Intervenciones para tratar la infección genital por Chlamydia trachomatis en el embarazo
Resumen
Antecedentes
La infección genital por Chlamydia trachomatis (C. trachomatis) puede provocar complicaciones en el embarazo como aborto espontáneo, trabajo de parto prematuro, bajo peso al nacer, rotura prematura de las membranas, aumento de la mortalidad perinatal, endometritis posparto, y conjuntivitis y neumonía por C. trachomatis. Esta revisión reemplaza una revisión anterior sobre este tema.
Objetivos
Establecer el tratamiento más efectivo y mejor tolerado para el tratamiento de la infección por Chlamydia genital para prevenir la infección materna y los resultados neonatales adversos.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) ClinicalTrials.gov, en la WHO International Clinical Trials Registry Platform (ICTRP) (26 junio 2017) y en listas de referencias de estudios recuperados.
Criterios de selección
Ensayos controlados aleatorios (ECA), así como estudios publicados en forma de resumen, que evaluaron intervenciones para el tratamiento de la infección genital por C. trachomatis en el embarazo. Se consideraron elegibles para inclusión los ECA grupales, aunque no se identificaron ensayos de este tipo. Los ensayos cuasialeatorios y los ensayos de diseño cruzado no fueron aptos para la inclusión en esta revisión.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los estudios para su inclusión, evaluaron la calidad de los ensayos y extrajeron los datos utilizando el formulario acordado. Se verificó la exactitud de los datos. La evidencia se evaluó mediante el enfoque GRADE.
Resultados principales
Se incluyeron 15 ensayos (con 1754 mujeres) aunque los metanálisis se basaron en menos números de estudios/mujeres. Todos los estudios incluidos se realizaron en Norteamérica entre 1982 y 2001. El riesgo de sesgo fue bajo en dos estudios en todos los dominios; en los otros estudios el riesgo fue variable. Se excluyeron otros cuatro estudios y un estudio está en curso.
Se incluyeron ocho comparaciones en esta revisión; tres compararon antibióticos (eritromicina, clindamicina, amoxicilina) versus placebo; cinco compararon un antibiótico versus otro antibiótico (eritromicina, clindamicina, amoxicilina, azitromicina). Ningún estudio informó regímenes diferentes de antibióticos.
Antibióticos versus placebo: la eritromicina (cociente de riesgos [CR] promedio 2,64; intervalo de confianza [IC] del 95%: 1,60 a 4,38; dos ensayos, 495 mujeres; I2 = 68%; evidencia de confiabilidad moderada) y la clindamicina (CR 4,08; IC del 95%: 2,35 a 7,08; un ensayo, 85 mujeres; evidencia de baja confiabilidad) se asociaron con mejor curación microbiológica en comparación con un control placebo. En un ensayo muy pequeño que comparó amoxicilina y placebo los resultados no estuvieron claros, pero la evidencia se consideró muy baja (CR 2,00; IC del 95%: 0,59 a 6,79; 15 mujeres).
Un antibiótico versus otro antibiótico: la amoxicilina logró poco o ningún cambio en la curación microbiológica en comparación con la eritromicina (CR 0,97; IC del 95%: 0,93 a 1,01; cuatro ensayos, 466 mujeres; evidencia de alta confiabilidad) y probablemente no hubo diferencias en comparación con la clindamicina (CR 0,96; IC del 95%: 0,89 a 1,04; un ensayo, 101 mujeres; evidencia de confiabilidad moderada) y la evidencia fue demuy baja confiabilidad cuando se comparó con la azitromicina, por lo que el efecto no es seguro (CR 0,89; IC del 95%: 0,71 a 1,12; dos ensayos, 144 mujeres; evidencia de muy baja confiabilidad). Al comparar la azitromicina versus la eritromicina (CR promedio 1,11; IC del 95%: 1,00 a 1,23; seis ensayos, 374 mujeres; I2 = 53%; evidencia de confiabilidad moderada) probablemente tuvieron una eficacia similar, aunque los resultados parecen favorecer a la azitromicina. La clindamicina versus la eritromicina (CR 1,06; IC del 95%: 0,97 a 1,15; dos ensayos, 173 mujeres; evidencia de baja confiabilidad) pueden dar lugar a un número similar de pacientes con curación microbiológica entre los grupos.
La evidencia se disminuyó debido a limitaciones en cuanto al diseño de los estudios y a la imprecisión en las estimaciones del efecto.
Antibióticos versus placebo: los efectos secundarios que incluyeron las náuseas, los vómitos y el dolor abdominal se informaron en dos estudios (495 mujeres), pero no hubo evidencia clara con respecto a si la eritromicina se asoció con más efectos secundarios que placebo y se observó un nivel alto de heterogeneidad (I2 = 78%) (CR promedio 2,93; IC del 95%: 0,36 a 23,76). Cuando la clindamicina se comparó con placebo en un estudio pequeño no hubo diferencias claras en el número de pacientes que presentaron efectos secundarios (5/41 versus 1/44) (CR 6,35; IC del 95%: 0,38 a 107,45; 62 mujeres). Los efectos secundarios informados fueron principalmente gastrointestinales y también incluyeron erupciones cutáneas que se resolvieron.
Un antibiótico versus otro antibiótico: no hubo diferencias claras en la incidencia de efectos secundarios (que incluyeron náuseas, vómitos, diarrea y dolor abdominal) cuando la amoxicilina se comparó con la azitromicina según los datos de un estudio pequeño (36 mujeres) (CR 0,56; IC del 95%: 0,24 a 1,31).
Sin embargo, la amoxicilina se asoció con menos efectos secundarios en comparación con la eritromicina según los datos de cuatro ensayos (513 mujeres) (CR 0,31; IC del 95%: 0,21 a 0,46; I2 = 27%). Los efectos secundarios fueron: náuseas, vómitos, diarrea, cólicos abdominales, erupción cutánea y reacción alérgica.
La azitromicina (CR 0,24; IC del 95%: 0,17 a 0,34; seis ensayos, 374 mujeres) y la clindamicina (CR 0,44; IC del 95%: 0,22 a 0,87; dos ensayos, 183 mujeres) se asociaron con una incidencia inferior de efectos secundarios en comparación con la eritromicina. Estos efectos secundarios incluyeron náuseas, vómitos, diarrea y cólicos abdominales.
Un estudio pequeño (101 mujeres) informó que no hubo diferencias claras en el número de pacientes con efectos secundarios cuando la amoxicilina se comparó con la clindamicina (CR 0,57; IC del 95%: 0,14 a 2,26; 107 mujeres). Los efectos secundarios informados incluyeron erupción cutánea y molestias gastrointestinales.
Los ensayos individuales informaron datos sobre la repetición de las infecciones, el parto prematuro, la rotura prematura de las membranas, la mortalidad perinatal y el bajo peso al nacer y no encontraron diferencias claras entre los tratamientos.
Muchos de los resultados secundarios de esta revisión no se informaron en los estudios incluidos.
Conclusiones de los autores
El tratamiento con agentes antibacterianos logra la curación microbiológica de la infección por C. trachomatis durante el embarazo. No hubo diferencias evidentes entre los agentes evaluados (amoxicilina, eritromicina, clindamicina, azitromicina) en cuanto a la eficacia (curación microbiológica y repetición de la infección) y las complicaciones en el embarazo (parto prematuro, rotura prematura de las membranas, bajo peso al nacer). La azitromicina y la clindamicina parecen provocar menos efectos secundarios que la eritromicina.
