Uso de la hialuronidasa como un complemento al bloqueo nervioso con anestésico local del ojo para aliviar el dolor intraoperatorio en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010368.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 marzo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: HR.
Co‐ordinating the review: HR.
Undertaking manual searches: KA and HR.
Screening search results: KA and HR.
Organizing retrieval of papers: DB, KA and HR.
Screening retrieved papers against inclusion criteria: KA and HR.
Appraising quality of papers: KA and HR.
Abstracting data from papers: KA and HR.
Writing to authors of papers for additional information: KA and HR.
Providing additional data about papers: HR.
Obtaining and screening data on unpublished studies: HR.
Data management for the review: KA and HR.
Entering data into Review Manager (5): KA and HR.
Review Manager 5 statistical data: KA, HR and CB.
Other statistical analysis not using Review Manager 5: CB.
Double entry of data: KA and HR.
Interpretation of data: KA and HR.
Statistical inferences: CB.
Assessment of risk of bias: KA and HR.
Writing the review: KA and HR.
Securing funding for the review: HR and CB.
Guarantor for the review: HR.
Responsible for reading and checking review before submission: KA and HR.
Sources of support
Internal sources
-
Moorfields Eye Hospital, UK.
Salary and facilities
External sources
-
No sources of support supplied
Declarations of interest
HR: no known conflict of interest.
KA: no known conflict of interest.
CB : no known conflict of interest.
DB : no known conflict of interest.
Acknowledgements
We thank Jane Cracknell, Managing Editor of the Cochrane Anaesthesia, Critical and Emergency Care Group (ACE), and Karen Hovhannisyan (former ACE trials search co‐ordinator). We would like to thank Andrew Smith (Content Editor), Marialena Trivella (Statistical Editor), Vibeke E Horstmann (Statistical Editor), Ana Licina, Jacques Ripart, Mahmoud B Alhassan (Peer Reviewers), Janet Wale (Consumer Editor), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of the protocol (Rüschen 2013) and the systematic review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Mar 02 | Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults | Review | Heinrich Rüschen, Kavitha Aravinth, Catey Bunce, Desta Bokre | |
| 2013 Feb 28 | Use of hyaluronidase as an adjunct to local anaesthetic eye blocks | Protocol | Heinrich Rüschen, Lee Adams, Catey Bunce | |
Differences between protocol and review
We made the following changes to the protocol (Rüschen 2013);
-
This review included only adults, we therefore stated "adults" in the title.
-
The phrase 'to reduce intraoperative pain' was added to the title to comply with PICO to reflect the review question.
-
Lee Adams was initially a registered author, but gave his agreement to be removed from the authors list at the protocol stage.
-
We added Kavitha Aravinth as second author.
-
We added Desta Bokre as fourth author.
-
We further stated in the primary objective that when considering studies for inclusion that we excluded studies that did not measure pain with a rating scale because spontaneous reporting of pain by the participant is not likely to produce reproducible results.
-
We stated the secondary objectives (incidence of harm, participant and surgical satisfaction and economic outcomes or cost calculations) in the Objectives section.
-
We stated in the protocol that intraoperative pain would be recorded using (VAS); however, we observed that included studies used either (VAS) or (VRS) to rate pain. As both are accepted and validated tools to measure pain, we analysed both these methods of measuring pain in the same manner.
-
We initially proposed to include only studies that described the first eye operation, but during the review process, we found no publication that described if the participants had a first or second eye operation.(See Types of participants and Unit of analysis issues).
-
We excluded adnexal surgery and any other eye surgery that was not intraocular. Anaesthetic techniques and surgical interventions for adnexal operations are inherently different from intraocular surgery and produce a very different pain profile.
-
We used GRADE‐pro to analyse the quality of evidence and to produce a 'Summary of Findings table'.
-
Collation of references: we added the following to the Selection of studies section. "Where studies had multiple publications, we planned to collate the reports of the same study so that each study, rather than each report, was the unit of interest for the review and such studies had a single identifier with multiple references.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Analgesia [*methods];
- Analgesics [*administration & dosage];
- Anesthesia, Local [methods];
- Anesthetics, Local [*administration & dosage];
- Hyaluronoglucosaminidase [*administration & dosage];
- Intraoperative Complications [*prevention & control];
- Pain Measurement;
- Pain, Procedural [*prevention & control];
- Patient Satisfaction;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adult; Humans;
PICO
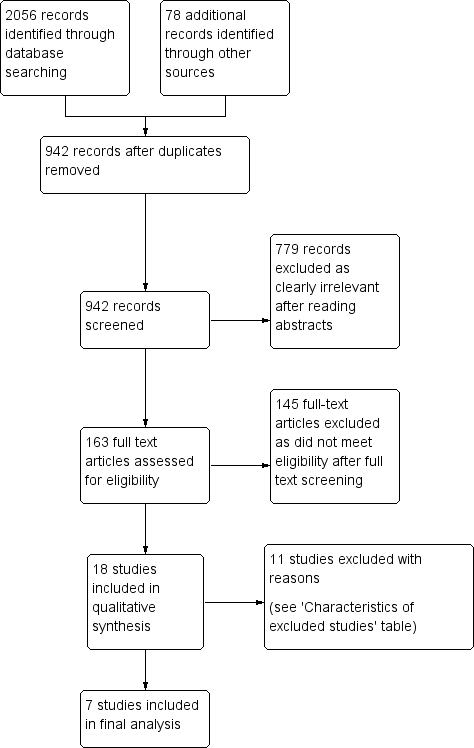
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
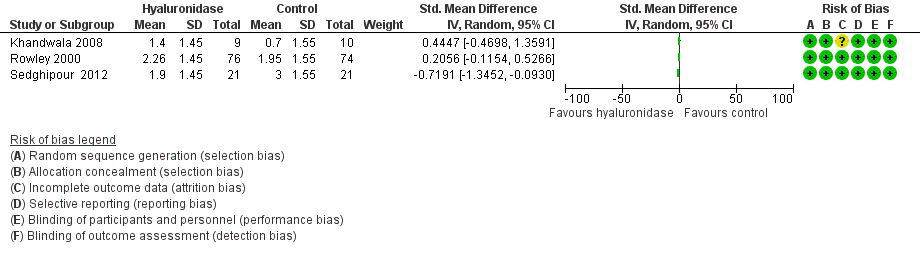
Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.1 Intraoperative pain (measured by analogue rating scales; reported continuous).

Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.2 Intraoperative pain (measured by analogue rating scales; reported dichotomous).

Comparison 1 Hyaluronidase versus control, Outcome 1 Intraoperative pain (reported continuous).

Comparison 1 Hyaluronidase versus control, Outcome 2 Intraoperative pain (reported dichotomous).
| Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults | ||||||
| Patients or population: adults (aged ≥ 18 years) undergoing ophthalmic surgery under local anaesthetic eye blocks. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no hyaluronidase | Risk with hyaluronidase | |||||
| Intraoperative pain (reported dichotomous) | RR 0.83 | 289 | ⊕⊕⊝⊝ | ‐ | ||
| 301 per 1000 | 250 per 1000 | |||||
| Intraoperative pain (reported continuous) | 3 trials looked at effect of hyaluronidase on reduction of intraoperative pain measured by rating scales. Results were reported as continuous data. 2 studies did not provide the SMD, which measures the effect in a clinical setting, the results could not be meta‐analysed and hence were reported narratively (Khandwala 2008; Rowley 2000). Among the 3 trials covering 211 participants (Khandwala 2008: Mean difference 0.70; Rowley 2000: Mean difference 0.31; Sedghipour 2012: Mean difference ‐1.10), only the Sedghipour study with 42 participants, which is a high quality study, showed a statistically significant (at the 5 % level) reduction in pain in the hyaluronidase group (P = 0.04). The remaining 2 studies with 169 participants showed no statistically significant (at the 5 % level) reduction of pain intraoperatively with hyaluronidase (Khandwala 2008: P = 0.5; Rowley 2000: n.s). These studies were also of high quality and low risk of bias. Khandwala and colleagues had an unclear attrition bias as 1/10 participants in the treatment group was dropped after randomization with no clear explanation. | ‐ | 211 | ⊕⊕⊝⊝ | ‐ | |
| Incidence of harm | None of the studies reported harms in relation to hyaluronidase. | ‐ | (0 studies) | ‐ | ‐ | |
| Participant satisfaction | Significantly better satisfaction in these well designed studies with low risk of bias (Remy 2008; Sedghipour 2012). The studies included 122 participants and showed higher satisfaction scores in the treatment group (P < 0.05). | ‐ | 122 | ⊕⊕⊕⊝ | ‐ | |
| Surgical satisfaction | Surgical satisfaction was reportedly superior with hyaluronidase in the larger 2 studies (Remy 2008: P < 0.001; Sedghipour 2012: P = 0.02) and not significantly different in 1 small study (Khandwala 2008: P = 0.96). | ‐ | 141 | ⊕⊕⊕⊝ | ‐ | |
| Economic outcomes or cost calculations | None of the included studies reported economic outcomes or cost calculations | ‐ | (0 RCTs) | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n.s: not statistically significant; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to marked heterogeneity with a calculated I2 > 50%. 2Downgraded one level for imprecision due to wide 95% confidence intervals, reflecting uncertainty in the direction of effect estimate. 3Downgraded one level for imprecision and inconsistency in measurement, lack of data and small sample size. 4Downgraded one level because of imprecision secondary to small sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraoperative pain (reported continuous) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Intraoperative pain (reported dichotomous) Show forest plot | 4 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |

