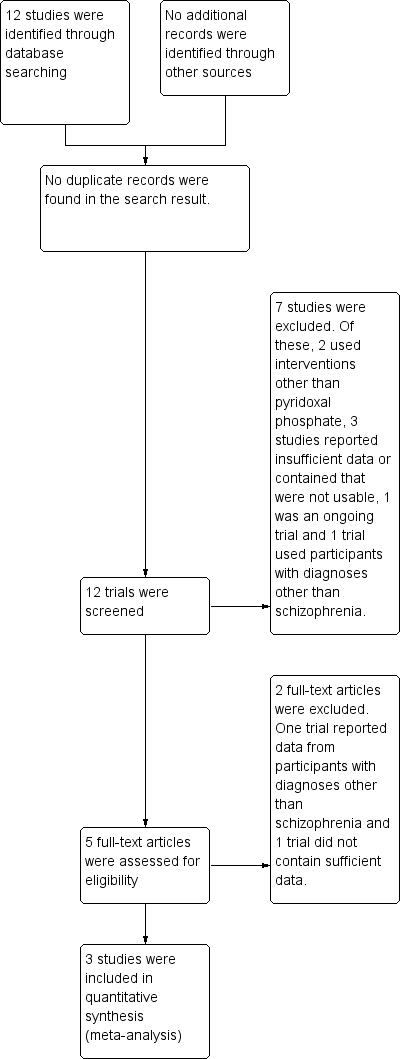
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
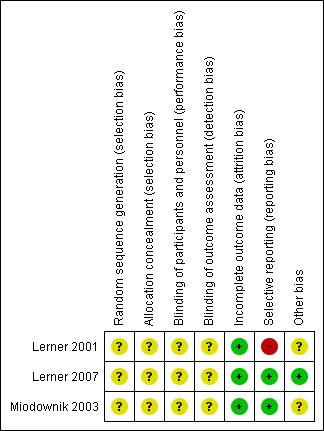
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline.
| Study | |
| Lerner 2001 | 400 mg daily |
| Lerner 2007 | 1200 mg daily |
| Miodownik 2003 | 400 mg daily |
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 3 Global: 2. Other adverse effects than tardive dyskinesia.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 4 Global: 3. Discontinuation of Vitamin B6.
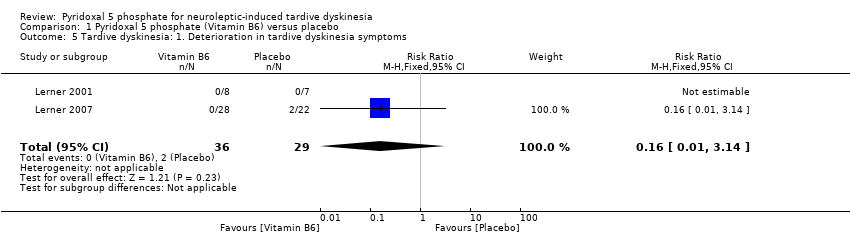
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms.
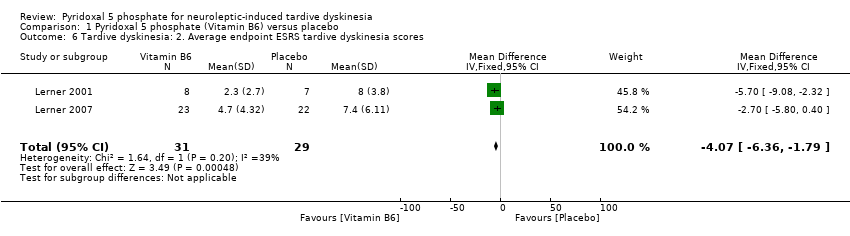
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores.
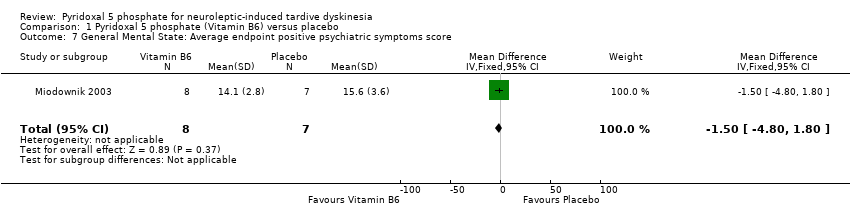
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 7 General Mental State: Average endpoint positive psychiatric symptoms score.
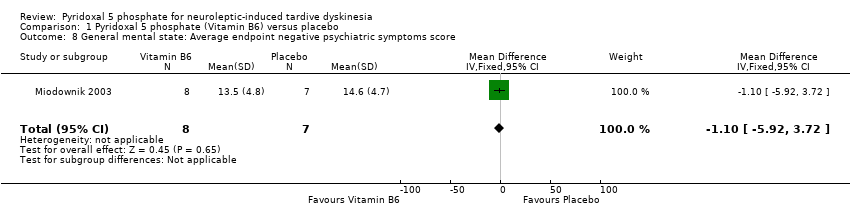
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 8 General mental state: Average endpoint negative psychiatric symptoms score.
| Method | Allocation: randomised ‐ clearly described generation of sequence and concealment of allocation. Design: Single phase, longer study duration. |
| Participants | People with schizophrenia or schizophrenia‐like disorder. History: History of tardive dyskinesia, fulfilling diagnostic criteria for tardive dyskinesia, stable on antipsychotic medication for at least 3 months. |
| Intervention | 1.Pyridoxal Phosphate (vitamin B6), any dose 2. Placebo |
| Outcomes | Tardive dyskinesia scores measured using AIMS (primary outcome) Deterioration of tardive dyskinesia symptoms Any other adverse effects Discontinuation of pyridoxal phosphate (with reasons) Psychiatric symptoms score using a standardised rating scale (PANSS, BPRS) Pyridoxal phosphate dose Plasma pyridoxal phosphate level Quality of life Satisfaction with care |
| AIMS: Abnormal Involuntary Movement Scale | |
| Pyridoxal 5 phosphate (vitamin B6) compared with Placebo for neuroleptic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with neuroleptic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pyridoxal 5 phosphate (vitamin B6) | |||||
| Clinical efficacy ‐ improvement (> 40%) in ESRS scores from baseline | Study population | RR 19.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Other adverse effects than tardive dyskinesia | Study population | RR 3.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Discontinuation of Vitamin B6 | Study population | RR 8.72 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Tardive dyskinesia: Deterioration in tardive dyskinesia symptoms | Study population | RR 0.16 | 65 | ⊕⊕⊝⊝ | ||
| 69 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 46 per 1000 | 7 per 1000 | |||||
| General mental state: Positive psychiatric symptom score | The mean general mental state: positive psychiatric symptom score in the control groups was | The mean general mental state: positive psychiatric symptom score in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| General mental state: Negative psychiatric symptoms | The mean general mental state: negative psychiatric symptoms in the control groups was | The mean general mental state: negative psychiatric symptoms in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Extrapyramidal Symptom Rating Scale (high scores = worse) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.97 [2.87, 139.19] |
| 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate Show forest plot | Other data | No numeric data | ||
| 3 Global: 2. Other adverse effects than tardive dyskinesia Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.20, 78.59] |
| 4 Global: 3. Discontinuation of Vitamin B6 Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.72 [0.51, 149.75] |
| 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.14] |
| 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐6.36, ‐1.79] |
| 7 General Mental State: Average endpoint positive psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐4.80, 1.80] |
| 8 General mental state: Average endpoint negative psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.92, 3.72] |

