Uso de la hialuronidasa como un complemento al bloqueo nervioso con anestésico local del ojo para aliviar el dolor intraoperatorio en adultos
Resumen
Antecedentes
La hialuronidasa se ha utilizado durante muchas décadas como un complemento a la solución anestésica local para mejorar la velocidad de inicio del bloqueo ocular y obtener mejor acinesia y analgesia. Con la evolución de las técnicas modernas de cirugía ocular, el inicio rápido y la acinesia ya no son requisitos fundamentales. Es necesario examinar la suposición de que el agregado de hialuronidasa a las inyecciones anestésicas locales confiere una mejor analgesia a los pacientes. No se han realizado revisiones sistemáticas recientes que proporcionen evidencia de que en realidad la hialuronidasa mejora la analgesia.
Objetivos
Evaluar si el agregado de hialuronidasa a las soluciones anestésicas locales para el uso en la anestesia oftálmica en pacientes adultos da lugar a una reducción del dolor percibido durante la operación y evaluar los efectos perjudiciales, la satisfacción quirúrgica y de los participantes y la repercusión económica.
Métodos de búsqueda
Se realizaron búsquedas sistemáticas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase y en otras cuatro bases de datos en junio 2017. Se buscaron ensayos relevantes en los registros de ensayos en www.ISRCTN.com, ClinicalTrials.gov y en www.clinicaltrialsregister.eu. No se impuso ninguna restricción de idioma.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios (ECA) que evaluaron el efecto de la hialuronidasa sobre el dolor presentado por pacientes adultos durante la cirugía intraocular mediante una escala de calificación.
Obtención y análisis de los datos
Dos autores de la revisión (HR y KA) extrajeron los datos de forma independiente y evaluaron la calidad metodológica mediante procedimientos estándar, según lo previsto por Cochrane.
Resultados principales
Se incluyeron siete ensayos con 500 participantes que estudiaron el efecto de la hialuronidasa sobre el dolor intraoperatorio. Cuatro de los siete ensayos, con 289 participantes, informaron el resultado primario de manera dicotómica y se procedió a metanalizar los hallazgos, que indicaron una heterogeneidad moderada que no fue posible explicar (I2 = 41% ). El cociente de riesgos (CR) agrupado de estos cuatro ensayos fue 0,83, con un intervalo de confianza del 95% que varió de 0,48 a 1,42. La reducción de las puntuaciones de dolor intraoperatorio en el grupo de hialuronidasa no fue estadísticamente significativa. En los tres ensayos que informaron el resultado primario de manera continua, los datos faltantes dificultaron el metanálisis. Para una exploración adicional de los datos se imputaron las desviaciones estándar de los otros estudios a partir de otro ECA incluido (Sedghipour 2012). Sin embargo, lo anterior dio lugar a heterogeneidad significativa entre las estimaciones de los estudios (I² = 76% ). La falta de informe de datos relevantes en dos de los tres ensayos restantes dificultó evaluar la dirección del efecto en un contexto clínico.
En general no hubo diferencias estadísticas con respecto a la reducción en las puntuaciones de dolor intraoperatorio entre el grupo de hialuronidasa y control. Los siete ensayos incluidos tuvieron un riesgo de sesgo bajo.
Según GRADE, se encontró que la calidad de la evidencia fue baja, que se disminuyó en los ensayos por riesgo grave de inconsistencia e imprecisión. Por lo tanto, los resultados deberían analizarse con cautela.
Las puntuaciones de satisfacción de los participantes fueron significativamente mayores en el grupo de hialuronidasa en dos ensayos de alta calidad con 122 participantes. La satisfacción quirúrgica también fue superior en dos de tres ensayos de alta calidad que incluyeron 141 participantes. Según GRADE, la calidad de la evidencia fue moderada para la satisfacción quirúrgica y de los participantes, y la calidad de los ensayos se disminuyó por imprecisión debido al pequeño tamaño de muestra. El riesgo de sesgo en estos ensayos era bajo.
En los estudios no se informaron efectos perjudiciales debido al agregado de hialuronidasa. Ningún estudio informó el costo de la hialuronidasa en el contexto de la cirugía ocular.
Conclusiones de los autores
Los efectos de agregar hialuronidasa al líquido anestésico local sobre los resultados de dolor en los pacientes sometidos a cirugía ocular no están claros debido a la baja calidad de la evidencia disponible. Se necesita un ECA bien diseñado para analizar la inconsistencia y la imprecisión entre los estudios, así como para determinar el efecto beneficioso de la hialuronidasa para mejorar la analgesia durante la cirugía ocular. La satisfacción quirúrgica y de los participantes es mayor con la hialuronidasa en comparación con los grupos control, como se demuestra en los estudios de calidad moderada. En los estudios no hubo efectos perjudiciales atribuidos al uso de la hialuronidasa. Si se considera que los efectos perjudiciales rara vez se definieron como una medida de resultado y que el número general de participantes fue pequeño, no es posible establecer conclusiones acerca de la incidencia de efectos perjudiciales de la hialuronidasa. Ninguno de los estudios realizó cálculos de los costos con respecto al uso de la hialuronidasa en el bloqueo anestésico local del ojo.
PICOs
Resumen en términos sencillos
Agregado de hialuronidasa al bloqueo anestésico local del ojo para aliviar el dolor durante la cirugía ocular en adultos.
Pregunta de la revisión
Se revisó la evidencia sobre la efectividad de agregar hialuronidasa a las soluciones para el bloqueo anestésico local del ojo (un fármaco sedante inyectado en el ojo para bloquear los nervios) para aliviar el dolor y aumentar la satisfacción quirúrgica y del paciente durante la cirugía ocular en adultos. También se buscaron informes sobre los efectos secundarios y el costo.
