Intervenciones para tratar la infección genital por Chlamydia trachomatis en el embarazo
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010485.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Cathy Cluver and Natalia Novikova are the guarantors of the review. Natalia Novikova developed the protocol, provided clinical and methodological perspectives and drafted the review. Catherine Cluver provided general advice on the protocol, assisted with assessment of studies for inclusion into the meta‐analysis, checked the trials for inclusion criteria, checked data entry, checked assessment of bias, performed the data analysis and edited the final versions of the review. David OA Eriksson and Kevin Bengtsson assessed the studies for inclusion into the meta‐analysis, extracted the data and assisted with data analysis. Göran K Lingman checked the data and provided advice on the review.
Declarations of interest
Natalia Novikova: none known.
Catherine Cluver: none known.
David OA Eriksson: received a small travel scholarship from the international department of Lund University to finance some of the costs for travelling from Sweden to South Africa, to be a part of this review.
Kevin Bengtsson: received a small travel scholarship from the international department of Lund University to finance some of the costs for travelling from Sweden to South Africa, to be a part of this review.
Göran K Lingman: none known.
Acknowledgements
The Cochrane Pregnancy and Childbirth Group and peer referees.
This project was supported by the National Institute for Health Research, via Cochrane infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Cochrane Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 22 | Interventions for treating genital <i>Chlamydia trachomatis</i> infection in pregnancy | Review | Catherine Cluver, Natalia Novikova, David OA Eriksson, Kevin Bengtsson, Göran K Lingman | |
| 2013 Apr 30 | Interventions for treating genital <i>Chlamydia trachomatis</i> infection in pregnancy | Protocol | Natalia Novikova, Catherine Cluver | |
Differences between protocol and review
There are some differences between our published protocol (Novikova 2013) and the full review; these are outlined below.
-
The contact person for the review has changed from Natalia Novikova to Cathy Cluver.
-
We have updated our methods text to include the use of GRADE and we have included eight 'Summary of findings' tables.
-
We have added the WHO International Clinical Trials Registry Platform (ICTRP) to sources searched.
Notes
This new review updates and supersedes an earlier review on this topic by Brocklehurst 1998.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Chlamydia trachomatis;
- Amoxicillin [therapeutic use];
- Anti‐Bacterial Agents [*therapeutic use];
- Azithromycin [therapeutic use];
- Chlamydia Infections [*drug therapy];
- Clindamycin [therapeutic use];
- Erythromycin [therapeutic use];
- Pregnancy Complications, Infectious [*drug therapy, microbiology];
- Randomized Controlled Trials as Topic;
- Reproductive Tract Infections [*drug therapy];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Erythromycin versus placebo, Outcome 1 Microbiological cure.

Comparison 1 Erythromycin versus placebo, Outcome 2 Preterm birth.

Comparison 1 Erythromycin versus placebo, Outcome 3 Preterm rupture of membranes.

Comparison 1 Erythromycin versus placebo, Outcome 4 Side effects of treatment.

Comparison 1 Erythromycin versus placebo, Outcome 5 Perinatal mortality.

Comparison 1 Erythromycin versus placebo, Outcome 6 Low birthweight.
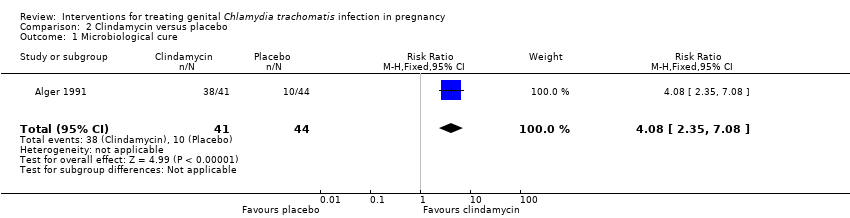
Comparison 2 Clindamycin versus placebo, Outcome 1 Microbiological cure.

Comparison 2 Clindamycin versus placebo, Outcome 2 Side effects of treatment.

Comparison 3 Amoxicillin versus placebo, Outcome 1 Microbiological cure.

Comparison 4 Amoxicillin versus azithromycin, Outcome 1 Microbiological cure.

Comparison 4 Amoxicillin versus azithromycin, Outcome 2 Repeated infection.

Comparison 4 Amoxicillin versus azithromycin, Outcome 3 Preterm birth.

Comparison 4 Amoxicillin versus azithromycin, Outcome 4 Side effects of treatment.

