Piridoxal 5 fosfato para la discinesia tardía inducida por neurolépticos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010501.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 abril 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esquizofrenia
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Adegoke Oloruntoba Adelufosi ‐ Data collection and interpretation, statistical analysis, writing the review.
Olukayode Abayomi ‐ Data collection and interpretation, assisted in writing the review.
Tunde Massey‐Ferguson Ojo ‐ Data collection, assisted in writing the review.
Sources of support
Internal sources
-
Reviews for Africa (Nigeria) Programme Fellowship, Nigeria.
External sources
-
UK Department for International Development (DFID) through the Effective Healthcare Research Consortium at the Liverpool School of tropical Medicine., UK.
Declarations of interest
The authors received no financial consideration from any parties for the preparation of this review.
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods sections of their reviews. This text was used as the basis of the protocol, with modifications where necessary.
Adegoke Oloruntoba Adelufosi was awarded a Reviews for Africa (Nigeria) Programme Fellowship funded by a grant from the UK Department for International Development (DFID) through the Effective Healthcare Research Consortium at the Liverpool School of tropical Medicine. This review was developed in part during the Reviews for Africa Programme protocol development course organised by the Nigerian Branch of South African Cochrane Centre, April 2013.
The search terms were developed by the Trials Search Co‐ordinator of the Cochrane Schizophrenia Group, Samantha Roberts.
We would like to thank Michael Wilson for peer reviewing this version of our review, his comments were most helpful.
We would also like to acknowledge and thank our copy editor, Heather Maxwell.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Apr 13 | Pyridoxal 5 phosphate for neuroleptic‐induced tardive dyskinesia | Review | Adegoke Oloruntoba Adelufosi, Olukayode Abayomi, Tunde Massey‐Ferguson Ojo | |
| 2013 Apr 30 | Pyridoxal 5 phosphate for neuroleptic‐induced tardive dyskinesia | Protocol | Adegoke Oloruntoba Adelufosi, Olukayode Abayomi, Tunde Massey‐Ferguson Ojo | |
Differences between protocol and review
There are two major differences between the protocol of this review and the final review viz;
1. Since the studies included in this review were generally of short duration, the classification of outcomes into short term, medium term and long term outcomes was eliminated. For the same reason, assessment of clinical improvement in tardive dyskinesia symptoms was changed from "at least six weeks of treatment" to "at least four weeks".
2. The definition of clinical efficacy for pyridoxal 5 phosphate was changed to > 40% improvement in tardive dyskinesia symptoms after at least four weeks of treatment. This was necessary for interpretation of results and to reflect the definitions of moderate to marked clinical improvement in tardive dyskinesia symptoms used in the included studies.
3. Due to the small sample sizes of studies included in this review and the possible bias that can be introduced by using a random‐effects model for data synthesis, we decided to use a fixed‐effect model for all our data analysis.
4. As at the time of writing this review, one pharmaceutical company was still recruiting participants for a trial on the safety and efficacy of pyridoxal 5 phosphate on treatment of tardive dyskinesia. Since the trial was still ongoing, we did not contact the pharmaceutical company as stated in the protocol of this review.
5. We did not conduct a sensitivity analyses as stated in the protocol due to the similarities in inclusion criteria, characteristics of intervention/comparator and study design among the included studies.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Aged; Female; Humans; Male; Middle Aged;
PICO
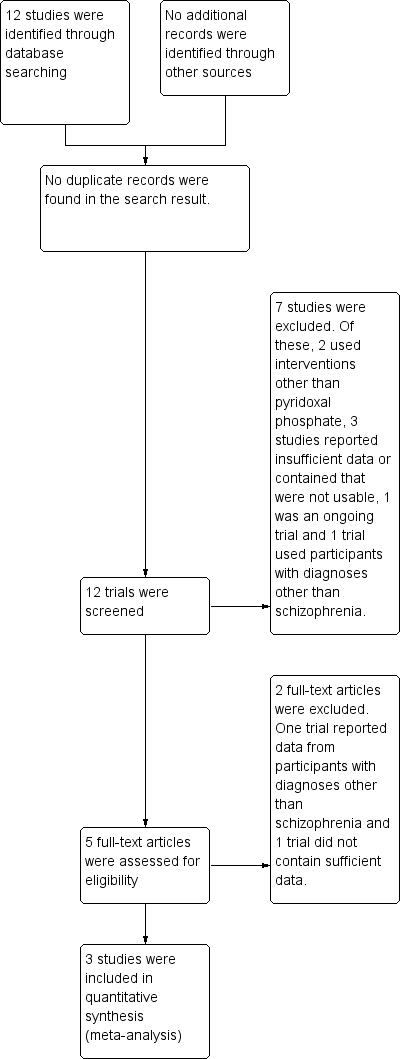
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
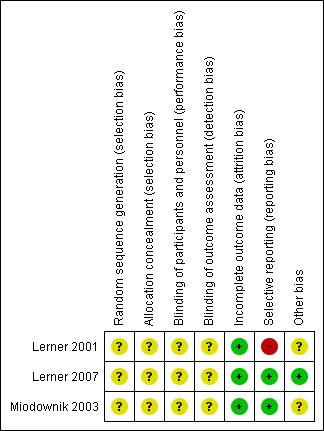
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline.
| Study | |
| Lerner 2001 | 400 mg daily |
| Lerner 2007 | 1200 mg daily |
| Miodownik 2003 | 400 mg daily |
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 3 Global: 2. Other adverse effects than tardive dyskinesia.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 4 Global: 3. Discontinuation of Vitamin B6.