Antecedentes
La hialuronidasa es una enzima (una proteína que regula una reacción química en el cuerpo) que ayuda a la diseminación del anestésico local en los tejidos alrededor del ojo. Se utiliza ampliamente como un agregado al bloqueo anestésico local del ojo para obtener un inicio más rápido de la anestesia y reducir o bloquear el movimiento del ojo (acinesia). Con las técnicas modernas de cirugía ocular, el inicio rápido y la acinesia ya no son requisitos fundamentales y a menudo la cirugía se puede realizar sin dolor con un anestésico tópico (en la superficie del ojo) solo. La hialuronidasa se ha asociado con efectos secundarios poco frecuentes. Por lo tanto, es necesario justificar el uso de la hialuronidasa, que fue el objetivo de esta revisión.
Fecha de la búsqueda
La revisión está actualizada hasta el 30 de junio de 2017.
Características de los estudios
En la revisión se incluyeron siete ensayos controlados aleatorios (estudios clínicos en los que los participantes se asignan al azar a uno de dos o más grupos de tratamiento). Estos ensayos incluyeron 500 pacientes adultos sometidos a cirugía ocular bajo anestesia local. Se examinó cualquier efecto adicional de agregar hialuronidasa al anestésico local sobre el dolor presentado durante la cirugía ocular. También se examinaron las puntuaciones de satisfacción quirúrgica y de los participantes, y si se informó algún efecto perjudicial después del uso de la hialuronidasa en la solución de inyección. Ninguno de los estudios informó sobre los costos.
Resultados clave
De los siete ensayos incluidos, se agruparon los resultados de cuatro (289 participantes) porque se informaron de una manera similar. Se encontró que el agregado de hialuronidasa no alivió significativamente el dolor durante la cirugía. En los tres ensayos restantes (211 participantes), la falta de datos informados en dos ensayos dificultó el agrupamiento de los resultados. El resultado general de examinar todos estos ensayos juntos indica que no hubo una reducción significativa del dolor con el uso de hialuronidasa en el bloqueo nervioso del ojo.
Se encontró evidencia de calidad moderada de dos ensayos (122 participantes) que indicó que el agregado de hialuronidasa aumentó las puntuaciones de satisfacción de los participantes. Tres estudios que incluyeron 141 participantes examinaron la satisfacción quirúrgica, que se informó que fue superior con la hialuronidasa en los dos estudios más grandes y no fue significativamente diferente en un estudio pequeño (19 participantes). Ninguno de los estudios incluidos informó algún efecto perjudicial de la hialuronidasa.
Calidad de la evidencia
Los ensayos incluidos que informaron el dolor durante la cirugía tuvieron bajo riesgo de sesgo. La calidad general de la evidencia fue baja debido a las variaciones en el efecto sobre la reducción del dolor. Se estableció contacto con todos los autores de los ensayos para solicitar más información sobre los mismos, pero los datos no estaban disponibles.
Los estudios de calidad moderada informaron una mayor satisfacción quirúrgica y de los participantes con la hialuronidasa.
La analgesia sola no tiene en cuenta el espectro completo de efectos beneficiosos de la hialuronidasa. También es probable que la comodidad de los pacientes con la cirugía ocular mejore por el inicio rápido y la reducción de los movimientos oculares debido a la hialuronidasa.
Authors' conclusions
Summary of findings
| Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults | ||||||
| Patients or population: adults (aged ≥ 18 years) undergoing ophthalmic surgery under local anaesthetic eye blocks. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no hyaluronidase | Risk with hyaluronidase | |||||
| Intraoperative pain (reported dichotomous) | RR 0.83 | 289 | ⊕⊕⊝⊝ | ‐ | ||
| 301 per 1000 | 250 per 1000 | |||||
| Intraoperative pain (reported continuous) | 3 trials looked at effect of hyaluronidase on reduction of intraoperative pain measured by rating scales. Results were reported as continuous data. 2 studies did not provide the SMD, which measures the effect in a clinical setting, the results could not be meta‐analysed and hence were reported narratively (Khandwala 2008; Rowley 2000). Among the 3 trials covering 211 participants (Khandwala 2008: Mean difference 0.70; Rowley 2000: Mean difference 0.31; Sedghipour 2012: Mean difference ‐1.10), only the Sedghipour study with 42 participants, which is a high quality study, showed a statistically significant (at the 5 % level) reduction in pain in the hyaluronidase group (P = 0.04). The remaining 2 studies with 169 participants showed no statistically significant (at the 5 % level) reduction of pain intraoperatively with hyaluronidase (Khandwala 2008: P = 0.5; Rowley 2000: n.s). These studies were also of high quality and low risk of bias. Khandwala and colleagues had an unclear attrition bias as 1/10 participants in the treatment group was dropped after randomization with no clear explanation. | ‐ | 211 | ⊕⊕⊝⊝ | ‐ | |
| Incidence of harm | None of the studies reported harms in relation to hyaluronidase. | ‐ | (0 studies) | ‐ | ‐ | |
| Participant satisfaction | Significantly better satisfaction in these well designed studies with low risk of bias (Remy 2008; Sedghipour 2012). The studies included 122 participants and showed higher satisfaction scores in the treatment group (P < 0.05). | ‐ | 122 | ⊕⊕⊕⊝ | ‐ | |
| Surgical satisfaction | Surgical satisfaction was reportedly superior with hyaluronidase in the larger 2 studies (Remy 2008: P < 0.001; Sedghipour 2012: P = 0.02) and not significantly different in 1 small study (Khandwala 2008: P = 0.96). | ‐ | 141 | ⊕⊕⊕⊝ | ‐ | |
| Economic outcomes or cost calculations | None of the included studies reported economic outcomes or cost calculations | ‐ | (0 RCTs) | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n.s: not statistically significant; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to marked heterogeneity with a calculated I2 > 50%. 2Downgraded one level for imprecision due to wide 95% confidence intervals, reflecting uncertainty in the direction of effect estimate. 3Downgraded one level for imprecision and inconsistency in measurement, lack of data and small sample size. 4Downgraded one level because of imprecision secondary to small sample size. | ||||||
Background
Description of the condition
Local anaesthesia for ophthalmic surgery can be provided by regional injection block or topical anaesthesia alone. The most frequently used anaesthetic injections are retrobulbar, peribulbar and sub‐Tenon's block.
