Piridoxal 5 fosfato para la discinesia tardía inducida por neurolépticos
Resumen
Antecedentes
La discinesia tardía es un trastorno del movimiento anormal crónico e incapacitante que afecta los músculos de la cara, el cuello, la lengua y los miembros. Es un efecto secundario habitual de la administración a largo plazo de medicación antipsicótica en los individuos con esquizofrenia y otros trastornos psicóticos relacionados. Aunque hasta la fecha no hay tratamientos efectivos conocidos para la discinesia tardía, algunos informes indican que el piridoxal 5 fosfato puede ser efectivo para reducir la gravedad de los síntomas de discinesia tardía.
Objetivos
Determinar la efectividad del piridoxal 5 fosfato (vitamina B6 o Piridoxina o Piridoxal fosfato) en el tratamiento de la discinesia tardía inducida por neurolépticos en pacientes con esquizofrenia y otros trastornos psicóticos relacionados.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos clínicos del Grupo Cochrane de Esquizofrenia (Cochrane schizophrenia group) (enero 2013) utilizando la frase: [*Pyridoxal* OR *Pyridoxine* OR *P5P* OR *PLP* OR *tardoxal* OR *Vitamin B6* O *Vitamin B 6* R in title, abstract or index terms of REFERENCE, or interventions of STUDY. Se realizaron búsquedas manuales de las referencias de los estudios identificados relevantes, y cuando fue necesario, se estableció contacto con los primeros autores de los estudios pertinentes.
Criterios de selección
Estudios descritos como ensayos controlados aleatorios que compararan la efectividad del piridoxal 5 fosfato con placebo en el tratamiento de la discinesia tardía inducida por neurolépticos entre los pacientes con esquizofrenia.
Obtención y análisis de los datos
Los autores de la revisión extrajeron los datos de cada estudio seleccionado de forma independiente. Para los datos dicotómicos se calcularon los cocientes de riesgos (CR) y sus intervalos de confianza (IC) del 95% sobre la base de un análisis de intención de tratar y según un modelo de efectos fijos. Para los datos continuos se calcularon las diferencias de medias (DM) con IC del 95% y, nuevamente, se utilizó un modelo de efectos fijos. Se evaluó el riesgo de sesgo de cada estudio incluido y se utilizó el enfoque GRADE (Grading of Recommendations Assessment, Development and Evaluation) para calificar la calidad de las pruebas.
Resultados principales
De los 12 registros recuperados mediante la búsqueda se incluyeron tres ensayos publicados en 2001; 2003 y 2007 con 80 pacientes hospitalizados de 18 a 71 años edad que presentaban esquizofrenia que habían ingresado a un establecimiento psiquiátrico y cuyo seguimiento se había realizado durante un periodo de nueve semanas a 26 semanas. En general, el piridoxal 5 fosfato produjo una mejoría significativa en los síntomas de discinesia tardía en comparación con placebo, evaluados mediante un cambio en las puntuaciones de la Extrapyramidal Symptoms Rating Scale (ESRS) desde el inicio hasta el final de la primera fase de los estudios incluidos (dos ECA, n = 65; CR 19,97; IC: 2,87 a 139,19; pruebas de baja calidad). La puntuación de la discinesia tardía al final del estudio (una medida de su gravedad) evaluada con la ESRS fue significativamente inferior entre los participantes que recibieron piridoxal 5 fosfato en comparación con los que recibieron placebo (dos ECA n = 60; DM ‐4,07; IC: ‐6,36 a ‐1,79; pruebas de baja calidad).
El hecho de si el piridoxal 5 fosfato dio lugar a más efectos secundarios (n = 65; dos ECA, CR 3,97; IC: 0,20 a 78,59; pruebas de baja calidad) o si causó un empeoramiento en los síntomas de discinesia tardía en comparación con placebo fue incierto (n = 65; dos ECA, CR 0,16; IC: 0,01 a 3,14; pruebas de baja calidad). Cinco participantes que recibieron piridoxal 5 fosfato se retiraron del estudio debido a que no estaban dispuestos a tomar más fármacos, mientras que ninguno de los participantes que recibió placebo interrumpió la medicación (n = 65; dos ECA, CR 8,72; IC: 0,51 a 149,75; pruebas de baja calidad).
No hubo diferencias significativas en las puntuaciones de los síntomas psiquiátricos positivos y negativos al final del estudio, medidos mediante la Positive and Negative symptoms Scale (PANSS) entre los participantes que recibieron piridoxal 5 fosfato y los que recibieron placebo. Para los síntomas positivos: (n = 15; un ECA, DM ‐1,50; IC: ‐4,80 a 1,80; pruebas de baja calidad). Para los síntomas negativos: (n = 15; un ECA, DM ‐1,10; IC: ‐5,92 a 3,72; pruebas de baja calidad).
Conclusiones de los autores
El piridoxal 5 fosfato puede tener algunos efectos beneficiosos en cuanto a la reducción de la gravedad de los síntomas de discinesia tardía entre los individuos con esquizofrenia. Sin embargo, la calidad de las pruebas que apoyan la efectividad del piridoxal 5 fosfato para el tratamiento de la discinesia tardía es baja, debido a los escasos estudios, los periodos de seguimiento cortos, los tamaños de la muestra pequeños y un cumplimiento inadecuado de las guías de informe estandarizadas para los ensayos controlados aleatorios entre los estudios incluidos.
PICO
Resumen en términos sencillos
Piridoxal 5 fosfato para la discinesia tardía inducida por neurolépticos.
Pregunta de la revisión.
Analizar los efectos del piridoxal 5 fosfato en el tratamiento del trastorno de movimiento de la discinesia tardía, que es causado por el uso a largo plazo de fármacos antipsicóticos en pacientes con esquizofrenia.
Antecedentes.
El tratamiento principal para la esquizofrenia son los fármacos antipsicóticos. Sin embargo, estos fármacos a veces provocan efectos secundarios graves e incapacitantes. La discinesia tardía es un trastorno del movimiento que causa contracciones en los músculos de la cara, el cuello, la lengua y los miembros. Puede ser causado por la administración de fármacos antipsicóticos durante un periodo prolongado. A menudo da lugar a estigmas, reducción en la calidad de vida y puede causar que los pacientes interrumpan el tratamiento con antipsicóticos. Aunque no hay tratamientos conocidos para la discinesia tardía, algunos informes indican que el piridoxal 5 fosfato puede reducir la discinesia tardía.
Características de los estudios.
Se realizó una búsqueda de estudios aleatorios relevantes en enero 2013. La revisión incluye tres estudios con 80 participantes. Todos los participantes presentaban discinesia tardía como resultado de la administración de antipsicóticos y se asignaron al azar a grupos de tratamiento. Un grupo recibió piridoxal 5 fosfato, el otro grupo recibió un placebo. Los antipsicóticos continuaron como tratamiento habitual en todos los ensayos.
Resultados clave.
Los pacientes que recibieron piridoxal 5 fosfato en estos estudios experimentaron una mejoría mayor del 40% en la discinesia tardía en comparación con los que recibieron placebo, por lo que la gravedad de la discinesia tardía fue menor. La experiencia de los efectos secundarios fue similar entre los grupos de tratamiento y los participantes que recibieron piridoxal 5 fosfato no presentaron más o menos efectos secundarios que los participantes del grupo placebo, ni experimentaron un empeoramiento mayor de los síntomas psiquiátricos que los del grupo placebo. Las pruebas de los estudios son débiles, aunque indican que el piridoxal 5 fosfato puede ser efectivo para el tratamiento de la discinesia tardía.
Calidad de la evidencia.
Las pruebas son débiles. El número de estudios y de participantes es escaso. La calidad de los estudios es baja. Podrían obtenerse mejores pruebas con ensayos mejor diseñados, realizados e informados.
Ben Gray, Investigador Superior Externo, McPin Foundation. http://mcpin.org/.
Authors' conclusions
Summary of findings
| Pyridoxal 5 phosphate (vitamin B6) compared with Placebo for neuroleptic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with neuroleptic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pyridoxal 5 phosphate (vitamin B6) | |||||
| Clinical efficacy ‐ improvement (> 40%) in ESRS scores from baseline | Study population | RR 19.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Other adverse effects than tardive dyskinesia | Study population | RR 3.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Discontinuation of Vitamin B6 | Study population | RR 8.72 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Tardive dyskinesia: Deterioration in tardive dyskinesia symptoms | Study population | RR 0.16 | 65 | ⊕⊕⊝⊝ | ||
| 69 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 46 per 1000 | 7 per 1000 | |||||
| General mental state: Positive psychiatric symptom score | The mean general mental state: positive psychiatric symptom score in the control groups was | The mean general mental state: positive psychiatric symptom score in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| General mental state: Negative psychiatric symptoms | The mean general mental state: negative psychiatric symptoms in the control groups was | The mean general mental state: negative psychiatric symptoms in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Extrapyramidal Symptom Rating Scale (high scores = worse) | ||||||
Background
Description of the condition
Since 1950 when the antipsychotic properties of chlorpromazine were first discovered (Lopez‐Munoz 2005), antipsychotic medications have been used effectively in treating the symptoms of schizophrenia, such as hallucinations and delusions. However, one of the drawbacks of long‐term antipsychotic medication use is a disabling, potentially irreversible movement disorder called tardive dyskinesia.
Tardive dyskinesia is a late onset, movement disorder often resulting from long‐term use of neuroleptic (antipsychotic) medications. It manifests as repetitive, involuntary movements affecting the face, jaw, tongue, extremities and the trunk. Abnormal movements in tardive dyskinesia may involve tongue darting, repetitive grimacing, chewing movements of the mouth, sucking movements or puckering of the lips and purposeless, irregular, jerking movements of the limbs. These symptoms are highly stigmatising and associated with poor quality of life in affected individuals (Ascher‐Svanum 2008; Browne 1996).
Risk factors for tardive dyskinesia in patients with schizophrenia include female gender (Yassa 1992), older age (Leung 2003; Niehaus 2008), cognitive impairment or neurological deficits (Waddington 1987), diagnosis of a mood disorder especially depression (Casey 1995; Kane 1986; Sachdev 1989), worsening of psychopathology (Tenback 2007) and diabetes mellitus (Casey 1995; Woerner 1993). Tardive dyskinesia is also higher among those taking first generation (conventional) antipsychotic drugs compared with those on second generation (atypical) antipsychotic medications (Corell 2008; Nasrallah 2006). However, neuroleptic medications are not the sole cause of tardive dyskinesia, as similar abnormal motor movements have been reported among patients with schizophrenia who were never exposed to neuroleptic medications (Ayehu 2014; Fenton 2000).
The exact pathogenesis of tardive dyskinesia is unknown and several theories about its aetiology has been proposed. Foremost is the dopamine receptor supersensitivity hypothesis which posits that long‐term receptor antagonism by antipsychotic drugs results in dopamine receptor supersensitivity and the movement disorder in tardive dyskinesia (Sachdev 1989; Teo 2012). The neurotoxicity hypothesis states that tardive dyskinesia results from the neurotoxic effects of free radicals that are released during dopamine metabolism. It is believed that these free radicals cause cellular degeneration of striatal GABA‐ergic neurons, leading to the loss of their inhibitory activities and the hyperkinetic state of tardive dyskinesia (Sachdev 1989). The use of antioxidants as possible treatment for tardive dyskinesia symptoms is based on the neurotoxicity hypothesis (Zhang 2004).
There are reports that pyridoxal 5 phosphate, a metabolite of vitamin B6 (pyridoxine) helps in alleviating these symptoms (Miodownik 2008).
Description of the intervention
Pyridoxal 5 phosphate is the metabolically active form of vitamin B6, a collective term for the chemically‐ and structurally‐related substances, pyridoxine, pyridoxamine and pyridoxal. Vitamin B6 is a naturally occurring vitamin that can be obtained from both animal and plant sources. Following ingestion, pyridoxine is absorbed from the upper small intestine, where it is transported to the liver and oxidised to form pyridoxal. It is then phosphorylated by pyridoxal kinase to pyridoxal 5 phosphate. Unlike pyridoxine, which causes peripheral neuropathy when high doses are given, pyridoxal 5 phosphate is not associated with any known adverse effects, even at doses as high as 1200 mg/day (Miodownik 2008; Schaumburg 1983).
How the intervention might work
Pyridoxal 5 phosphate is a coenzyme in the decarboxylation of DOPA to dopamine and other neurotransmitters such as serotonin and gamma amino butyric acid (GABA). Although its actual mechanism of action is not clear, 9t is speculated that vitamin B6 may reduce the symptoms of tardive dyskinesia through its effects on the biogenic amines, mainly dopamine, GABA, serotonin and by scavenging free radicals (Lerner 2007).
Why it is important to do this review
Tardive dyskinesia is very disabling and disfiguring and its occurrence is associated with poor treatment adherence (Barnes 1993) and low quality of life (Ascher‐Svanum 2008). The reported prevalence of tardive dyskinesia ranged from 3% to 70% with a median of 24% among patients on long‐term use of neuroleptic medications (Yassa 1992).The annual incidence is 5.2% in those with a first episode of schizophrenia placed on neuroleptic medications, the rate increasing to about 20% at five years (Chakos 1996). Several drugs have been used in an attempt to treat tardive dyskinesia, but little evidence exists to support their efficacy. Such interventions include melatonin (Nelson 2003), cholinergic agonists (Caroff 2001), vitamin E (Adler 1998), calcium channel blockers (Fay‐McCarthy 1997) and clozapine (Bassitt 1998). While the evidence for the effectiveness for some of these interventions has been evaluated (McGrath 2001; Soares‐Weiser 2004; Tammenmaa 2002), no evaluation has taken place for pyridoxal 5 phosphate. Thus, it is uncertain if pyridoxal 5 phosphate is effective in the treatment of neuroleptic‐induced tardive dyskinesia. This review may provide evidence for its efficacy in the treatment of tardive dyskinesia among patients with schizophrenia and other psychotic disorders.
Objectives
To determine the effectiveness of pyridoxal 5 phosphate (vitamin B6, pyridoxine, pyridoxal phosphate) in the treatment of neuroleptic‐induced tardive dyskinesia among patients with schizophrenia and other related psychotic disorders.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials in this review. Quasi‐randomised studies, such as those allocating by alternate days of the week were not eligible for inclusion. We planned that if people were given additional treatments to pyridoxal 5 phosphate, we would only include the data if the adjunct treatment was evenly distributed between groups and it was only pyridoxal 5 phosphate that was randomised.
Types of participants
People with schizophrenia or other chronic mental illness, diagnosed by any criteria, irrespective of gender, age or nationality, who:
-
require the use of antipsychotics for more than three months;
-
developed tardive dyskinesia (diagnosed by any criteria at baseline and at least one other occasion) during antipsychotic treatment; and
-
for whom the dose of antipsychotic medication has been stable for one month or more (the same applied for those free of antipsychotics).
We included only trials where the majority of participants had a diagnosis of schizophrenia.
Types of interventions
-
Pyridoxal 5 phosphate (pyridoxal phosphate, pyridoxine, vitamin B6): any dose or means of administration.
-
Placebo or no intervention.
Types of outcome measures
Primary outcomes
1. Clinical efficacy: Clinical efficacy was defined as an improvement in the symptoms of tardive dyskinesia of more than 40%, on any peer‐reviewed scale, after at least four weeks of intervention
Secondary outcomes
1. Global Outcomes
1.1 Death due to suicide or other causes
1.2 Average endpoint dose of pyridoxal 5 phosphate or vitamin B6
1.3 Any adverse effects (other than deterioration of tardive dyskinesia symptoms or change in mental state)
1.4 Average time to discontinuation of pyridoxal 5 phosphateand reasons for discontinuation
2. Tardive dyskinesia
2.1 Deterioration in tardive dyskinesia symptoms
2.2 Average endpoint tardive dyskinesia score
3. General mental state changes
3.1 Any deterioration in psychiatric symptoms (such as delusions and hallucinations)
3.2 Average endpoint psychiatric symptoms score
4. 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADE profiler (GRADEPRO) to import data from RevMan 5 (Review Manager) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision‐making. We selected all of our listed outcomes for inclusion in the 'Summary of findings' table.
