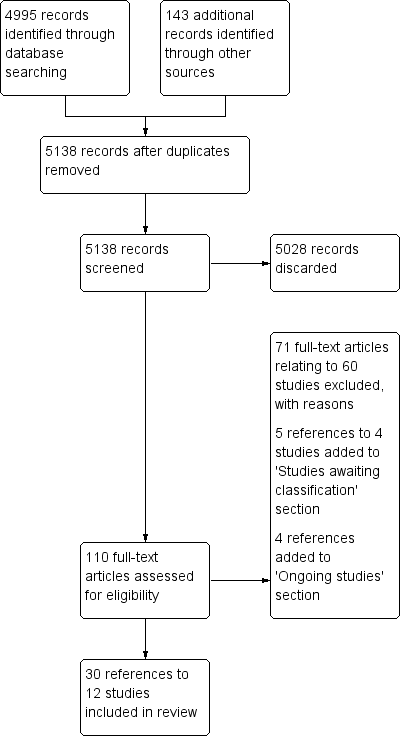
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) |
| Any event | All grades: n = 431 (97%);
Grades 3‐4: n = 395 (89%) | All grades: n = 434 (97%);
Grades 3‐4: n = 389 (87%) |
| Dysphagia | All grades: n = 364 (82%);
Grades 3‐4: n = 235 (53%) | All grades: n = 384 (86%);
Grades 3‐4: n = 255 (57%) |
| Mucositis | All grades: n = 364 (82%);
Grades 3‐4: n = 191 (43%) | All grades: n = 322 (72%);
Grades 3‐4: n = 147 (33%) |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%);
Grades 3‐4: n = 89 (20%) | All grades: n = 63 (14%);
Grades 3‐4: n = 4 (1%) |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%);
Grades 3‐4: n = 111 (25%) | All grades: n = 353 (79%);
Grades 3‐4: n = 67 (15%) |
| Fatigue | All grades: n = 289 (65%);
Grades 3‐4: n = 62 (14%) | All grades: n = 268 (60%);
Grades 3‐4: n = 40 (9%) |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) |
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) |
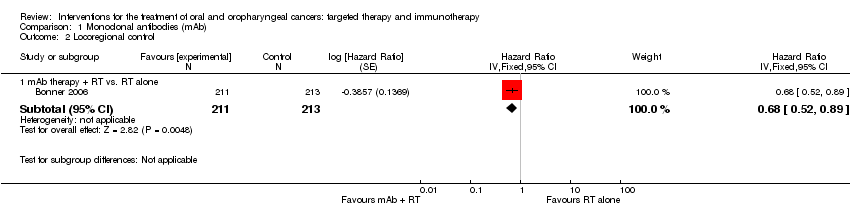
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps |
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) |
| Dry mouth | n = 9 (17%) | n = 12 (23%) |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) |
| Odynophagia | n = 4 (7%) | n = 6 (12%) |