During a local anaesthetic injection for ophthalmic surgery, the objective is to deliver local anaesthetic fluid to the sensory and motor nerve fibres in the orbit. There is a large variation of techniques, anaesthetic mixtures and instruments in use to achieve this. Some of these techniques have been compared in Cochrane Reviews; for example, peribulbar versus retrobulbar block (Alhassan 2015), and topical anaesthesia alone versus sub‐Tenon's block (Guay 2015). Schein 2000 undertook a comprehensive systematic review of anaesthetic interventions.
Unfortunately, there is always a proportion of blocks that fail to provide adequate analgesia or akinesia. To improve the quality of the anaesthetic block, various adjuncts to the local anaesthetic fluid have been introduced.
Description of the intervention
Atkinson 1949 first described the addition of hyaluronidase to local anaesthetic fluid with the intention of improving the speed of onset of analgesia and akinesia. Subsequently, hyaluronidase has commonly been added to local anaesthetic injection fluid for this purpose.
How the intervention might work
For any local anaesthetic block to work, the local anaesthetic fluid needs to spread through the orbital cavity to reach the relevant motor and sensor fibres. A complex system of connective tissue membranes divides the orbital space, thereby impeding the spread of local anaesthetic fluid (Koornneef 1988).
Buhren 2016 described the molecular mechanisms of how hyaluronidase spreads through this connective tissue barrier, in the most recent review on this topic. The main components of connective tissue are the fibrous proteins collagen and elastin as well as proteoglycans located in the extracellular matrix. Glycosamine‐glycans attach to proteoglycans in a characteristic manner giving the connective tissue its viscoelastic properties. The most common glycosamine‐glycan is hyaluronic acid, it is a linear glycosaminoglycan disaccharide composed of alternating units of N‐acetyl‐D‐glucosamine and D‐glucuronic acid via alternating ß(1‐4) and ß(1‐3) glycosidic bonds. Hyaluronidase (hyaluronoglucosaminidase) is an enzyme that facilitates the spread of local anaesthetic fluid through connective tissue by degrading hyaluronic acid into smaller fragments and hydrolyzing the disaccharides at hexosaminidic ß(1‐4) linkages.
Meyer 1934 first extracted hyaluronidase. Preparations contain purified ovine testicular hyaluronidase as a dehydrated sterilized solid for reconstitution before use. Brand names of animal‐derived hyaluronidase include Hydase, Vitrase, Amphadase, Wydase and Hyalase. Apart from a preparation of ovine testicular hyaluronidase, a recombinant human hyaluronidase is also available as Hylenex. It is produced by genetically engineered Chinese hamster ovary cells containing a DNA plasmid encoding for a soluble fragment of human hyaluronidase (Hylenex® Prescribing Information 2016). The exact chemical structure of this enzyme is unknown. The approximate molecular weight is 61,000 daltons (Borders 1968).
Hyaluronidase also alters the pH of a local anaesthetic due to the presence of phosphate buffers within the preparation. The pH of plain bupivacaine solution is changed from 5.3 to 6.3 following the addition of hyaluronidase, and it may maintain local anaesthetic solubility during the process of alkalinization (Roberts 1993). This alkalinization may also explain any improved anaesthesia and akinesia.
A reduction in time to onset of surgical anaesthesia is considered desirable to facilitate patient throughput. The action of hyaluronidase may promote rapid onset of anaesthesia and akinesia. The minimum and maximum effective doses of hyaluronidase are unknown. The doses used range from 0.75 IU/mL to 300 IU/mL (Dempsey 1997).
Why it is important to do this review
We conducted this systematic review to explore the uncertainty about the benefits of using hyaluronidase in local anaesthetic mixtures to provide analgesia during eye surgery. There is considerable variation of practice, and the studies in this area show conflicting results.
Furthermore, the use of hyaluronidase increases the cost of the anaesthetic and has been associated with adverse allergic reactions in a small number of cases. For example, Kempeneers 1992, described five people who developed an orbital pseudotumour as a complication of retrobulbar anaesthesia. Allergic reactions can range from local reactions to anaphylactic (systemic allergy) shock. When hyaluronidase is added to a local anaesthetic agent, the wider spread of the local anaesthetic solution also increases its absorption and removal in the bloodstream. This shortens the duration of action of the local anaesthetic and tends to increase the incidence of systemic reactions. Some hyaluronidase products contain bovine ingredients, and due to the theoretical concerns about transmissible spongiform encephalopathies, the World Health Organization (WHO) issued guidelines regarding the use of bovine materials in the manufacture of biological and pharmaceutical products (WHO 2010).
The absence of hyaluronidase in ophthalmic regional blockade has also been associated with adverse events. An interruption in hyaluronidase supply was associated with a cluster of postoperative diplopia (double vision) Brown 1999. It was postulated that the absence of hyaluronidase caused the local anaesthetic to loculate in close proximity to the extraocular muscles and cause clinically significant myotoxicity. When hyaluronidase was unavailable once again in 2000, Brown 2001 published a repeated cluster of diplopia cases. The omission of hyaluronidase in local anaesthesia fluid leads to clinically important rises in intraocular pressure. This is thought to be due to the decreased removal and dispersal of local anaesthetic fluid from the periocular compartment (Dempsey 1997).