Todos los estudios de esta revisión se realizaron en Norteamérica, lo que puede limitar la generalizabilidad de los resultados. Además, las poblaciones de estudio pueden diferir en ámbitos de bajos recursos y, por lo tanto, estos resultados solamente son aplicables a ámbitos de buenos recursos. Además, los ensayos de esta revisión se realizaron principalmente en los años noventa y antes de los años 2000: la resistencia a los antibióticos puede haber cambiado desde entonces.
Se requieren aún más estudios bien diseñados, con tamaños apropiados de la muestra y en diversos ámbitos para evaluar de forma adicional las intervenciones para el tratamiento de la infección por C. trachomatis en el embarazo y determinar los agentes que logran una mejor curación microbiológica con efectos secundarios mínimos. Dichos estudios podrían brindar información sobre los resultados enumerados en esta revisión.
PICO
Resumen en términos sencillos
Tratamiento de la infección genital por Chlamydia trachomatis en el embarazo
¿Cuál es el problema?
Esta revisión intentó evaluar si el tratamiento de la infección por Chlamydia durante el embarazo curó la infección y evitó las complicaciones en las mujeres y los recién nacidos, sin provocar efectos secundarios. Esta nueva revisión reemplaza una revisión anterior sobre este tema.
¿Por qué es esto importante?
La Chlamydia trachomatis es una infección bacteriana de transmisión sexual. Es más común en las mujeres más jóvenes. Las mujeres pueden tener la infección sin saberlo. En las embarazadas, la Chlamydia trachomatis genital puede provocar complicaciones en el embarazo como el trabajo de parto prematuro, el parto prematuro, la rotura prematura de las membranas, el bajo peso al nacer de los lactantes y la infección en el útero después del parto. Los recién nacidos que contraen la Chlamydia trachomatis durante el nacimiento pueden desarrollar infección en los pulmones y los ojos.
Encontrar un tratamiento efectivo con efectos secundarios mínimos es muy importante si se consideran las complicaciones que pueden ocurrir con la infección por Chlamydia trachomatis sin tratar en el embarazo.
¿Qué evidencia se encontró?
Se buscó evidencia (junio 2017) y se incluyeron 15 estudios en la revisión. Los estudios tuvieron un riesgo mixto de sesgo y fueron de calidad limitada, a menudo con un escaso número de participantes. Tres estudios compararon antibióticos (eritromicina, clindamicina y amoxicilina) con placebo. Los otros estudios compararon diferentes antibióticos entre sí.
Todos los estudios informaron la curación de la Chlamydia, sobre la base de la eliminación de las bacterias, con un antibiótico. La eritromicina (evidencia de calidad moderada a partir de dos estudios, 495 mujeres) y la clindamicina (evidencia de baja calidad a partir de un estudio, 85 mujeres) parecieron ser más efectivas que placebo. La calidad de la evidencia para la amoxicilina versus placebo (un estudio, 15 mujeres) fue muy baja, por lo que no es posible tener seguridad con respecto a los resultados.
Cuando se compararon diferentes antibióticos entre sí, ningún antibiótico fue significativamente mejor que otro para la curación de la Chlamydia en los estudios examinados: amoxicilina versus azitromicina (evidencia de muy baja calidad a partir de dos estudios, 144 mujeres), amoxicilina versus eritromicina (evidencia de alta calidad a partir de cuatro estudios, 466 mujeres), azitromicina versus eritromicina (evidencia de calidad moderada a partir de seis estudios, 374 mujeres), clindamicina versus eritromicina (evidencia de baja calidad a partir de dos estudios, 173 mujeres), amoxicilina versus clindamicina (evidencia de calidad moderada a partir de un estudio, 101 mujeres). Solamente ensayos individuales evaluaron la repetición de las infecciones, el parto prematuro, la rotura prematura de las membranas, la mortalidad perinatal y el bajo peso al nacer y no encontraron diferencias claras entre los diferentes tipos de antibióticos examinados.
Los efectos secundarios fueron más frecuentes con la eritromicina (dos estudios, 495 mujeres) y la clindamicina (un estudio, 85 mujeres) que con placebo. La amoxicilina provocó menos efectos secundarios que la azitromicina (un estudio, 36 mujeres) o la eritromicina (cuatro estudios, 513 mujeres) y la azitromicina causó menos efectos secundarios que la eritromicina (seis estudios, 374 mujeres). La amoxicilina y la clindamicina produjeron un número similar de efectos secundarios en un estudio (107 mujeres).
¿Qué significa esto?
El tratamiento con antibióticos de la infección por Chlamydia parece ser efectivo durante el embarazo. No hay una diferencia clara entre la amoxicilina, la eritromicina, la clindamicina y la azitromicina en la curación de la infección ni en el parto prematuro, la rotura prematura de las membranas y el bajo peso al nacer. La azitromicina y la clindamicina parecen provocar menos efectos secundarios que la eritromicina.
Los estudios incluidos se realizaron en América del Norte. El diagnóstico de la Chlamydia todavía es un problema en ámbitos de bajos recursos debido a los costos. Se concluye que se necesitan estudios bien diseñados con un tamaño de la muestra apropiado, realizados en ámbitos diferentes, para evaluar aún más los efectos del tratamiento de la infección por Chlamydia en el embarazo. La resistencia a los antibióticos estudiados podría haber cambiado desde que se realizaron los estudios incluidos en esta revisión. En concreto, los estudios de investigación futuros podrían informar sobre resultados relacionados con el objetivo de esta revisión y orientarse a los antibióticos, como la amoxicilina y la clindamicina, que pueden ser efectivos para la curación de la Chlamydia con efectos secundarios mínimos.
Authors' conclusions
Summary of findings
| Erythromycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Erythromycin | |||||
| Microbiological cure | Study population | Average RR 2.64 | 495 | ⊕⊕⊕⊝ | ||
| 344 per 1000 | 908 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Statistical Heterogeneity (I2 > 60%). (Inconsistency: ‐1) 2 One included study has design limitations but contributed < 40% weight. (Not downgraded) | ||||||
| Clindamycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 4.08 | 85 | ⊕⊕⊝⊝ | ||
| 227 per 1000 | 927 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The included study had design limitation (Design limitations: ‐1) 2 Wide confidence interval and small sample size (Imprecision: ‐1) | ||||||
| Amoxicillin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 2.00 | 15 | ⊕⊝⊝⊝ | ||
| 333 per 1000 | 667 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The included study had design limitation (Design limitations: ‐1) 2 Wide confidence intervals crossing the line of no effect, few events, and small sample size (Imprecision: ‐2) | ||||||
| Amoxicillin compared to azithromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with azithromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.89 | 144 | ⊕⊝⊝⊝ | ||
| 716 per 1000 | 637 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study contributing to over 68% of weight to pooled analysis had some design limitations (Design limitations: ‐1) 2 Wide confidence intervals crossing the line of no effect and small size (Imprecision: ‐2) | ||||||