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
1. Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group’s Trials Register (January 2013) using the phrase:
[*Pyridoxal* OR *Pyridoxine* OR *P5P* OR *PLP* OR *tardoxal* OR *Vitamin B6* O *Vitamin B 6* R in title, abstract or index terms of REFERENCE, or interventions of STUDY]
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches of journals and conference proceedings (see Group Module). Incoming trials are assigned to relevant existing or new review titles.
Searching other resources
1. Reference searching
We inspected references of all identified studies for more studies.
2. Personal communication
We contacted the first author of each included study for more information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review authors AOA and OA independently inspected citations from the searches and identified relevant abstracts. A random 20% sample was independently re‐inspected by TMO to ensure reliability. Where disputes arose, the full report was acquired for more detailed scrutiny. Full reports of the abstracts meeting the review criteria were obtained and inspected by AOA and OA. Again, a random 20% of reports was re‐inspected by TMO in order to ensure reliable selection.There were no disagreements as to the inclusion or otherwise of any study among the review authors.
Data extraction and management
1. Extraction
Review authors AOA and OA extracted data from all included studies. In addition, to ensure reliability, TMO independently extracted data from a random sample of the included studies. Disagreements were discussed, decisions documented and, where necessary, the authors of studies were contacted for clarification. Data presented only in graphs and figures were extracted whenever possible, but were included only if two review authors independently had the same result. We contacted authors of studies through an open‐ended request in order to obtain missing information or for clarification where necessary.
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and
b) the measuring instrument has not been written or modified by one of the trialists for that particular trial.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data in our analyses.
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion:
a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors;
b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996);
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We presented and entered all useable change data into analyses.
2.7 Direction of graphs
We entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for pyridoxal 5 phosphate.If we had to enter data so the area to the left of the line indicated a favourable outcome for the control group, this was noted in the relevant graphs.
Assessment of risk of bias in included studies
Review authors AOA and OA independently assessed risk of bias by using criteria described in the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If inadequate details of randomisation and other characteristics of trials were provided, we contacted the authors of the studies in order to obtain further information.
The level of risk of bias was noted in both the text of the review and in the 'summary of findings Table for the main comparison
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The number needed to treat to benefit/harm (NNTB/H) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'summary of findings Table for the main comparison, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated the mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, where scales of very considerable similarity were used, we presumed there was a small difference in measurement, and calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992), whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
We did not find any cluster trials in this search. If we had, we would have employed the methods below.
Where clustering is not accounted for in primary studies, we planned to present data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, if cluster trials are included, we will seek to contact the first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We sought statistical advice and were advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported, it will be assumed to be 0.1 (Ukoumunne 1999).
Where cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data from the first phase of cross‐over studies.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). For any particular outcome, where more than 50% of data was unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table by down‐rating quality. Finally, we also downgraded quality within the 'summary of findings Table for the main comparison where loss was 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome is between 0% and 50% and where these data are not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ was used for those who did not.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported, we reproduced these.
3.2 Standard deviations
When only the standard error (SE) was reported, SDs were calculated by the formula SD = SE * square root (n).
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently methods such as multiple imputation or mixed effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, we prefer to use the more sophisticated approaches. E.g. MMRM or multiple‐imputation is preferred to LOCF and completer analyses would only be presented if some kind of ITT data are not available at all. Moreover, we addressed this issue in the item "incomplete outcome data" of the risk of bias tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies was investigated by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, was interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Higgins 2011). Had substantial levels of heterogeneity been found in the primary outcome, we would have explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011). We tried to locate protocols of the included randomised trials. Had protocols been available, outcomes in the protocol and in the published report would have been compared. Consequently, outcomes listed in the methods section of the trial report were compared with actually reported results.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We therefore chose to use a fixed‐effect method for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We did not undertake any subgroup analyses due to lack of data.
1.2 Clinical state, stage or problem
We undertook this review and provided an overview of the effects of pyridoxal 5 phosphate for people with neuroleptic‐induced tardive dyskinesia in general.
2. Investigation of heterogeneity
We would have reported inconsistency where it was high. First, we would have investigated whether data had been entered correctly. Second, where data were correct, the graphs would have been visually inspected and outlying studies were removed to see if homogeneity was restored.
We decided that if unanticipated clinical or methodological heterogeneity were obvious, we would simply state hypotheses regarding these for future reviews or versions of this review. We did not plan to undertake analyses relating to these.
Sensitivity analysis
1. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption/s and used data only from people who completed the study to that point. When there was a substantial difference, we reported the results and discussed them, but continued to employ our assumption.
2. Risk of bias
If we had found trials with a high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome, we would have analysed the effects of excluding trials that were judged to be at high risk of bias. If the exclusion of trials at high risk of bias does not substantially alter the direction of effect or the precision of the effect estimates, then data from these trials would have been included in the analysis.