Therefore, use of hyaluronidase must be justified, and data must be available for clinicians and patients to make an informed decision regarding the efficacy of hyaluronidase addition. The results of this review should allow justification (or not) for the use of adjuvant hyaluronidase to improve the quality of anaesthesia and analgesia.
Objectives
To ascertain if adding hyaluronidase to local anaesthetic solutions for use in ophthalmic anaesthesia in adults results in a reduction of perceived pain during the operation and to assess harms, participant and surgical satisfaction and economic impact.
Methods
Criteria for considering studies for this review
Types of studies
We included:
-
Randomized controlled trials (RCTs) and quasi‐randomized controlled clinical trials either published or unpublished;
-
Studies if they compared equal volumes and concentrations of local anaesthetic with and without adjuvant hyaluronidase administered with the injection;
-
Studies when other adjuvants such as adrenaline were used, only if the adjuvant was present in both the control and hyaluronidase intervention;
Types of participants
We included:
-
Adults (aged 18 years and older) presenting for ophthalmic surgery under ophthalmic anaesthetic block;
-
Participants receiving sub‐Tenon's, peribulbar, retrobulbar or other types of local anaesthetic;
-
Participants receiving sedation but documented this fact;
We excluded:
-
Participants receiving adnexal surgery and any other eye surgery that was not intraocular;
-
Participants who received general anaesthesia;
Types of interventions
Ophthalmic local anaesthetic blocks comparing adjuvant hyaluronidase to an otherwise equal anaesthetic and surgery without hyaluronidase.
We considered any dose of hyaluronidase in the intervention group and any dose or type of local anaesthetic agent.
We included studies that used any number of injections to anaesthetise the eye if the number of injections was equal in the treatment and control groups.
Types of outcome measures
Primary outcomes
-
Intraoperative pain, as measured by analogue rating scales. We excluded studies that reported pain, but did not measure pain formally using analogue rating scales, as they did not provide sufficiently useful information on the outcome. We excluded studies if they reported that 'supplementary injections' were primarily given to achieve akinesia, for example, if a certain immobility score was not reached.
Secondary outcomes
-
Incidence of harm (reported as a narrative).
-
Participant and surgical satisfaction, as documented by scoring systems.
-
Economic outcomes or cost calculations (reported as a narrative).
Search methods for identification of studies
Electronic searches
We carried out systematic searches in:
-
The Cochrane Central Register of Controlled Trials( CENTRAL, 2007 Issue 6; Appendix 1);
-
Ovid MEDLINE (1946 to 30 June 2017; Appendix 2);
-
Ovid Embase (1947 to 30 June 2017; Appendix 3);
-
Web of Science (1900 to 30 June 2017; Appendix 4);
-
Scopus (1823 to 30 June 2017; Appendix 5);
-
CINAHL Plus (EBSCOhost, 1937 to 30 June 2017; Appendix 6);
-
LILACS (1982 to 30 June 2017; Appendix 7);
We broke down our research question into four key searchable concepts: " Eye," "Surgery," " Local Anaesthesia" and "Hyaluronidase".This strategy ensured that we retrieved studies on eye surgery where local anaesthesia was applied along with hyaluronidase. We conducted searches for each concept using free text terms and MeSH terms wherever possible. When we carried out free text searches, we applied synonyms, derivative forms and singular/plural forms for each concept. Detailed search steps are documented in the 'Appendices'.
We applied no language restrictions.
Searching other resources
We searched the reference lists of all eligible trials and reviews and used any trials that fit the inclusion criteria.
We searched the registers at www.controlled‐trials.com, www.ISRCTN.com and www.clinicaltrialsregister.eu for relevant trials.
We contacted specialists in the field, authors of the included trials and pharmaceutical manufacturers for any unpublished data.
The search of these other resources was completed by 30 June 2017.
Data collection and analysis
Selection of studies
Two review authors (KA and HR) independently reviewed the trials identified from the search strategy, removed duplicates and documented the reason for each trial being excluded (see Characteristics of excluded studies table). We resolved any disagreements with the studies by input from a third review author CB). We presented information regarding methods, participants, setting, interventions and outcomes in the Characteristics of included studies table. Where studies had multiple publications, we planned to collate the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references. (See Differences between protocol and review).
Data extraction and management
Two review authors (KA and HR) independently extracted and collected data on a paper form. After initial piloting this form was assessed and agreed for usability. A copy of this form is in Appendix 8. We (KA and HR) resolved any discrepancies in data extracted by discussion with a third review author (CB) as a final arbiter. In the case of additional information being required, HR or KA contacted the authors of the relevant trial.
Assessment of risk of bias in included studies
To assess the risk of bias, two review authors (KA and HR) independently assessed the studies included in the review according to the criteria described by Higgins 2011. We assessed the following aspects as being at either 'low risk', 'high risk' or 'unclear risk' of bias. We assessed the risk of bias for the following components of each trial.
-
Random sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Masking of participants and personnel (performance bias).
-
Masking of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Other bias.
We included a 'Risk of bias' table as part of the Characteristics of included studies table based on Cochrane's tool for assessing the risk of bias, from the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8:Higgins 2011). See Appendix 8 (data collection form) and Appendix 10 ('Risk of bias' table).
Measures of treatment effect
Intraoperative pain
We noted intraoperative pain measured using visual analogue scales (VAS) or verbal rating scales (VRS) when available and interpreted them as continuous data. We used the greatest intraoperative score reported. We planned to use the standardized mean difference (SMD) as an effect measure and 95% confidence intervals (CIs) to allow for the fact that different studies might have used different scales. However, because the only studies detected used the same scaling method, there was no need to use the SMD. If authors documented non‐normality of their data, we extracted medians and interquartile ranges and collated this information. (See Differences between protocol and review).