| Amoxicillin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.97 | 466 | ⊕⊕⊕⊕ | One study contributing to 24% of weight had some design limitation. (not downgraded) | |
| 954 per 1000 | 925 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Azithromycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Azithromycin | |||||
| Microbiological cure | Study population | Average RR 1.11 | 374 | ⊕⊕⊕⊝ | ||
| 825 per 1000 | 916 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies have design limitations (Design limitations: ‐1) 2 Statistical heterogeneity at 53% (I2 < 60%) (not downgraded) | ||||||
| Clindamycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 1.06 | 173 | ⊕⊕⊝⊝ | ||
| 905 per 1000 | 959 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study contributing to over 40% of weight to pooled analysis had some design limitations (Design limitations: ‐1) 2 Small sample size (Imprecision: ‐1) | ||||||
| Amoxicillin compared to clindamycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with clindamycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.96 | 101 | ⊕⊕⊕⊝ | ||
| 979 per 1000 | 940 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The pooled effect was based on one study with a small sample size (Imprecision: ‐1) | ||||||
Background
The prevalence of chlamydial infection in pregnancy is between 2% to 30% depending on the patient's age and risk factors (Berggren 2011; Much 1991). It is particularly common in women younger than 25 years of age (Walker 2012). Genital Chlamydia trachomatis (C.trachomatis) infection has been shown to be associated with pregnancy complications such as miscarriage (Nigro 2011), preterm labour (Pararas 2006; Rours 2011), low birthweight (Attenburrow 1985) and increased perinatal mortality (Silva 2011). There may also be an association with preterm rupture of membranes (Blas 2007) and postpartum endometritis (Ismail 1987). If the mother is untreated, 20% to 50% of newborn babies may develop chlamydial conjunctivitis (Kakar 2010), and another 10% to 20% may develop C.trachomatis pneumonia (Rours 2009). Vaginal birth is associated with the highest risk of transmission of chlamydial infection, however, there is a small risk of acquiring the infection even in infants born by caesarean section with premature rupture of membranes and intact membranes (Pammi 2012; Yu 2009).
Genital C.trachomatis infection is detected by nucleic acid amplification test (NAAT) on the specimens of genital secretions or urine. This test has replaced tissue culture of C.trachomatis (Jespersen 2005).
Description of the condition
Genital C.trachomatis infection is a common bacterial sexually transmitted infection. The majority of women infected with this bacteria are asymptomatic and, therefore, may be more likely to transmit the infection because they do not seek treatment for the infection, which may result in a longer duration of the infection. The sequelae of C.trachomatis genital infection range from cervicitis to pelvic inflammatory disease, perihepatitis, ectopic pregnancy and infertility (Zenilman 2012). We have described complications of pregnancy and diseases of newborn related to genital Chlamydia infection in the Background section above.
C.trachomatis is a small gram‐negative intracellular bacterium with a two‐phased life‐cycle, which includes the form that infects new cells, (e.g. the small elementary body) and the active form (e.g. the reticulate body). The life‐cycle is about two to three days, and, therefore, sustained high serum minimum inhibitory concentration of antimicrobial agents is needed to achieve eradication of the infection, which can be achieved by long‐acting antimicrobials treatment or prolonged treatment. The incubation period of C.trachomatis infection varies between seven and 14 days (Zenilman 2012).
Description of the intervention
There are various treatment regimens for the management of chlamydial infection during pregnancy, however, there is no consensus on the most effective and safest option. In some, the hosts' immune system may even clear the infection.
According to the Centers for Disease Control and Prevention (CDC) guideline followed by many countries around the world, the recommended regimens for treatment of genital chlamydial infection in pregnancy are azithromycin (1 g orally given as a single dose) or amoxicillin (500 mg orally three times daily for seven days) (Workowski 2010). The alternative regimen according to the CDC guideline is erythromycin (500 mg or 250 mg orally four times daily for seven days), or erythromycin ethylsuccinate (800 mg orally four times daily for seven days, or 400 mg orally four times daily for 14 days) (Workowski 2010). Erythromycin is associated with a high degree of gastrointestinal side effects (primarily nausea) and the compliance may be an issue in such cases (Workowski 2010).
Women who present in labour but were not treated for a prior positive chlamydial test are advised to be treated immediately with one of the above regimens. However, such late treatment is unlikely to substantially decrease the risk of transmission of Chlamydia to the newborn.
Clindamycin is another alternative drug for treatment of genital C.trachomatis infection. Despite it being safe in pregnancy, clindamycin is not used widely due to its cost (Miller 2000).
Other antibiotics such as doxycycline, levofloxacin, ofloxacin, and erythromycin estolate are used for the treatment of genital C.trachomatis outside of pregnancy. These drugs are contraindicated in pregnancy and lactation (Workowski 2010).
Azithromycin is believed to be the superior agent in comparison to other antibiotics for treatment of chlamydial infection but new research has emerged suggesting that there is a higher failure rate with azithromycin treatment of chlamydial infection than previously believed (Schwebke 2011). One of the explanations for this recent finding is a higher sensitivity of NAAT in comparison to that previously used in the tissue culture as a test of cure (Handsfield 2011), although it does not explain the similar cure rates reported after doxycycline treatment with both of these tests. Another explanation for treatment failure is heterotopic resistance with high Chlamydia loads which leads to treatment failures (Horner 2006). Re‐infection is also a cause of treatment failure (Horner 2006).
Cure rates of C.trachomatis in women who are pregnant are lower than in non‐pregnant women. The reasons behind this is a generally higher failure rate of treatment with amoxicillin, which has been traditionally used for treatment of C.trachomatis infection during pregnancy. A test of cure has always been recommended for all pregnant women and is performed no earlier than three weeks after treatment is initiated (Workowski 2010).
The previous Cochrane review on interventions for treating genital C.trachomatis infection in pregnancy found that amoxicillin was as effective as erythromycin (odds ratio (OR) 0.54, 95% confidence interval (CI) 0.28 to 1.02) (Brocklehurst 1998). Amoxycillin was found to be better tolerated than erythromycin (OR 0.16, 95% CI 0.09 to 0.30). Clindamycin and azithromycin were reported to be effective, however, the numbers of women included in trials were small (Brocklehurst 1998). New studies have been published in this area, therefore, it is important to update this review, which was done under new authorship.