3. Fixed and random effects
All data were synthesised using a fixed‐effect model.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
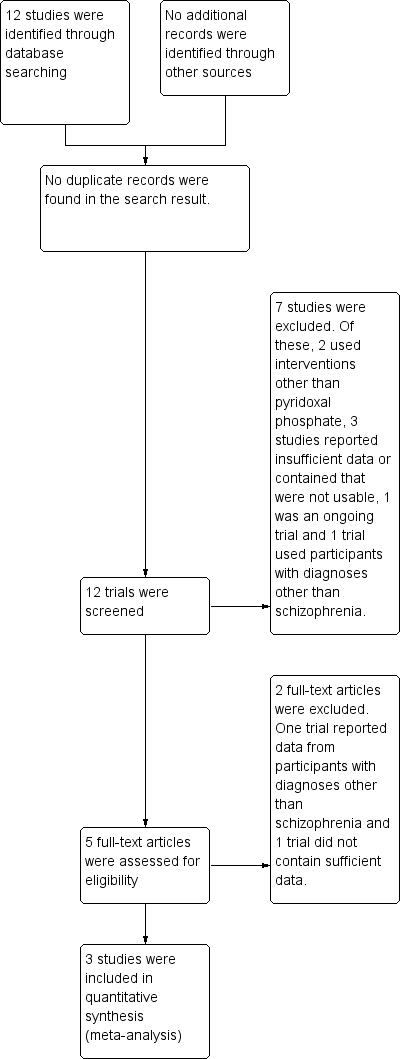
Using the search criteria, the electronic database search carried out in January 2013 yielded 12 trials No additional relevant references were identified from the reference lists of the published trials. See Figure 1.

Study flow diagram.
Included studies
The review included three randomised, double blind, controlled studies published in 2001, 2003 and 2007 (Lerner 2001; Miodownik 2003; Lerner 2007). For more details, see Characteristics of included studies and the accompanying 'Risk of bias' tables.
1. Setting
The three studies included in this review were conducted at the mental health unit of Be'er Sheva Mental Health Centre in Israel.
2. Length of Trials
All three included studies had cross‐over designs. The included trials varied in duration; from nine weeks (Lerner 2001; Miodownik 2003), to 26 weeks (Lerner 2007), including a wash‐out period of either one week (Lerner 2001; Miodownik 2003) or two weeks (Lerner 2007).
3. Participants
There were a total of 80 participants in the included trials. Participants in all the studies were inpatients of a mental health facility with diagnoses of schizophrenia and schizoaffective disorders. The diagnoses were either based on DSM IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) criteria for schizophrenia or schizoaffective disorders (Lerner 2007) or ICD‐10 (International Statistical Classification of Diseases, 10th Revision) criteria (Miodownik 2003). One of the studies did not specify a diagnostic criteria (Lerner 2001).
In total there were more women (n = 44) than men (n = 36) in the included studies. Participants' ages ranged from 18 to 60 years (Miodownik 2003) to 28 to 71 years (Lerner 2001) and 20 to 66 years (Lerner 2007).
4. Trial Size
The overall trial size in the three included studies was small. The total number of participants in each trial ranged from 15 each in Lerner 2001 and Miodownik 2003 to 50 in Lerner 2007.
5. Interventions
Participants in the three included studies either received vitamin B6 or placebo, in addition to their ongoing medication regimens, which remained unchanged throughout the study durations. The maximal daily dose of vitamin B6 used in the included studies ranged from 400 mg (Lerner 2001; Miodownik 2003) to 1200 mg (Lerner 2007).
6. Outcomes
Details of scales providing useable data are described below:
6.1 Tardive dyskinesia
6.1.1. Extrapyramidal Symptom Rating Scale (ESRS) ‐ Chouinard 2005
The ESRS is a measure of the severity of movement disorders in patients with schizophrenia taking antipsychotic medications. The ESRS measures four types of drug‐induced movement disorders viz: parkinsonism, akathisia, dystonia and tardive dyskinesia. Each of these movement disorders can be scored from normal (score of zero) to extremely severe (score of six), according to the symptom severity.
ESRS was used to assess the severity of tardive dyskinesia symptoms and clinical efficacy of the interventions by the studies included in this review (Lerner 2001; Lerner 2007, Miodownik 2003). The assessments were performed at baseline, repeated either every week (Lerner 2001; Miodownik 2003), or at least every two weeks (Lerner 2007). A 20% reduction in ESRS scores from baseline to week four (Lerner 2001; Miodownik 2003) or week 12 (Lerner 2007) was taken to represent no clinical response, 21% to 40% as minimal improvement, 41% to 60% as moderate improvement, and more than 61% as marked improvement.
6.2 General mental state changes
6.2.1 Positive And Negative Symptoms Scale (PANSS) ‐ Kay 1987
The PANSS is a 30‐item seven‐point rating scale divided into positive (seven items), negative (seven items) and general psychopathology (16 items) sub‐scales. It was designed to assess the clinical symptoms of schizophrenia by a clinician rater, using a semi‐structured interview. Each item on the PANSS is scored on a seven‐point Likert scale ranging from one to seven and the rating can generally be completed in 30 to 40 minutes. The PANSS can reliably be administered to assess symptoms improvement and exacerbations.
PANSS was used in one of the included trials (Miodownik 2003) to assess the severity of psychiatric symptoms among participants taking vitamin B6 and placebo.