In the case of absolute numbers of participants experiencing pain where a rating scale was used but reported in a dichotomous manner (data as pain or no pain), we used risk ratios (RR) with 95% CI as a measure of effect. We collated this information and reported it. While there was evidence of moderate heterogeneity (I2 = 41%) we presented a meta‐analysis but urge caution interpreting this.
To avoid multiplicity, we restricted meta‐analyses to the primary outcomes but this would not be at the cost of presenting the totality of evidence should there be more RCTs providing information with regards to adverse effects.
Incidence of harm
We would have reported adverse events due to the use of hyaluronidase (e.g. allergic reactions) as a narrative, but as expected, there were no reports of such adverse events, probably because of their rarity and the relatively small number of participants in each trial.
Participant and surgical satisfaction
We reported participant and surgical satisfaction scores narratively. We would also have noted as narrative if other validated tools had been used.
Economic outcomes or cost calculation
We planned to report economic outcomes or cost calculations narratively.
Unit of analysis issues
We anticipated that most trials would involve one eye per participant, and even if both eyes were included, our outcomes were primarily measured at the participant rather than eye level. It was very unlikely that both eyes were operated on simultaneously with different anaesthetic procedures. (See Differences between protocol and review).
Dealing with missing data
We contacted authors and asked them to provide missing data. We imposed a time limit of two months and follow‐up on one occasion. Irrespective of the type of data, we reported dropout rates in the 'Risk of bias' tables within the Characteristics of included studies table and noted whether or not authors had compared characteristics of participants who had complete data sets against those that did not. We investigated studies that had missing data, whether or not imputing for missing cases impacted greatly on the interpretation of findings. We attempted to impute the data, but the values obtained would not be consistently reflected within the trials due to the variation in sample size.
Assessment of heterogeneity
We assessed all studies for clinical and methodological heterogeneity. We examined the I2 statistic and it's 95% CI to assess inconsistency between studies as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the thresholds advised by Higgins 2011, for the interpretation of the I2 statistic. We found substantial inconsistency in our primary outcome when assessed as a continuous score (I2 = 76%) but less when assessed as a dichotomous measure (I2 =41%). We attempted to investigate causes for this by exploring factors such as the type and duration of surgery, anaesthetic intervention (hyaluronidase dosage, type and volume of anaesthetic fluid) but the number of studies contributing to the meta‐analysis was small. Despite the heterogeneity, we presented a meta‐analysed outcome for the dichotomized outcome but urge caution in its interpretation.
Assessment of reporting biases
We assessed publication bias and small study effects in a qualitative manner using a funnel plot. We planned to test for funnel plot asymmetry if there had been a meta‐analysis with more than 10 studies included.
Data synthesis
We performed the analysis using Review Manager 5 (RevMan 2014).
While we found some evidence of heterogeneity (for the dichotomous outcome), we meta‐analysed results using a random‐effects model as per our original intentions. A random‐effects model was chosen because we believe that each trial estimates an intervention effect that follows a distribution across studies.
Subgroup analysis and investigation of heterogeneity
We planned no subgroup analyses.
Sensitivity analysis
We carried out sensitivity analyses to explore the robustness of the results to key methodological decisions that we made in our review. We examined whether or not excluding studies at risk of bias impacted on our findings, and since we had missing data, we attempted to impute and examine whether or not analysing intention‐to‐treat data differed considerably from the available case meta‐analysis.
GRADE assessment of quality of evidence
We adopted the GRADE system postprotocol (Rüschen 2013) to rate the quality of evidence for each outcome (Guyatt 2011). GRADE assessment classifies the quality of evidence into four categories; high, moderate, low and very low. The overall assessment considers the study design, risk of bias, imprecision, inconsistency, indirectness, publication bias, large effect size, dose‐response effect and presence of confounding factors to rate the evidence. We applied the principles of GRADE to assess the quality of evidence specific to each outcome in our review.
-
Intraoperative pain, as measured by analogue rating scales.
-
Incidence of harm.
-
Participant and surgical satisfaction, as documented by scoring systems.
-
Economic outcomes or cost calculations.
The GRADE software from GRADEpro GDT generated the 'Summary of findings' table. The GRADE approach ensures the confidence which one can have in the estimate of effect from the outcomes being assessed in the included studies.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
See Figure 1 for the study flow diagram.

Study flow diagram.
We identified 942 references after removal of duplicates. Two review authors (HR and KA) independently read and analysed the abstracts of all references and if needed, full papers. We excluded 779 references as clearly irrelevant to the review. We obtained the full papers for the remaining 163 references and again analysed them independently. We identified 18 trials, and 11 trials were further excluded for reasons documented in the 'Characteristics of excluded studies' table. The review includes seven trials.
Included studies
Design
We included seven RCTs published from 1995 to 2012 with 500 participants (Bowman 1997; Brydon 1995; Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002). One study was published in Portuguese and translated into English (Shiroma 2002).
Characteristics of study population
The review included 500 participants.The participants were adults (aged 18 years or older) presenting for ophthalmic surgery undergoing a retrobulbar, peribulbar or sub‐Tenon block. The mean age in the studies ranged from 66 to 77 years. Studies were balanced with regards to gender.
Setting
Four studies were conducted in the UK (Bowman 1997; Brydon 1995; Khandwala 2008; Rowley 2000). The remaining three studies were based in Germany (Remy 2008), Brazil (Shiroma 2002), and Iran (Sedghipour 2012).
Intervention
The seven trials studied the effect of adding hyaluronidase to a local anaesthetic mixture with the primary outcome measure of reduction of intraoperative pain. The participants were divided into a treatment group (hyaluronidase) and a control group (no hyaluronidase). The doses of hyaluronidase used ranged from 15 IU/mL to 150 IU/mL.