How the intervention might work
Irradicating genital chlamydial infection during pregnancy with antibacterial drugs may lead to the following:
-
treatment of symptoms and sequelae of genital chlamydial infection such as discharge, cervicitis, pelvic inflammatory disease, tubal disease and infertility;
-
a decrease in perinatal complications such as preterm labour and early pregnancy loss, preterm rupture of membranes;
-
a decrease in transmission of the infection to the fetus or newborn and, therefore, prevention of intrauterine infection, neonatal conjunctivitis and pneumonia during pregnancy;
-
prevention of postpartum infection such as endometritis.
Why it is important to do this review
It is important to assess the different interventions for treating genital C.trachomatis in order to establish whether effective treatment of this infection improves perinatal outcomes and decreases maternal complications. This new review updates and replaces an earlier Cochrane review on this topic (Brocklehurst 1998).
Objectives
To establish the most efficacious and best‐tolerated therapy for treatment of genital chlamydial infection in preventing maternal infection and adverse neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials. Cluster‐randomised trials will be eligible for inclusion in this review in the future updates if identified. Quasi‐randomised trials and trials using cross‐over design were not eligible for inclusion. We included studies published in abstract form.
Types of participants
Pregnant women with a confirmed C.trachomatis infection.
Types of interventions
-
Any antibiotic versus no treatment or placebo for genital C.trachomatiss infection in pregnancy
-
One antibiotic versus another antibiotic
-
Different antibacterial regimens
Types of outcome measures
Primary outcomes
-
Microbiological cure ‐ negative Chlamydia test at least three weeks after treatment of the mother
Secondary outcomes
A. Maternal
-
Repeated infection
-
Preterm labour
-
Preterm birth
-
Preterm rupture of membranes
-
Chorioamnionitis
-
Postpartum endometritis
-
Sepsis
-
Prolonged hospital stay of the mother
-
Side effects of treatment
-
Maternal satisfaction with treatment
B. Fetal/neonatal
-
Perinatal mortality
-
Neonatal conjunctivitis
-
Neonatal pneumonia
-
Fetal anomalies
-
Low birthweight
-
Apgar score less than seven at five minutes
C. Cost
-
Cost of treatment
Search methods for identification of studies
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (26 June 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies)
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (26 June 2017) for unpublished, planned and ongoing trial reports. The search terms we used are given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed all the potential studies identified as a result of the search strategy for inclusion. Two review authors assessed the quality and extracted the data using the agreed form. Discrepancies were resolved through discussion with a third review author when needed. We entered data into Review Manager software and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Studies published only in abstract form were included if they otherwise satisfied inclusion criteria. The authors of such studies were contacted if any additional information was required.
Data extraction and management
We designed a form to extract data. For eligible studies, review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third assessor. We entered data into Review Manager software (RevMan 2014), and checked for accuracy.
When information regarding any of the above is unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
A cut‐off point of 20% was used to assess the level of missing data as adequate for different outcomes.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update , we assessed the quality of the evidence for all comparisons using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the main outcome of microbiological cure.
We used GRADEpro GDT to import data from Review Manager 5.3 (RevMan 2014) to create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this version of the review. If we identify any cluster‐randomised trials for inclusion in future updates, we will include them in our analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the studies and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will acknowledged heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This study design is not eligible for inclusion in this review.
Other unit of analysis issues
We identified the trials with more than two treatment groups and included each pair‐wise comparison separately, but with shared intervention groups divided out approximately evenly among the comparisons. For dichotomous outcomes, both the number of events and the total number of patients were divided up. For continuous outcomes, only the total number of participants were divided up and the means and standard deviations left unchanged (Cochrane Handbook for Systematic Reviews of Interventions 16.5.4).
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis (see Sensitivity analysis).
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
We would have excluded studies with more than 20% missing data.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots and will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out ant of the planned subgroup analyses as the outcomes only had a few included trials. In future updates if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We would consider carrying out the following subgroup analyses.
-
Women with a first episode versus women with recurrent (previously treated in pregnancy) genital C.trachomatis infection
-
Women in the first half (before 20 weeks) versus women in the second half (including 20 weeks and after 20 weeks) of pregnancy
The following outcome would be used in subgroup analysis.
-
Microbiological cure negative Chlamydia test after treatment for the mother
We would have assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We would have reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Sensitivity analyses were not performed as there were no aspects of the review that may have affected the results, for example, the risk of bias associated with the quality of some of the included trials. We would have undertaken analysis of the primary outcome separately for trials with low risk of bias and high and unknown risk of bias (allocation concealment) if needed. Sensitivity analysis would have been carried out to explore the effects of random‐effects analyses for outcomes with statistical heterogeneity. We would also have carried out sensitivity analysis to investigate the effect of the randomisation unit if we had included cluster‐randomised controlled trials along with the individually‐randomised trials.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved 23 reports of 20 trials and we retrieved no other studies from other sources (see: Figure 1). We included 15 studies, excluded four, and one is ongoing (Okunola 2013).

Study flow diagram.
Included studies
We included 15 studies into the meta‐analysis with a total of 1754 women. Meta‐analyses were mostly based on fewer numbers of studies.
Methods
All the trials were randomised control trials of pregnant women with confirmed Chlamydia trachomatis (C.trachomatis) infection.
Populations and settings
All of the included studies were undertaken in North America (14 in USA and one in Canada). One study took place in 1982, and the rest took place in the nineties and early 2000s.
Interventions and comparisons
Two studies compared erythromycin and placebo (Alger 1991; Martin 1997). One study compared clindamycin and placebo (Alger 1991). One study compared amoxicillin versus placebo (Bell 1982). Two studies compared azithromycin and amoxicillin (Jacobson 2001; Kacmar 2001). Four studies compared amoxicillin and erythromycin (Alary 1994; Magat 1993; Silverman 1994; Turrentine 1995). Six studies compared erythromycin and azithromycin (Adair 1998; Bush 1994; Edwards 1996; Gunter 1996; Rosenn 1995; Wehbeh 1998). Two studies compared clindamycin and erythromycin (Alger 1991; Turrentine 1995). One study compared amoxicillin and clindamycin (Turrentine 1995).
Funding sources
Adair 1998, Edwards 1996, and Turrentine 1995 had drugs donated by a pharmaceutical company at no cost. Alger 1991 was funded by a grant from the Upjohn company.
Alary 1994 was funded by a grant from the National Health Research and Development Program. Kacmar 2001 was funded by a NIH grant. Martin 1997 was funded by a National Institute of Child Health and Human Development grant. Bell 1982 was supported by a US Public Health Service grant.
Wehbeh 1998 was funded by local departmental funds.
Bush 1994, Gunter 1996, Jacobson 2001, Magat 1993, Rosenn 1995, and Silverman 1994 did not disclose any funding sources.
Trial authors' declarations of interest
Declarations of interest were not mentioned in any of the included studies.
Excluded studies
Reasons for exclusion are as follows.
-
El‐Shourbagy 2011 ‐ this study examines the rate of pre‐eclampsia in groups of treated and non‐treated Chlamydia pneumoniae infections in pregnancy.
-
McGregor 1990 ‐ this study included pregnant women with various genital tract infections and not only Chlamydia trachomatis. The data for Chlamydia trachomatis infection were not presented separately.
-
Nadafi 2005 ‐ this study included women with positive and negative Chlamydia test, it was a cohort study, sequence generation was not clear. The data for women with positive and negative Chlamydia test are presented together.
-
Zulkarneev 1998 ‐ this study was not a randomised controlled trial.
Risk of bias in included studies