Comparison 5 Amoxicillin versus erythromycin, Outcome 1 Microbiological cure.

Comparison 5 Amoxicillin versus erythromycin, Outcome 2 Side effects of treatment.

Comparison 6 Azithromycin versus erythromycin, Outcome 1 Microbiological cure.

Comparison 6 Azithromycin versus erythromycin, Outcome 2 Repeated infection.

Comparison 6 Azithromycin versus erythromycin, Outcome 3 Preterm birth.

Comparison 6 Azithromycin versus erythromycin, Outcome 4 Preterm rupture of membranes.

Comparison 6 Azithromycin versus erythromycin, Outcome 5 Side effects of treatment.

Comparison 7 Clindamycin versus erythromycin, Outcome 1 Microbiological cure.

Comparison 7 Clindamycin versus erythromycin, Outcome 2 Side effects of treatment.

Comparison 8 Amoxicillin versus clindamycin, Outcome 1 Microbiological cure.

Comparison 8 Amoxicillin versus clindamycin, Outcome 2 Side effects of treatment.
| Erythromycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Erythromycin | |||||
| Microbiological cure | Study population | Average RR 2.64 | 495 | ⊕⊕⊕⊝ | ||
| 344 per 1000 | 908 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Statistical Heterogeneity (I2 > 60%). (Inconsistency: ‐1) 2 One included study has design limitations but contributed < 40% weight. (Not downgraded) | ||||||
| Clindamycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 4.08 | 85 | ⊕⊕⊝⊝ | ||
| 227 per 1000 | 927 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The included study had design limitation (Design limitations: ‐1) 2 Wide confidence interval and small sample size (Imprecision: ‐1) | ||||||
| Amoxicillin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 2.00 | 15 | ⊕⊝⊝⊝ | ||
| 333 per 1000 | 667 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The included study had design limitation (Design limitations: ‐1) 2 Wide confidence intervals crossing the line of no effect, few events, and small sample size (Imprecision: ‐2) | ||||||
| Amoxicillin compared to azithromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with azithromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.89 | 144 | ⊕⊝⊝⊝ | ||
| 716 per 1000 | 637 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study contributing to over 68% of weight to pooled analysis had some design limitations (Design limitations: ‐1) 2 Wide confidence intervals crossing the line of no effect and small size (Imprecision: ‐2) | ||||||
| Amoxicillin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.97 | 466 | ⊕⊕⊕⊕ | One study contributing to 24% of weight had some design limitation. (not downgraded) | |
| 954 per 1000 | 925 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Azithromycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Azithromycin | |||||
| Microbiological cure | Study population | Average RR 1.11 | 374 | ⊕⊕⊕⊝ | ||
| 825 per 1000 | 916 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies have design limitations (Design limitations: ‐1) 2 Statistical heterogeneity at 53% (I2 < 60%) (not downgraded) | ||||||
| Clindamycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with erythromycin | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 1.06 | 173 | ⊕⊕⊝⊝ | ||
| 905 per 1000 | 959 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study contributing to over 40% of weight to pooled analysis had some design limitations (Design limitations: ‐1) 2 Small sample size (Imprecision: ‐1) | ||||||
| Amoxicillin compared to clindamycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with clindamycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.96 | 101 | ⊕⊕⊕⊝ | ||
| 979 per 1000 | 940 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The pooled effect was based on one study with a small sample size (Imprecision: ‐1) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.60, 4.38] |
| 2 Preterm birth Show forest plot | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.46] |
| 3 Preterm rupture of membranes Show forest plot | 1 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.43] |
| 4 Side effects of treatment Show forest plot | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.36, 23.76] |
| 5 Perinatal mortality Show forest plot | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.32, 28.74] |
| 6 Low birthweight Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [2.35, 7.08] |
| 2 Side effects of treatment Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [0.65, 44.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.59, 6.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| 2 Repeated infection Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.55] |
| 3 Preterm birth Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.43, 3.20] |
| 4 Side effects of treatment Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 4 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.01] |
| 2 Side effects of treatment Show forest plot | 4 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.21, 0.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 6 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 2 Repeated infection Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.32, 5.73] |
| 3 Preterm birth Show forest plot | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.29, 2.10] |
| 4 Preterm rupture of membranes Show forest plot | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.48] |
| 5 Side effects of treatment Show forest plot | 6 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.17, 0.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 2 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.97, 1.15] |
| 2 Side effects of treatment Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.22, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Microbiological cure Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 2 Side effects of treatment Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.14, 2.26] |