All seven trials assessed pain objectively using either the VAS or the VRS and compared a group with hyaluronidase to a group without hyaluronidase (Bowman 1997; Brydon 1995; Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002).
One trial included three arms in their study looking at effects of no hyaluronidase and effects of hyaluronidase at 50 IU/mL and 150 IU/mL (Brydon 1995). We combined the results of the groups with different doses of hyaluronidase and compared them with the no hyaluronidase group.
Funding sources
Two trials reported no conflict of interest and received no funding support for the trials (Khandwala 2008; Remy 2008). There was no clear documentation of reported conflict of interest or funding support from the remaining five trials (Bowman 1997; Brydon 1995; Rowley 2000; Sedghipour 2012; Shiroma 2002).
We attempted to contact the authors of all the trials for additional data but received no clarification.
For more details about the included trials, see the Characteristics of included studies table.
Excluded studies
We excluded 11 studies after analysis (Berg 2001; Crawford 1994; Guise 1999; House 1991; Johansen 1993; Lange 1989; Moharib 2002; Morsman 1992; Ramanathan 1999; Sarvela 1992; Soares 2002).
Three studies reported that "supplementary injections" were primarily given to achieve akinesia, for example, if a certain immobility score was not reached. Pain during surgery was not assessed or reported specifically.(Crawford 1994; House 1991; Soares 2002).
One study did not mask the relevant part of the trial (Morsman 1992).
Four studies did not assess pain or discomfort using a rating scale, and the inclusion criteria were ultimately not met (Berg 2001; Guise 1999; Johansen 1993; Moharib 2002). We excluded these studies because they did not measure the outcome measure of "pain by rating scale". They reported an unstructured description of pain. This is unlikely to provide useful information about the levels of pain during the operation.
One study was published as a poster presentation (Ramanathan 1999). We contacted the author for more details about randomization, masking and results but none was made available.
We excluded one study because there was no randomization, this was not immediately obvious (Lange 1989).
We excluded one study because the relevant part of the study was not randomized and only compared different concentrations of hyaluronidase (Sarvela 1992).
See Characteristics of excluded studies table.
Awaiting classification
We found no studies awaiting classification.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We judged the quality of studies according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Overall, the selected studies were of low risk; however, there was a lack of concise information in the methodology of randomization and withdrawal description. Please refer to Figure 2 and Figure 3 for a summary of risk of bias assessment for the selected studies and a 'Risk of bias' graph that represents the studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All seven included studies reported that allocation was randomized, but only three studies provided any detail about the method of random allocation (Khandwala 2008; Rowley 2000; Sedghipour 2012). These three studies were at low risk of allocation bias. The studies used random number tables, stratified lottery system or computer generated randomization. The remaining four studies were at unclear risk of bias.
With regard to allocation concealment, four of the seven studies used "coded syringes" (Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012). We classed these at low risk of bias, but an experienced operator might have recognized the formation of tiny bubbles in the mixture indicating hyaluronidase content.
Most studies implied that participants, personnel and assessors were unaware of the composition of the anaesthetic solution because the syringes were coded by an uninvolved third party, but exact details were rarely given.
In this context, only one study used a placebo control, with inactive Hyalase (Remy 2008).
Blinding
Five studies were classified as low risk for performance and detection bias (Bowman 1997; Khandwala 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002). These studies had described double masking where neither the participant nor the caregiver was aware of the contents of the syringe. The outcome assessors were also masked, which further reduced the risk of bias by masking.
Incomplete outcome data
Withdrawal of participants after randomization was rarely reported in detail.
Six studies were at low risk as there was a description of no withdrawals; therefore, we had more confidence in the intention‐to‐treat analysis for these studies (Bowman 1997; Brydon 1995; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002).
Of the included seven studies, one described the withdrawal of participants after randomization (Khandwala 2008). One participant was withdrawn from the treatment group due to incomplete data. No further details were given. Considering the low number of participants in this trial (10 in each of two arms) the exclusion may represent bias.
Selective reporting
We found all seven studies at low risk of reporting bias. Pain was stated as an outcome measure at the start of the trials. We did not attempt to obtain research protocols (Bowman 1997; Brydon 1995; Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002).
Other potential sources of bias
We found no other potential sources of bias.
Effects of interventions
Primary outcomes
1. Intraoperative pain, as measured by analogue rating scales
Seven trials with 500 participants looked at the effect of hyaluronidase on the reduction of intraoperative pain. Four of these trials with 289 participants reported intraoperative pain in a dichotomous manner (Bowman 1997; RR 0.82, 95% CI 0.38 to 1.75; Brydon 1995; RR 1.00, 95% CI 0.20 to 5.00; Remy 2008; RR 0.47, 95% CI 0.24 to 0.92; Shiroma 2002; RR 1.45, 95% CI 0.70 to 3.00). We calculated the (RR) as a measure of effect. The I2 statistic was 41%, which represents moderate heterogeneity. We proceeded to meta‐analyse the results and found that the pooled RR was 0.83 (95% CI 0.48 to 1.42). Therefore, there was no statistically significant reduction of pain scores in the hyaluronidase group.
Three studies involving 211 participants reported pain objectively using rating scales and presented continuous data (Khandwala 2008; Rowley 2000; Sedghipour 2012). Sedghipour 2012 described 42 participants in a high quality study and provided SDs for their data. They found a significant reduction of pain in the hyaluronidase group (P = 0.04). The other two trials did not report SDs (Khandwala 2008; Rowley 2000). We interchanged the SDs from Sedghipour 2012, but this resulted in significant heterogeneity among the studies (I²= 76%), so we did not perform a meta‐analysis. See Figure 4 and Figure 5.

Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.1 Intraoperative pain (measured by analogue rating scales; reported continuous).

Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.2 Intraoperative pain (measured by analogue rating scales; reported dichotomous).
Looking at the overall studies taking into account the results of the meta‐analysis and the individual studies that reported the continuous outcome, there was no statistically significant reduction in intraoperative pain with hyaluronidase in the local anaesthetic mixture. However, this has to be interpreted with caution.
We adopted the GRADEpro method of analysing the quality of evidence and produced summary of findings Table for the main comparison. We found the quality of studies was low. We downgraded the quality of evidence due to concerns regarding inconsistency in the direction and magnitude of effect across the studies (I2 = 41% and 76%). We looked at the individual studies for factors that could have contributed to the heterogeneity and found that there was no wide variability between characteristics of participants, interventions and outcome measures. We tried to establish if the heterogeneity was due to the dose of hyaluronidase, the volume of injection or number of participants, but there was insufficient data provided to enable a valid analysis. The level of imprecision was another reason for downgrading the quality of evidence. Only Rowley 2000, provided a rationale for the selected sample size that would yield the specific effect measure.
Secondary outcomes
1. Incidence of harm
None of the included studies measured or reported the incidence of harm from hyaluronidase.
2. Participant and surgical satisfaction, as documented by scoring systems
Two studies analysing 122 participants looking at participant satisfaction reported that the investigator and participant assessment scores were significantly higher in the hyaluronidase group (P < 0.05)(Remy 2008; Sedghipour 2012). The studies assessed satisfaction in different ways so prohibiting meta‐analysis Remy 2008 used a five level VAS to assess participant efficacy and tolerability at the end of surgery and at the final visit, while Sedghipour 2012 captured data as a dichotomous 'satisfied' or 'unsatisfied'. Using the GRADE system to assess the quality of evidence, we found the studies were of moderate quality with low risk of bias.
We found three studies involving 141 participants that measured surgical satisfaction scores (Khandwala 2008; Remy 2008; Sedghipour 2012). Sedghipour 2012 reported that surgical satisfaction with intraoperative anaesthesia was 85.7% in the hyaluronidase group compared to 52.5% in the control group (P = 0.02). Remy 2008 reported a P value of less than 0.001 for surgical satisfaction in the hyaluronidase group. Khandwala 2008 found no difference in the quality of the surgical field between groups (P = 0.96). These studies assessed surgeon satisfaction as they had assessed participant satisfaction while Khandwala 2008 simply asked surgeons to rate surgical conditions on a VAS from 0 (worst) to 10 (best). Since each had assessed satisfaction using a different method, no meta‐analysis was conducted. There was low risk of bias with regards to the method of randomization with Sedghipour 2012, and there was incomplete outcome data reporting with Khandwala 2008. Overall, we found the quality of evidence with these two studies to be moderate. See Figure 2.
3. Economic outcomes or cost calculations
None of the included studies reported on the economic impact of using hyaluronidase.
Discussion
The use of hyaluronidase in ophthalmic surgery remains a topic of debate. The perceived advantages of adding hyaluronidase include shortened time to onset of the block and improved akinesia, and as investigated in this review: analgesia. The apparent disadvantages of hyaluronidase include the additional cost and possible adverse reactions.
Most modern surgical techniques are no longer essentially dependent on akinesia. Anaesthesia itself is readily provided by eye blocks without hyaluronidase. Even topical anaesthesia is often deemed sufficient during routine cataract surgery. We systematically reviewed the literature on the benefits of hyaluronidase for analgesia in ophthalmic surgery.
Summary of main results
We reviewed evidence from seven RCTs involving 500 participants regarding the reduction of pain during intraocular surgery by adding hyaluronidase to the local anaesthetic fluid. We found that the reduction of intraoperative pain by hyaluronidase was not statistically significant. The quality of evidence was low. We assessed the literature in this field as having a low risk of bias, but we had concerns regarding heterogeneity across the studies. With regards to the outcomes of participant and surgeon satisfaction, the moderate quality studies show an advantage of using hyaluronidase. (See summary of findings Table for the main comparison).
Overall completeness and applicability of evidence
We are confident that our search strategy obtained all available studies. The results of this review are applicable to all adults undergoing intraocular surgery who would want to make an informed decision regarding the use of hyaluronidase as an adjunct in eye blocks to reduce intraoperative pain. We found that the use of hyaluronidase is beneficial in terms of participant and surgical satisfaction. Such benefit from using hyaluronidase was not statistically significant with regards to intraoperative reduction of pain.
We consider that most of the authors gave priority to akinesia as an outcome measure over analgesia, probably due to the perceived importance for the safe conduct of surgery. This priority has now receded as the majority of surgeons can carry out most operations without depending on fully established akinesia. Profound akinesia will still be necessary for more difficult operations and training situations. Hyaluronidase may be necessary to achieve akinesia in such situations.
Quality of the evidence
We found the overall quality of evidence to be low due imprecision and inconsistency of the results. There was moderate heterogeneity (I² = 41%). Therefore, we downgraded the quality of evidence by one level. We looked for possible causes for the variation such as sample size, dose of hyaluronidase or characteristics of participants but data were sparse.
We found the overall risk of bias in the studies to be low. However, there was an unclear risk with regards to methods of randomization and concealment in a few studies.
As for imprecision, failure to estimate the sample size needed to make an effect by six of the seven studies led to the quality of evidence to be downgraded by one level.
Potential biases in the review process
A potential bias arises from the narrow spectrum of the review question: "Does hyaluronidase improve pain control during eye surgery?" Hyaluronidase is used for a variety of indications. For example, if the speed of onset of anaesthesia is increased by hyaluronidase and the eye is much more akinetic at the beginning of the operation, participant and surgeon comfort will also likely be increased. Therefore, the narrow aspect of analgesia alone does not consider the full spectrum of beneficial effects from hyaluronidase.