Excluded studies
Overall, eight studies were excluded from the review. Of these four randomised double blind controlled studies reported insufficient data and some data obtained following communication with study authors were not usable (Lerner 1999; Lerner 2002; Lerner 2007a; Lerner 2009). Two studies did not use vitamin B6/pyridoxal phosphate (Xiao 2002, Greenberg 2003), while two studies had participants with diagnoses other than schizophrenia/schizoaffective disorders (Venegas 2006, NCT00202280).
Ongoing studies
One study was still ongoing as at July 2013 (NCT00917293).
Risk of bias in included studies
For a graphical overview please see Figure 2 and Figure 3.
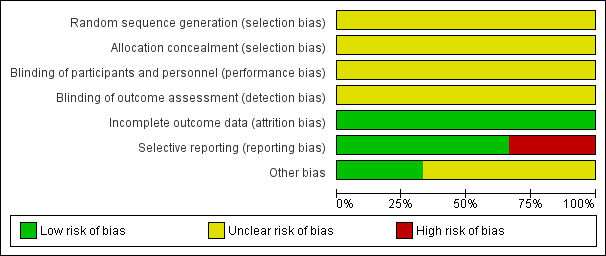
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

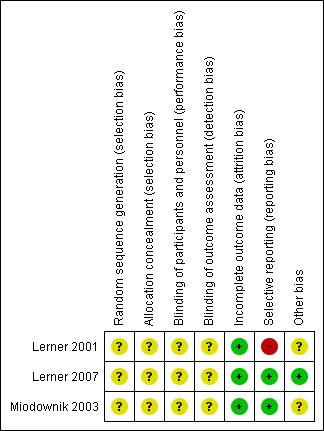
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included studies were described as randomised placebo‐controlled studies. However, none of the included studies described a specific method of randomisation or of allocation concealment, making allocation bias to be unclear.
Blinding
Although the included studies were described as double‐blind, placebo‐controlled studies, none explicitly described how this was undertaken and how the investigators, participants and outcome raters were blinded.
Incomplete outcome data
All participants randomised at the beginning of the three studies included were accounted for, making their attrition bias to be low.
Selective reporting
Two of the included studies (Lerner 2007; Miodownik 2003) accounted for all outcomes at the study completion. Lerner 2001 did not account for all outcomes with the specific number of participants who showed none, minimal, moderate or marked improvement in their tardive dyskinesia symptoms from both arms of the study not reported by this study, making the risk of bias to be high. Specific outcomes such as the number of participants who withdrew due to side effects were not accounted for by the second included study (Lerner 2001), thus making the risk of bias to be high. Also, due to inadequate reporting, it was difficult to ascertain from which arm of the study the numbers of participants who left the study early came from. For Lerner 2001, using a flow chart would have been an appropriate measure to accurately reflect these numbers.
Other potential sources of bias
1. Funding
Only one of the included studies reported financial sponsorship from a research institution (Lerner 2007). It was not clear if the other studies (Lerner 2001; Miodownik 2003) received funding from any sponsor.
2. Plasma level of pyridoxal 5 phosphate
The three studies included in this review did not mention who measured the plasma level of pyridoxal 5 phosphate and did not describe the extent to which the raters were independent.
Effects of interventions
Although three studies were included in this review, in some instances the data reported by Miodownik 2003 were not useable, hence only outcomes from the other two included studies (Lerner 2001; Lerner 2007) were reported.
1. COMPARISON: Pyridoxal 5 phosphate (vitamin B6) versus placebo
1.1 Clinical efficacy (Extrapyramidal Symptom Rating Scale (ESRS), high = worse)
1.1.1 Improvement in tardive dyskinesia symptoms from baseline
Lerner 2001 reported a 68.6% (SD 23%) reduction in ESRS symptoms from baseline to week four among participants taking vitamin B6, indicating marked improvement. In Lerner 2007, 15 (65%) of the 23 participants treated with vitamin B6 demonstrated > 40% reduction in their ESRS scores from baseline to week 12, indicating moderate to marked improvement. No clinical response to placebo was observed among participants in both studies, implying that placebo failed to produce not more than 20% reduction in ESRS scores from baseline to either week four or week 12 (Lerner 2001; Lerner 2007).The pooled summary result showed that pyridoxal 5 phosphate was clinically efficacious compared to placebo in reducing tardive dyskinesia symptoms (2 RCTs, n = 65, risk ratio (RR) 19.97, 95% confidence interval (CI) 2.87 to 139.19).
1.2 Global
1.2.1 Death due to suicide or other causes
None of the included studies reported any data on this outcome.
1.2.2 Average endpoint dose of pyridoxal 5 phosphate
Lerner 2001 and Miodownik 2003 reported an endpoint vitamin B6 dose of 400 mg daily, while Lerner 2007 reported an endpoint vitamin B6 dose of 1200 mg daily.
1.2.3 Other adverse effects than tardive dyskinesia
Adverse effects other than the deterioration of tardive dyskinesia symptoms were reported in two of the included studies (Lerner 2001; Lerner 2007). There was no significant difference in adverse effects reported by participants on pyridoxal 5 phosphate when compared with those on placebo (2 RCTs, n=65, RR 3.97, 95% CI 0.20 to 78.59).
1.2.4 Discontinuation of treatment
Lerner 2007 reported 5 participants on pyridoxal 5 phosphate who discontinued their medications because they were unwilling to take additional medications. None of the participants in Lerner 2001 study discontinued their treatment. The pooled summary estimate did not show any significant difference between participants on pyridoxal 5 phosphate who discontinued their medications compared to those on placebo. (2 RCTs, n= 65, RR 8.72, 95% CI 0.51 to 149.75). The time to discontinuation of pyridoxal 5 phosphate was not stated.