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eleven studies had low risk of selection bias, e.g. three studies used number of blocks for allocation (Adair 1998; Alary 1994; Rosenn 1995), four studies used computer‐generated randomisation for allocation (Bush 1994; Jacobson 2001; Martin 1997; Turrentine 1995), four studies used random number tables (Edwards 1996; Kacmar 2001; Magat 1993; Silverman 1994). Four studies had unclear risk of selection bias, e.g. allocation method was not described (Alger 1991; Bell 1982; Gunter 1996; Wehbeh 1998).
Nine studies had a low risk of bias for allocation sequence. Six used sealed opaque envelopes (Adair 1998; Bush 1994; Jacobson 2001, Kacmar 2001; Rosenn 1995; Silverman 1994). One study used identical treatment packs (Alary 1994). In two trials the medications were dispensed by the pharmacy to prevent the healthcare practitioners knowing which medication and which dose were allocated (Magat 1993; Turrentine 1995). There was an unclear risk in six studies as allocation concealment was not described (Alger 1991; Bell 1982; Edwards 1996; Gunter 1996; Martin 1997; Wehbeh 1998).
Blinding
Performance bias
Blinding of participants and personnel was performed four studies (Alary 1994; Alger 1991; Martin 1997; Turrentine 1995).
Five studies did not implement blinding of participants or personnel and were assessed as high risk (Adair 1998; Edwards 1996; Jacobson 2001; Magat 1993; Wehbeh 1998).
Six studies did not describe performance blinding (Bell 1982; Bush 1994; Gunter 1996; Kacmar 2001; Rosenn 1995; Silverman 1994).
Assessment bias
Blinding of outcome assessment was unclear in 13 studies (Adair 1998; Alger 1991; Bell 1982; Bush 1994; Edwards 1996; Gunter 1996; Jacobson 2001; Kacmar 2001; Magat 1993; Martin 1997; Rosenn 1995; Silverman 1994; Wehbeh 1998).
Assessment bias was assessed as low risk in two studies were staff taking cultures were blinded to treatment group (Alary 1994; Turrentine 1995).
Incomplete outcome data
No studies had significant attrition bias. All losses to follow‐up were described. One study (Bell 1982) had high attrition for the final outcome reporting data for only 71% of participants. Five studies are at unclear risk of attrition bias due to insufficient information given in the study report (Gunter 1996), and some unexplained loss to follow‐up (Jacobson 2001; Kacmar 2001; Martin 1997; Silverman 1994).
Selective reporting
One study was published only in abstract form and states that it is an ongoing trial but no further information has been published (Gunter 1996). The remaining 14 studies were rated as being at low risk of reporting bias.
Other potential sources of bias
Two studies had unexplained different mean gestational ages in women in the two treatment arms (Edwards 1996; Magat 1993). The remaining 13 studies were assessed as being at a low risk of other bias.
Effects of interventions
See: Summary of findings for the main comparison Erythromycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy; Summary of findings 2 Clindamycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy; Summary of findings 3 Amoxicillin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy; Summary of findings 4 Amoxicillin compared to azithromycin for treating genital Chlamydia trachomatis infection in pregnancy; Summary of findings 5 Amoxicillin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy; Summary of findings 6 Azithromycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy; Summary of findings 7 Clindamycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy; Summary of findings 8 Amoxicillin compared to clindamycin for treating genital Chlamydia trachomatis infection in pregnancy
Erythromycin versus placebo (comparison 1)
Primary outcome
Microbiological cure
Erythromycin appears to improve microbiological cure in comparison to placebo (moderate‐certainty evidence, summary of findings Table for the main comparison; (average risk ratio (RR) 2.64, 95% confidence interval (CI) 1.60 to 4.38; 495 women; studies = two; I² = 68%; Analysis 1.1)). There was evidence of substantial heterogeneity between the studies (I² = 68%) in effect size; both studies found erythromycin improved microbiological cure.
Secondary outcomes
Preterm birth
There was no clear difference in preterm births (RR 0.90, 95% CI 0.56 to 1.46; 405 women; studies = one; Analysis 1.2).
Preterm rupture of membranes
There was no clear difference in preterm rupture membranes between the treatment groups (RR 0.83, 95% CI 0.48 to 1.43; 389 women; studies = one; Analysis 1.3).
Side effects of treatment
We are uncertain if erythromycin results in a higher incidence of side effects when compared to placebo (average RR 2.93, 95% CI 0.36 to 23.76; 495 women; studies = two; I² = 78%; Analysis 1.4). There was substantial heterogeneity between the studies, I² = 78%. The side effects reported included nausea, appetite loss (Martin 1997), vomiting, diarrhoea, and abdominal pain (Alger 1991).
Perinatal mortality
There was no clear difference in perinatal deaths between the groups (RR 3.01, 95% CI 0.32 to 28.74; 405 women; studies = one; Analysis 1.5).
Low birthweight
There was no clear difference in low birthweight between the groups (RR 0.77, 95% CI 0.42 to 1.40; 400 women; studies = one; Analysis 1.6).
Other secondary outcomes
No studies assessed the other secondary outcomes.
Clindamycin versus placebo (comparison 2)
Primary outcome
Microbiological cure
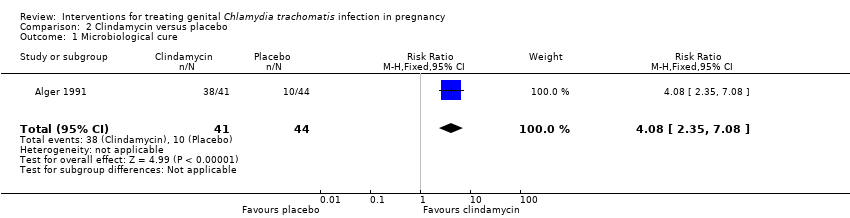
Clindamycin appears to improve microbiological cure in comparison to placebo (low‐certainty evidence, summary of findings Table 2; (RR 4.08, 95% CI 2.35 to 7.08; 85 women; studies = one; Analysis 2.1)). One study (Alger 1991), which was funded by a pharmaceutical company contributed to this comparison.
Secondary outcomes
Side effects of treatment
There was no clear difference in side effects between the two groups (RR 5.37, 95% CI 0.65 to 44.01; 85 women; studies = one; Analysis 2.2). The side effects included a rash and mild gastrointestinal complaints including nausea and vomiting, abdominal pain, cramps and diarrhoea.
Other secondary outcomes
No studies assessed the other secondary outcomes.
Amoxicillin versus placebo (comparison 3)
Primary outcome
Microbiological cure
It is uncertain whether amoxicillin improves microbiological cure in comparison to placebo but the certainty of this evidence is very low (summary of findings Table 3; (RR 2.00, 95% CI 0.59 to 6.79; 15 women; studies = one; Analysis 3.1)).
Secondary outcomes
No secondary outcomes were reported for this outcome in the included studies.
Amoxicillin versus azithromycin (comparison 4)
Primary outcome
Microbiological cure
It is uncertain whether amoxicillin improves or reduces microbiological cure in comparison to azithromycin because the certainty of this evidence is very low (summary of findings Table 4 ; (RR 0.89, 95% CI 0.71 to 1.12; 144 women; studies = two; Analysis 4.1)).
Secondary outcomes
Repeated infection
There was no clear difference for the outcome of repeated infections between amoxicillin and azithromycin in the single included study (RR 0.42, 95% CI 0.02 to 9.55; 34 women; studies = one; Analysis 4.2).
Preterm birth
There was no clear difference in the incidence of preterm birth between amoxicillin and azithromycin (RR 1.17, 95% CI 0.43 to 3.20; 90 women; studies = one; Analysis 4.3).
Side effects of treatment
There was no clear difference in side effects between the two groups (RR 0.56, 95% CI 0.24 to 1.31; 36 women; studies = one; Analysis 4.4). Side effects reported included nausea, vomiting, diarrhoea and abdominal pain.
Other secondary outcomes
None were reported.
Amoxicillin versus erythromycin (comparison 5)
Primary outcome
Microbiological cure
Amoxicillin makes little or no difference to microbiological cure in comparison to erythromycin (high‐certainty evidence, summary of findings Table 5; (RR 0.97, 95% CI 0.93 to 1.01; 466 women; studies = 4; Analysis 5.1)).
Secondary outcomes
Side effects of treatment
Amoxicillin was associated with reduced incidence of side effects in comparison to erythromycin (RR 0.31, 95% CI 0.21 to 0.46; 513 women; studies = four; I² = 27%; Analysis 5.2). Side effects associated with erythromycin use included nausea, vomiting, diarrhoea, abdominal cramping, rash, and an allergic reaction.