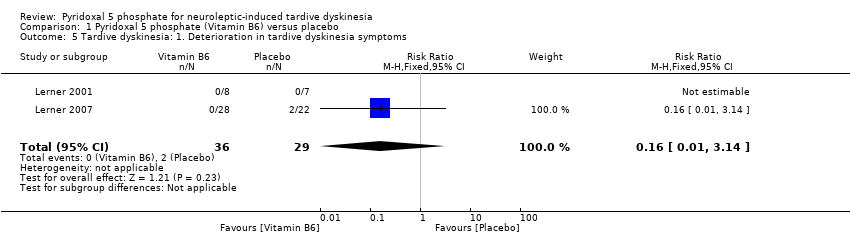
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms.
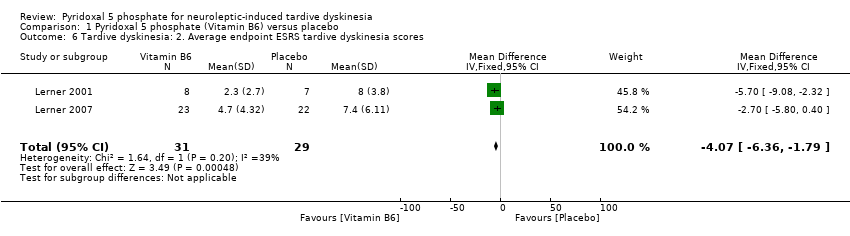
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores.
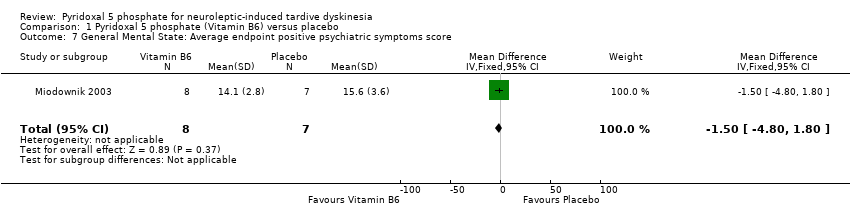
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 7 General Mental State: Average endpoint positive psychiatric symptoms score.
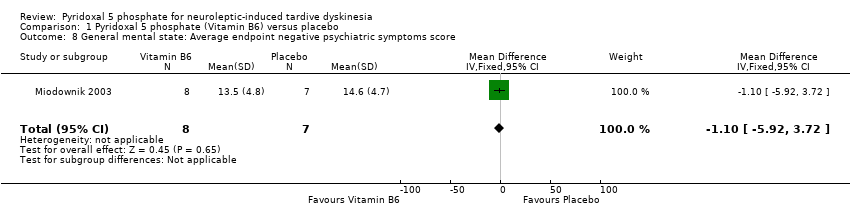
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 8 General mental state: Average endpoint negative psychiatric symptoms score.
| Method | Allocation: randomised ‐ clearly described generation of sequence and concealment of allocation. Design: Single phase, longer study duration. |
| Participants | People with schizophrenia or schizophrenia‐like disorder. History: History of tardive dyskinesia, fulfilling diagnostic criteria for tardive dyskinesia, stable on antipsychotic medication for at least 3 months. |
| Intervention | 1.Pyridoxal Phosphate (vitamin B6), any dose 2. Placebo |
| Outcomes | Tardive dyskinesia scores measured using AIMS (primary outcome) Deterioration of tardive dyskinesia symptoms Any other adverse effects Discontinuation of pyridoxal phosphate (with reasons) Psychiatric symptoms score using a standardised rating scale (PANSS, BPRS) Pyridoxal phosphate dose Plasma pyridoxal phosphate level Quality of life Satisfaction with care |
| AIMS: Abnormal Involuntary Movement Scale | |
| Pyridoxal 5 phosphate (vitamin B6) compared with Placebo for neuroleptic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with neuroleptic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pyridoxal 5 phosphate (vitamin B6) | |||||
| Clinical efficacy ‐ improvement (> 40%) in ESRS scores from baseline | Study population | RR 19.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Other adverse effects than tardive dyskinesia | Study population | RR 3.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Discontinuation of Vitamin B6 | Study population | RR 8.72 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Tardive dyskinesia: Deterioration in tardive dyskinesia symptoms | Study population | RR 0.16 | 65 | ⊕⊕⊝⊝ | ||
| 69 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 46 per 1000 | 7 per 1000 | |||||
| General mental state: Positive psychiatric symptom score | The mean general mental state: positive psychiatric symptom score in the control groups was | The mean general mental state: positive psychiatric symptom score in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| General mental state: Negative psychiatric symptoms | The mean general mental state: negative psychiatric symptoms in the control groups was | The mean general mental state: negative psychiatric symptoms in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Extrapyramidal Symptom Rating Scale (high scores = worse) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.97 [2.87, 139.19] |
| 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate Show forest plot | Other data | No numeric data | ||
| 3 Global: 2. Other adverse effects than tardive dyskinesia Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.20, 78.59] |
| 4 Global: 3. Discontinuation of Vitamin B6 Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.72 [0.51, 149.75] |
| 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.14] |
| 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐6.36, ‐1.79] |
| 7 General Mental State: Average endpoint positive psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐4.80, 1.80] |
| 8 General mental state: Average endpoint negative psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.92, 3.72] |