1.3 Tardive dyskinesia (ESRS, high = worse)
1.3.1 Deterioration in tardive dyskinesia symptoms
Lerner 2007 reported deterioration of tardive dyskinesia symptoms in two study participants on placebo. However, the pooled summary did not show any significant difference in deterioration of tardive dyskinesia symptoms in those on placebo compared to those on pyridoxal 5 phosphate (2 RCTS, n = 65, RR 0.16, 95% CI 0.01 to 3.14).
1.3.2 Average endpoint ESRS tardive dyskinesia score
The pooled summary result from two of the included studies (Lerner 2001; Lerner 2007) showed a beneficial effect of pyridoxal 5 phosphate when compared with placebo (2 RCTs, n = 60, mean difference (MD) ‐4.07, 95% CI ‐6.36 to ‐1.79). Miodownik 2003 reported the endpoint ESRS tardive dyskinesia scores on an unscaled graph, thereby making the data unable to be used.
1.4 General mental state changes (Positive and Negative Symptoms Scale (PANSS), high = worse)
1.4.1 Deterioration in psychiatric symptoms (e.g. hallucinations and delusions)
None of the included studies reported data on this outcome
1.4.2 Average endpoint PANSS psychiatric symptoms score
One of the included studies (Miodownik 2003) reported average endpoint psychiatric symptoms scores for participants on vitamin B6 and placebo, assessed with the PANSS. The pooled summary result for positive symptoms of schizophrenia did not show any significant differences between participants on vitamin B6 compared to those on placebo (1 RCT, n = 15, MD ‐1.50, CI ‐4.80 to 1.80). The pooled summary estimate showed no significant improvements in negative symptoms of schizophrenia (such as social withdrawal, lack of motivation) among participants on vitamin B6 compared to those on placebo (1 RCT, n = 15, MD ‐1.10, 95% CI ‐5.92 to 3.72).
Discussion
Summary of main results
The aim of this review was to determine the effectiveness of pyridoxal 5 phosphate (vitamin B6 or Pyridoxine or Pyridoxal phosphate) in the treatment of neuroleptic‐induced tardive dyskinesia among patients with schizophrenia and other related psychotic disorders. The review included all relevant randomised controlled trials obtained from the Cochrane Schizophrenia Group's Trial Register as at January 2013.
However, it should be noted that there are few studies included in this review, the size of the studies is small and all outcomes of interest were rated as 'low' quality evidence (also see summary of findings Table for the main comparison).
Evidence from the three available studies in this review showed that pyridoxal 5 phosphate may be superior to placebo in reducing tardive dyskinesia symptoms among patients with schizophrenia on antipsychotic medications. The number of participants on pyridoxal 5 phosphate who had > 40% improvement in their tardive dyskinesia symptoms at the end of the study was significantly higher than those on placebo. However, its effect for other outcomes of interest are unclear. There was no significant difference in other adverse effects experienced by those on pyridoxal 5 phosphate compared to those on placebo and pyridoxal 5 phosphate did not cause significant worsening or improvement of psychiatric symptoms among study participants.
Overall completeness and applicability of evidence
The sample size of trials and the number of participants included in this review are small, furthermore the duration of follow‐up is short, thereby making generalisation of the evidence obtained on the efficacy of pyridoxal 5 phosphate compared with placebo in the treatment of tardive dyskinesia difficult and limited.
Quality of the evidence
There is a dearth of studies on the effectiveness of pyridoxal 5 phosphate in the treatment of tardive dyskinesia. The quality of evidence for the efficacy of pyridoxal 5 phosphate provided by the studies included in this review is low. This review evaluated evidence from three studies with a total of 80 participants, assessed over a period of nine to 26 weeks in a cross‐over study design. The quality of evidence from future studies could be significantly improved with larger sample sizes to increase their power, as well as longer duration of follow‐up for study participants, especially since tardive dyskinesia symptoms often run a fluctuating course (APA 1992). In addition, adherence to guidelines for reporting randomised controlled trials that emphasise the explicit description of the processes of randomisation, allocation concealment and blinding etc could help improve the quality of future studies on this subject. Such reporting guidelines include the CONSORT (Consolidated Standards of Reporting Trials) statement (Moher 2001). The evidence provided by the studies included in this review should be interpreted in the light of the limitations mentioned above.
Potential biases in the review process
No known potential biases in the review process were identified by the authors of this review.
Agreements and disagreements with other studies or reviews
A recent systematic review presented as a conference proceeding, comprising 105 randomised controlled trials which assessed the evidence for various natural medicines in reducing antipsychotics side effects in schizophrenia, reported vitamin B6 to be effective in reducing medication‐induced tardive dyskinesia (Hoenders 2014). However, no quantitative data were reported in the abstract of the systematic review and no full text of the review is available yet. To the best knowledge of the authors of this review, there are no other reviews to which the findings of this review could be compared.
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline.
| Study | |
| Lerner 2001 | 400 mg daily |
| Lerner 2007 | 1200 mg daily |
| Miodownik 2003 | 400 mg daily |
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 3 Global: 2. Other adverse effects than tardive dyskinesia.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 4 Global: 3. Discontinuation of Vitamin B6.