Other secondary outcomes
None were reported.
Azithromycin versus erythromycin (comparison 6)
Primary outcome
Microbiological cure
It appears that azithromycin probably improves microbiological cure in comparison to erythromycin (moderate‐certainty evidence, summary of findings Table 6; (average RR 1.11, 95% CI 1.00 to 1.23; participants = 374; studies = six; I² = 53%; Analysis 6.1)), however, there was substantial heterogeneity between the included studies (I² = 53%) and the lower confidence interval just touches the line of no effect.
Secondary outcomes
Repeated infection
There was no clear difference between azithromycin and amoxicillin for the outcome of repeated infections (RR 1.37, 95% CI 0.32 to 5.73; 85 women; studies = one; Analysis 6.2).
Preterm birth
There was no clear difference in the rate of preterm birth between azithromycin and amoxicillin (RR 0.77, 95% CI 0.29 to 2.10; 126 women; studies = one; Analysis 6.3).
Preterm rupture of membranes
There was no clear difference for the outcome of preterm rupture of membranes between azithromycin and amoxicillin (RR 0.62, 95% CI 0.15 to 2.48; 126 women; studies = one; Analysis 6.4).
Side effects of treatment
Fewer women in the azithromycin group experienced side effects in comparison to women receiving erythromycin (RR 0.24, 95% CI 0.17 to 0.34; 374 women; studies = six; Analysis 6.5). These side effects were mostly gastrointestinal in origin and included nausea, vomiting, diarrhoea and abdominal cramping.
Other secondary outcomes
None were reported.
Clindamycin versus erythromycin (comparison 7)
Primary outcome
Microbiological cure
Clindamycin may make little or no difference on microbiological cure in comparison to erythromycin (low‐certainty evidence,summary of findings Table 7; (RR 1.06, 95% CI 0.97 to 1.15; 173 women; studies = two; Analysis 7.1)).
Secondary outcomes
Side effects of treatment
Women in the clindamycin group experienced less side effects in comparison to erythromycin (RR 0.44, 95% CI 0.22 to 0.87; 183 women; studies = two; Analysis 7.2). These side effects were mostly gastrointestinal in origin and included nausea, vomiting, diarrhoea and abdominal cramping.
Other secondary outcomes
None were reported.
Amoxicillin versus clindamycin (comparison 8)
Primary outcome
Microbiological cure
Amoxicillin probably makes little or no difference on microbiological cure in comparison to clindamycin (moderate‐certainty evidence, summary of findings Table 8; (RR 0.96, 95% CI 0.89 to 1.04; 101 women; studies = one; Analysis 8.1)).
Secondary outcomes
Side effects of treatment
There was no clear difference in number of side effects associated with amoxicillin and clindamycin (RR 0.57, 95% CI 0.14 to 2.26; 107 women; studies = one; Analysis 8.2). The side effects reported included rash and gastrointestinal complaints.
Other secondary outcomes
None were reported.
Discussion
Summary of main results
Fifteen studies involving 1754 women were included in this review but our meta‐analyses are based on fewer numbers of studies/women. We excluded four studies and one study is ongoing.
Erythromycin (moderate‐certainty evidence) and clindamycin (low‐certainty evidence) were associated with a higher incidence of microbiological cure in comparison to placebo. Results were unclear in one very small study comparing amoxicillin placebo but the evidence was graded very‐low certainty.
There is no clear difference in microbiological cure between the assessed agents compared to each other: amoxicillin versus azithromycin (very low‐certainty evidence); amoxicillin versus erythromycin (high‐certainty evidence); azithromycin versus erythromycin (moderate‐certainty evidence); clindamycin versus erythromycin (low‐certainty evidence); amoxicillin versus clindamycin (moderate‐certainty evidence). There was no clear difference in repeat infections for amoxicillin versus azithromycin, or azithromycin versus erythromycin. Most secondary outcomes were not reported in any of the included studies.
Antibacterial treatment of genital Chlamydia trachomatis (C.trachomatis) infection was associated with side effects which were more common with the use of erythromycin and clindamycin than placebo as would be expected. Amoxicillin and clindamycin were associated with less side effects than azithromycin and erythromycin. Azithromycin caused less side effects than erythromycin. Side effects associated with erythromycin, azithromycin and clindamycin included nausea, vomiting, abdominal cramping and diarrhoea. Clindamycin use was occasionally associated with a non severe rash.
There were only a few studies that assessed the outcomes of preterm birth, preterm rupture of membranes and low birthweight. No studies assessed chorioamnionitis, postpartum endometritis, sepsis, prolonged hospital stay, maternal satisfaction, neonatal conjunctivitis, neonatal pneumonia, fetal anomalies, low birthweight and Apgar scores.
Overall completeness and applicability of evidence
All of the included studies were undertaken in North America (14 in USA and 1 in Canada) in 1982 and the mid to late nineties and early 2000s. Antibiotic resistance may have changed since these studies were performed. Study populations could differ in low‐resource settings and the results are therefore only applicable to well‐resourced settings. C.trachomatis testing remains a challenge in low‐resource settings because of the cost, and the treatment of genital infection is still based on a syndromic approach (South African STI guideline 2015). There was little or no information on the outcomes of preterm labour, preterm birth, preterm rupture of membranes, chorioamnionitis, postpartum endometritis, sepsis, prolonged hospital stay, maternal satisfaction with treatment, perinatal mortality, neonatal conjunctivitis, neonatal pneumonia, fetal anomalies, low birthweight, Apgar score less than seven at five minutes and cost of treatment.
Quality of the evidence
We assessed the included studies for risk of bias. Two studies (Alary 1994; Turrentine 1995) were assessed to be at low risk of bias in all domains. The remaining studies had varying risks of bias; blinding of participants and outcome assessors was unclear, not reported, or not attempted in most studies. We carried out formal assessments of quality of the evidence using GRADEpro for the review's primary outcome of microbiological cure. For this outcome, the evidence was graded from very low to high certainty for the different comparisons: amoxicillin versus placebo and versus azithromycin were graded very low quality; clindamycin versus placebo, and versus erythromycin were graded low quality; erythromycin versus placebo, azithromycin versus erythromycin, and amoxicillin versus clindamycin were graded moderate quality; amoxicillin versus erythromycin was graded high quality. Evidence was downgraded for limitations in study designs, inconsistency, and imprecision in effect estimates.
Potential biases in the review process
Evidence in this review was derived from studies identified in a detailed search process. Trials comparing interventions to treat C. trachomatis infection in pregnancy that have not been published may not have been identified. We attempted to minimise bias in the review process by having two review authors independently extract data.
Agreements and disagreements with other studies or reviews
We did not find any publications which included meta‐analysis of published studies, but we have identified two recent reviews/guidelines addressing the treatment of C.trachomatis during pregnancy.
CDC guidelines (CDC 2015) and the up‐to‐date review (Marrazzo 2016) recommends the treatment of C.trachomatis infection in pregnancy with azithromycin based on clinical practice as it is safe and effective. Recommended alternatives suggested by both documents are amoxicillin and erythromycin. A test of cure is recommended in pregnant women three to four weeks after treatment and again three months later. Resistance to amoxicillin is highlighted, however, it is referenced with respect to animal studies only. The review and guideline did not suggest clindamycin as an alternative, but according to limited data from this review it could be considered as a treatment option.

