Blood pressure targets for the treatment of people with hypertension and cardiovascular disease
Abstract
Background
This is the first update of the review published in 2017. Hypertension is a prominent preventable cause of premature morbidity and mortality. People with hypertension and established cardiovascular disease are at particularly high risk, so reducing blood pressure to below standard targets may be beneficial. This strategy could reduce cardiovascular mortality and morbidity but could also increase adverse events. The optimal blood pressure target in people with hypertension and established cardiovascular disease remains unknown.
Objectives
To determine if 'lower' blood pressure targets (≤ 135/85 mmHg) are associated with reduction in mortality and morbidity as compared with 'standard' blood pressure targets (≤ 140 to 160/90 to 100 mmHg) in the treatment of people with hypertension and a history of cardiovascular disease (myocardial infarction, angina, stroke, peripheral vascular occlusive disease).
Search methods
For this updated review, the Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials up to February 2018: Cochrane Hypertension Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from 1946), Embase (from 1974), and Latin American Caribbean Health Sciences Literature (LILACS) (from 1982), along with the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov. We also contacted authors of relevant papers regarding further published and unpublished work. We applied no language restrictions.
Selection criteria
We included randomized controlled trials (RCTs) that included more than 50 participants per group and provided at least six months' follow‐up. Trial reports had to present data for at least one primary outcome (total mortality, serious adverse events, total cardiovascular events, cardiovascular mortality). Eligible interventions involved lower targets for systolic/diastolic blood pressure (≤ 135/85 mmHg) compared with standard targets for blood pressure (≤ 140 to 160/90 to 100 mmHg).
Participants were adults with documented hypertension and adults receiving treatment for hypertension with a cardiovascular history for myocardial infarction, stroke, chronic peripheral vascular occlusive disease, or angina pectoris.
Data collection and analysis
Two review authors independently assessed search results and extracted data using standard methodological procedures expected by Cochrane.
Main results
We included six RCTs that involved a total of 9484 participants. Mean follow‐up was 3.7 years (range 1.0 to 4.7 years). All RCTs provided individual participant data.
We found no change in total mortality (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.91 to 1.23) or cardiovascular mortality (RR 1.03, 95% CI 0.82 to 1.29; moderate‐quality evidence). Similarly, we found no differences in serious adverse events (RR 1.01, 95% CI 0.94 to 1.08; low‐quality evidence) or total cardiovascular events (including myocardial infarction, stroke, sudden death, hospitalization, or death from congestive heart failure) (RR 0.89, 95% CI 0.80 to 1.00; low‐quality evidence). Studies reported more participant withdrawals due to adverse effects in the lower target arm (RR 8.16, 95% CI 2.06 to 32.28; very low‐quality evidence). Blood pressures were lower in the lower target group by 8.9/4.5 mmHg. More drugs were needed in the lower target group, but blood pressure targets were achieved more frequently in the standard target group.
Authors' conclusions
We found no evidence of a difference in total mortality, serious adverse events, or total cardiovascular events between people with hypertension and cardiovascular disease treated to a lower or to a standard blood pressure target. This suggests that no net health benefit is derived from a lower systolic blood pressure target. We found very limited evidence on adverse events, which led to high uncertainty. At present, evidence is insufficient to justify lower blood pressure targets (≤ 135/85 mmHg) in people with hypertension and established cardiovascular disease. More trials are needed to examine this topic.
PICO
Plain language summary
Blood pressure targets in people with cardiovascular disease
Review question
We assessed whether lower blood pressure goals are better than standard blood pressure goals for people with high blood pressure who also have heart or vascular problems.
Background
Many people with heart or vascular problems also have high blood pressure. Some clinical guidelines recommend a lower blood pressure goal (135/85 mmHg or lower) for people with previous heart or vascular problems than for with those without (≤ 140 to 160 mmHg systolic and ≤ 90 to 100 mmHg diastolic are standard blood pressure goals). It is unclear whether lower goals lead to overall health benefits.
Search date
We searched for evidence up to February 2018.
Study characteristics
For this updated review, we included six trials with 9484 participants who were followed‐up from one year to 4.7 years. We analyzed data to detect differences between lower and standard blood pressure goals in terms of numbers of deaths and numbers of serious adverse events (leading to hospital admission).
Key results
We found no differences in total numbers of deaths, heart or vascular deaths, total heart problems, or vascular problems, nor in total serious harms, between lower and standard blood pressure goal approaches. Based on very little information, we found more dropouts resulting from drug‐related harms in the lower blood pressure target group and no overall health benefit among people in the lower target group.
Quality of the evidence
The best available evidence does not support lower blood pressure goals over standard goals in people with elevated blood pressure and heart or vascular problems. More new trials are needed to examine this question. Overall, the quality of evidence was assessed as low to moderate according to the GRADE assessment.