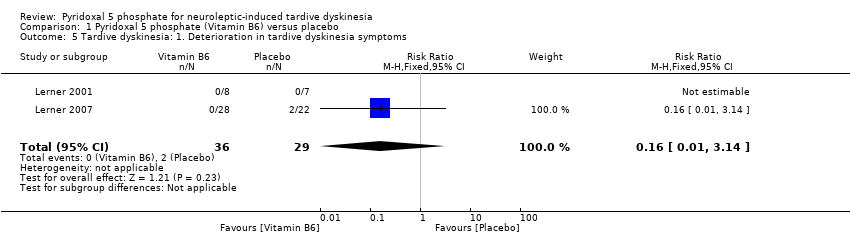
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms.
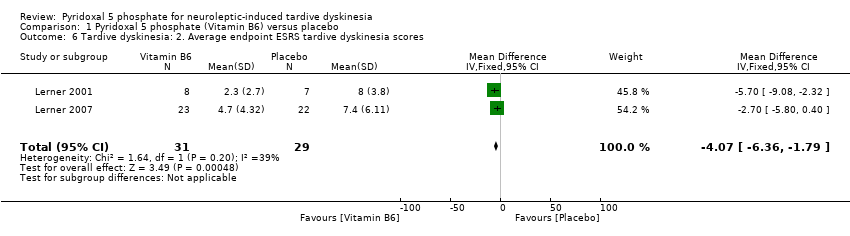
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores.
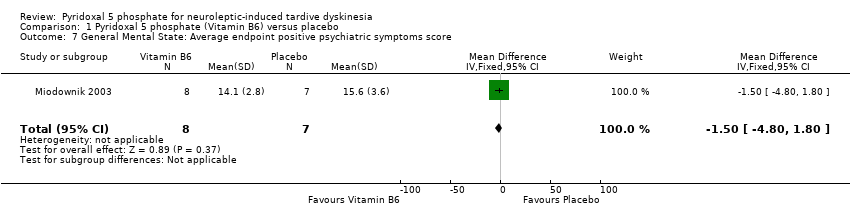
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 7 General Mental State: Average endpoint positive psychiatric symptoms score.
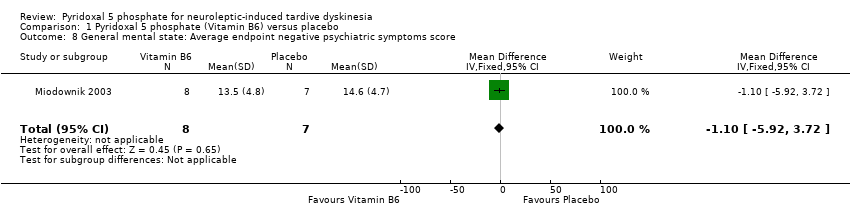
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 8 General mental state: Average endpoint negative psychiatric symptoms score.
| Method | Allocation: randomised ‐ clearly described generation of sequence and concealment of allocation. Design: Single phase, longer study duration. |
| Participants | People with schizophrenia or schizophrenia‐like disorder. History: History of tardive dyskinesia, fulfilling diagnostic criteria for tardive dyskinesia, stable on antipsychotic medication for at least 3 months. |
| Intervention | 1.Pyridoxal Phosphate (vitamin B6), any dose 2. Placebo |
| Outcomes | Tardive dyskinesia scores measured using AIMS (primary outcome) Deterioration of tardive dyskinesia symptoms Any other adverse effects Discontinuation of pyridoxal phosphate (with reasons) Psychiatric symptoms score using a standardised rating scale (PANSS, BPRS) Pyridoxal phosphate dose Plasma pyridoxal phosphate level Quality of life Satisfaction with care |
| AIMS: Abnormal Involuntary Movement Scale | |
| Pyridoxal 5 phosphate (vitamin B6) compared with Placebo for neuroleptic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with neuroleptic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pyridoxal 5 phosphate (vitamin B6) | |||||
| Clinical efficacy ‐ improvement (> 40%) in ESRS scores from baseline | Study population | RR 19.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Other adverse effects than tardive dyskinesia | Study population | RR 3.97 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Global: Discontinuation of Vitamin B6 | Study population | RR 8.72 | 65 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Tardive dyskinesia: Deterioration in tardive dyskinesia symptoms | Study population | RR 0.16 | 65 | ⊕⊕⊝⊝ | ||
| 69 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 46 per 1000 | 7 per 1000 | |||||
| General mental state: Positive psychiatric symptom score | The mean general mental state: positive psychiatric symptom score in the control groups was | The mean general mental state: positive psychiatric symptom score in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| General mental state: Negative psychiatric symptoms | The mean general mental state: negative psychiatric symptoms in the control groups was | The mean general mental state: negative psychiatric symptoms in the intervention groups was | 15 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Extrapyramidal Symptom Rating Scale (high scores = worse) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.97 [2.87, 139.19] |
| 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate Show forest plot | Other data | No numeric data | ||
| 3 Global: 2. Other adverse effects than tardive dyskinesia Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.20, 78.59] |
| 4 Global: 3. Discontinuation of Vitamin B6 Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.72 [0.51, 149.75] |
| 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms Show forest plot | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.14] |
| 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐6.36, ‐1.79] |
| 7 General Mental State: Average endpoint positive psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐4.80, 1.80] |
| 8 General mental state: Average endpoint negative psychiatric symptoms score Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.92, 3.72] |