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Erythromycin versus placebo, Outcome 1 Microbiological cure.

Comparison 1 Erythromycin versus placebo, Outcome 2 Preterm birth.

Comparison 1 Erythromycin versus placebo, Outcome 3 Preterm rupture of membranes.

Comparison 1 Erythromycin versus placebo, Outcome 4 Side effects of treatment.

Comparison 1 Erythromycin versus placebo, Outcome 5 Perinatal mortality.

Comparison 1 Erythromycin versus placebo, Outcome 6 Low birthweight.
Comparison 2 Clindamycin versus placebo, Outcome 1 Microbiological cure.

Comparison 2 Clindamycin versus placebo, Outcome 2 Side effects of treatment.

Comparison 3 Amoxicillin versus placebo, Outcome 1 Microbiological cure.

Comparison 4 Amoxicillin versus azithromycin, Outcome 1 Microbiological cure.

Comparison 4 Amoxicillin versus azithromycin, Outcome 2 Repeated infection.

Comparison 4 Amoxicillin versus azithromycin, Outcome 3 Preterm birth.

Comparison 4 Amoxicillin versus azithromycin, Outcome 4 Side effects of treatment.

Comparison 5 Amoxicillin versus erythromycin, Outcome 1 Microbiological cure.

Comparison 5 Amoxicillin versus erythromycin, Outcome 2 Side effects of treatment.

Comparison 6 Azithromycin versus erythromycin, Outcome 1 Microbiological cure.

Comparison 6 Azithromycin versus erythromycin, Outcome 2 Repeated infection.

Comparison 6 Azithromycin versus erythromycin, Outcome 3 Preterm birth.

Comparison 6 Azithromycin versus erythromycin, Outcome 4 Preterm rupture of membranes.

Comparison 6 Azithromycin versus erythromycin, Outcome 5 Side effects of treatment.

Comparison 7 Clindamycin versus erythromycin, Outcome 1 Microbiological cure.

Comparison 7 Clindamycin versus erythromycin, Outcome 2 Side effects of treatment.

Comparison 8 Amoxicillin versus clindamycin, Outcome 1 Microbiological cure.

Comparison 8 Amoxicillin versus clindamycin, Outcome 2 Side effects of treatment.
| Erythromycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Erythromycin | |||||
| Microbiological cure | Study population | Average RR 2.64 | 495 | ⊕⊕⊕⊝ | ||
| 344 per 1000 | 908 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Statistical Heterogeneity (I2 > 60%). (Inconsistency: ‐1) 2 One included study has design limitations but contributed < 40% weight. (Not downgraded) | ||||||
| Clindamycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 4.08 | 85 | ⊕⊕⊝⊝ | ||
| 227 per 1000 | 927 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The included study had design limitation (Design limitations: ‐1) 2 Wide confidence interval and small sample size (Imprecision: ‐1) | ||||||
| Amoxicillin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 2.00 | 15 | ⊕⊝⊝⊝ | ||
| 333 per 1000 | 667 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The included study had design limitation (Design limitations: ‐1) 2 Wide confidence intervals crossing the line of no effect, few events, and small sample size (Imprecision: ‐2) | ||||||
| Amoxicillin compared to azithromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with azithromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.89 | 144 | ⊕⊝⊝⊝ | ||
| 716 per 1000 | 637 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study contributing to over 68% of weight to pooled analysis had some design limitations (Design limitations: ‐1) 2 Wide confidence intervals crossing the line of no effect and small size (Imprecision: ‐2) | ||||||
| Amoxicillin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.97 | 466 | ⊕⊕⊕⊕ | One study contributing to 24% of weight had some design limitation. (not downgraded) | |
| 954 per 1000 | 925 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Azithromycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Azithromycin | |||||
| Microbiological cure | Study population | Average RR 1.11 | 374 | ⊕⊕⊕⊝ | ||
| 825 per 1000 | 916 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies have design limitations (Design limitations: ‐1) 2 Statistical heterogeneity at 53% (I2 < 60%) (not downgraded) | ||||||
| Clindamycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 1.06 | 173 | ⊕⊕⊝⊝ | ||
| 905 per 1000 | 959 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study contributing to over 40% of weight to pooled analysis had some design limitations (Design limitations: ‐1) 2 Small sample size (Imprecision: ‐1) | ||||||
| Amoxicillin compared to clindamycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with clindamycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.96 | 101 | ⊕⊕⊕⊝ | ||
| 979 per 1000 | 940 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The pooled effect was based on one study with a small sample size (Imprecision: ‐1) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.60, 4.38] |
| 2 Preterm birth Show forest plot | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.46] |
| 3 Preterm rupture of membranes Show forest plot | 1 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.43] |
| 4 Side effects of treatment Show forest plot | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.36, 23.76] |
| 5 Perinatal mortality Show forest plot | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.32, 28.74] |
| 6 Low birthweight Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [2.35, 7.08] |
| 2 Side effects of treatment Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [0.65, 44.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.59, 6.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| 2 Repeated infection Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.55] |
| 3 Preterm birth Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.43, 3.20] |
| 4 Side effects of treatment Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 4 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.01] |
| 2 Side effects of treatment Show forest plot | 4 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.21, 0.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 6 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 2 Repeated infection Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.32, 5.73] |
| 3 Preterm birth Show forest plot | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.29, 2.10] |
| 4 Preterm rupture of membranes Show forest plot | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.48] |
| 5 Side effects of treatment Show forest plot | 6 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.17, 0.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 2 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.97, 1.15] |
| 2 Side effects of treatment Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.22, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 2 Side effects of treatment Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.14, 2.26] |