This review found only a relatively small number of studies (seven) with a small number of participants (500). However, can systematic reviews with such sparse data be trusted (Afshari 2017)? During this Cochrane Review, we adhered to all essential requirements such as publishing a protocol, incorporating risk of bias assessment, searching for unpublished data and many other review tools as laid out in the Cochrane framework (Higgins 2011).
According to our protocol, we excluded studies that did not assess pain in a structured manner (using rating scales). Because of this, we excluded unstructured assessments that may have shown results. We consider that results from these trials would have a high risk of reporting bias and therefore, would not produce a reliable, useful effect.
Lack of reported data also led to certain included trials being excluded from the meta‐analysis. We were unable to obtain clarification about these issues from authors of the included studies.
Readers of this review may be interested in the incidence of adverse effects of hyaluronidase use. Adverse effects such as allergy to hyaluronidase are extremely rare. None of the trials we analysed reported any adverse events related to hyaluronidase, but we would like to highlight that our review would not have reliably captured the incidence of very rare adverse events due to an overall small number of participants.
We have acknowledged and taken into account the inherent methodological limitation of our systematic review and in our opinion addressed them adequately.
Agreements and disagreements with other studies or reviews
Our findings match that of the review of Schein 2000. Their review reported the same problems with the available literature that we found. Those are;
-
very few studies reported data on the effect of hyaluronidase on pain;
-
high levels of inconsistency among the included studies.
Schein's review was written in 2000, and despite many additional studies having been published since, there is still no certainty on the effect of hyaluronidase use for analgesia.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.1 Intraoperative pain (measured by analogue rating scales; reported continuous).

Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.2 Intraoperative pain (measured by analogue rating scales; reported dichotomous).

Comparison 1 Hyaluronidase versus control, Outcome 1 Intraoperative pain (reported continuous).
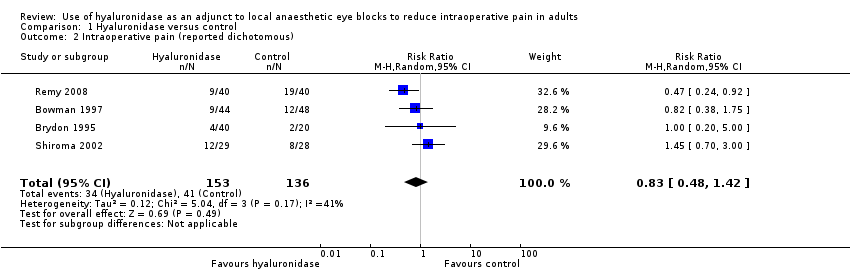
Comparison 1 Hyaluronidase versus control, Outcome 2 Intraoperative pain (reported dichotomous).
| Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults | ||||||
| Patients or population: adults (aged ≥ 18 years) undergoing ophthalmic surgery under local anaesthetic eye blocks. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no hyaluronidase | Risk with hyaluronidase | |||||
| Intraoperative pain (reported dichotomous) | RR 0.83 | 289 | ⊕⊕⊝⊝ | ‐ | ||
| 301 per 1000 | 250 per 1000 | |||||
| Intraoperative pain (reported continuous) | 3 trials looked at effect of hyaluronidase on reduction of intraoperative pain measured by rating scales. Results were reported as continuous data. 2 studies did not provide the SMD, which measures the effect in a clinical setting, the results could not be meta‐analysed and hence were reported narratively (Khandwala 2008; Rowley 2000). Among the 3 trials covering 211 participants (Khandwala 2008: Mean difference 0.70; Rowley 2000: Mean difference 0.31; Sedghipour 2012: Mean difference ‐1.10), only the Sedghipour study with 42 participants, which is a high quality study, showed a statistically significant (at the 5 % level) reduction in pain in the hyaluronidase group (P = 0.04). The remaining 2 studies with 169 participants showed no statistically significant (at the 5 % level) reduction of pain intraoperatively with hyaluronidase (Khandwala 2008: P = 0.5; Rowley 2000: n.s). These studies were also of high quality and low risk of bias. Khandwala and colleagues had an unclear attrition bias as 1/10 participants in the treatment group was dropped after randomization with no clear explanation. | ‐ | 211 | ⊕⊕⊝⊝ | ‐ | |
| Incidence of harm | None of the studies reported harms in relation to hyaluronidase. | ‐ | (0 studies) | ‐ | ‐ | |
| Participant satisfaction | Significantly better satisfaction in these well designed studies with low risk of bias (Remy 2008; Sedghipour 2012). The studies included 122 participants and showed higher satisfaction scores in the treatment group (P < 0.05). | ‐ | 122 | ⊕⊕⊕⊝ | ‐ | |
| Surgical satisfaction | Surgical satisfaction was reportedly superior with hyaluronidase in the larger 2 studies (Remy 2008: P < 0.001; Sedghipour 2012: P = 0.02) and not significantly different in 1 small study (Khandwala 2008: P = 0.96). | ‐ | 141 | ⊕⊕⊕⊝ | ‐ | |
| Economic outcomes or cost calculations | None of the included studies reported economic outcomes or cost calculations | ‐ | (0 RCTs) | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n.s: not statistically significant; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to marked heterogeneity with a calculated I2 > 50%. 2Downgraded one level for imprecision due to wide 95% confidence intervals, reflecting uncertainty in the direction of effect estimate. 3Downgraded one level for imprecision and inconsistency in measurement, lack of data and small sample size. 4Downgraded one level because of imprecision secondary to small sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intraoperative pain (reported continuous) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Intraoperative pain (reported dichotomous) Show forest plot | 4 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |

