Не‐хирургические вмешательства в лечении тяжелых менструальных кровотечений (меноррагии) у женщин с нарушениями свертываемости крови
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010338.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 26 noviembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Sujoy Ray: conceptualising the review topic; developing and drafting the final version of the protocol; searching for additional references and subsequently developing and drafting the final version of the review.
Amita Ray: conceptualising the review topic; developing the protocol; providing citations and full text articles for the 'Background' and 'Objectives' sections and drafting the final version of the review.
Sources of support
Internal sources
-
None, Other.
External sources
-
South Asian Cochrane Network and Centre, BV Moses and ICMR centre for Advanced Research and Training Christian Medical College Vellore, Tamilnadu, India, India.
Declarations of interest
None known.
Acknowledgements
We gratefully acknowledge the contribution of Ms Gurpreet Rana, Medical Librarian, University of Michigan, USA for designing and conducting the Embase search for this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Nov 10 | Non‐surgical interventions for treating heavy menstrual bleeding (menorrhagia) in women with bleeding disorders | Review | Sujoy Ray, Amita Ray | |
| 2014 Nov 26 | Non‐surgical interventions for treating heavy menstrual bleeding (menorrhagia) in women with bleeding disorders | Review | Sujoy Ray, Amita Ray | |
| 2013 Jan 31 | Non‐surgical interventions for treating heavy menstrual bleeding (menorrhagia) in women with bleeding disorders | Protocol | Sujoy Ray, Amita Ray, Aneesh Thomas George | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Blood Coagulation Disorders [complications, *drug therapy];
- Deamino Arginine Vasopressin [adverse effects, *therapeutic use];
- Hemostatics [adverse effects, *therapeutic use];
- Menorrhagia [*drug therapy, etiology];
- Randomized Controlled Trials as Topic;
- Tranexamic Acid [adverse effects, *therapeutic use];
Medical Subject Headings Check Words
Adult; Female; Humans;
PICO

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 DDAVP Vs Placebo, outcome: 1.1 Adverse effects.

Forest plot of comparison: 2 Desmopressin versus Tranaxemic Acid, outcome: 2.1 Adverse effects.

Comparison 1 Desmopressin versus placebo, Outcome 1 Adverse effects.
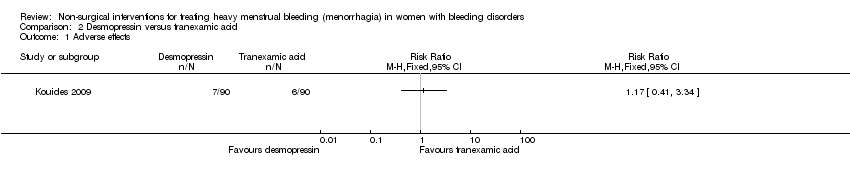
Comparison 2 Desmopressin versus tranexamic acid, Outcome 1 Adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