Authors' conclusions
Summary of findings
| Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity | ||||||
| Patient or population: cardiovascular disease with high blood pressure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard blood pressure target | Risk with lower blood pressure target | |||||
| Total mortality | Study population | RR 1.06 | 9484 | ⊕⊕⊕⊝ | ||
| 68 per 1000 | 72 per 1000 | |||||
| Serious adverse events | Study population | RR 1.01 | 9484 | ⊕⊕⊝⊝ | ||
| 252 per 1000 | 255 per 1000 | |||||
| Total cardiovascular events | Study population | RR 0.89 | 9484 | ⊕⊕⊝⊝ | ||
| 127 per 1000 | 113 per 1000 | |||||
| Cardiovascular mortality | Study population | RR 1.03 (0.82 to 1.29) | 9484 (6 RCTs) | ⊕⊕⊕⊝ | ||
| 31 per 1000 | 32 per 1000 (25 to 40) | |||||
| Withdrawals due to adverse effects | Study population | RR 8.16 | 690 | ⊕⊝⊝⊝ | ||
| 7 per 1000 | 60 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level owing to serious imprecision (95% CI is wider than the minimal important difference). bDowngraded one level owing to incomplete available data. cDowngraded one level owing to high risk of bias. dDowngraded two levels because only two of the smaller studies reported this outcome. | ||||||
Background
Description of the condition
Hypertension (high blood pressure) is one of the most preventable causes of premature morbidity and mortality worldwide. Researchers described hypertension as the second leading risk factor for the global burden of disease in 2013 (Forouzanfar 2015). Hypertension is a major risk factor for ischemic and hemorrhagic stroke, myocardial infarction, heart failure, chronic kidney disease, peripheral vascular disease (PVD), cognitive decline, and premature death (NICE 2016).
Historically more emphasis was placed on diastolic than on systolic blood pressure as a predictor of cardiovascular morbidity and fatal events. However a large number of observational studies have revealed that both systolic and diastolic blood pressures show a graded independent relationship with mortality and morbidity (ESH‐ESC 2013). Untreated hypertension may be associated with a progressive rise in blood pressure, possibly culminating in a treatment‐resistant state caused by associated vascular and kidney damage (NICE 2016).
Epidemiological studies suggest that the risk associated with high blood pressure is a continuous relationship, and for blood pressures above 115/70 mmHg, the risk of cardiovascular events doubles for every 20/10 mmHg rise in blood pressure. This suggests that for every 20 mmHg lower systolic blood pressure (SBP) or 10 mmHg lower diastolic blood pressure (DBP), the risk of a cardiovascular event is reduced by about 50% (Lewington 2002).
Blood pressure is normally distributed within a population, and there is no natural cutoff point above which hypertension definitively exists and below which it does not. In any individual person, systolic and/or diastolic blood pressure may be elevated. Diastolic pressure is more commonly elevated among people younger than 50 years. With aging, systolic hypertension becomes a more significant problem as a result of progressive stiffening and loss of compliance of larger arteries (NICE 2016).
Cardiovascular disease remains the leading cause of death around the world (Townsend 2016). Cardiovascular disease accounts for more deaths than all communicable, neonatal, maternal, and nutritional disorders combined, and double the number of deaths caused by cancers. Globally, cardiovascular disease accounts for approximately 17 million deaths annually ‐ nearly one‐third of the total number of deaths. Among these, complications of hypertension account for 9.4 million deaths worldwide every year. Despite this, between 1990 and 2013, age‐standardized death rates fell by 22% for cardiovascular and circulatory diseases, mainly as the result of trends in high‐ and middle‐income countries (GBD 2013). Both ischemic heart disease (IHD) and cerebrovascular disease are considered to be major cardiovascular diseases, resulting in 130 million disability‐adjusted life‐years lost in 2010 (WHO 2010).
Thus, cardiovascular secondary prevention is considered to be a key issue. People who have had atherosclerotic stroke should be included among those deemed to be at high risk (20% over 10 years) of further atherosclerotic coronary events. A significant percentage of those who have a first myocardial infarction are expected to experience recurrent myocardial infarction, heart failure, stroke, or fatal coronary heart disease (CHD). In fact, within five years of a first myocardial infarction, around 20% to 30% of the population aged over 65 years will experience recurrent myocardial infarction or fatal CHD (Mozaffarian 2015).
Description of the intervention
Clinicians use target blood pressures in clinical practice to make treatment decisions related to the intensity of antihypertensive therapy for each patient.
The standard blood pressure target has generally been an arbitrary threshold blood pressure above which treatment is recommended. Over time, this threshold has become lower. The standard systolic blood pressure target declined from a target of ≤ 160 mmHg to a target of ≤ 140 mmHg, and the diastolic blood pressure target has decreased from ≤ 100 mmHg to ≤ 90 mmHg in people aged up to 80 years (ESH‐ESC 2007; NICE 2016). Even lower blood pressure targets have been proposed for people with a history of cardiovascular events (AHA 2007; ESH‐ESC 2007; JNC‐7 2003).
More recently, a review of available evidence led to a reappraisal of some recommendations made by international guidelines, particularly among older people and those with diabetes or previous cardiovascular disease (ESH‐ESC 2013; JNC‐8 2014; Joint ESC 2016). However, the last update of the US Guidelines has turned again to recommend more intensive goals (ACC‐AHA 2017).
How the intervention might work
Some evidence suggests that for people at high risk, thresholds for antihypertensive treatment should be lower than for those at lower risk. It has also been suggested that to maximize the cost‐effectiveness of hypertension management, the intensity of the therapeutic approach should be graded as a function of total cardiovascular risk (ESH‐ESC 2007). However, we noted a trend toward homogenizing blood pressure goals. For example, European guidelines on hypertension recommend a goal < 140/90 mmHg in most clinical situations (ESH‐ESC 2013).
People with a history of cardiovascular disease are considered to represent a high‐risk population. The effect of lowering blood pressure values in these people could include greater absolute reduction in morbidity and mortality but could also be associated with an absolute increase in adverse events.
Reducing blood pressure to below standard targets through drug therapy has been recommended in guidelines as a strategy for those with a history of cardiovascular disease. Nevertheless, lower may not always be better. Researchers have described a J‐curve for blood pressure in coronary artery disease (Bangalore 2010; Messerli 2006). Bangalore 2010 reported that for people with coronary artery disease, low blood pressure (< 110 to 120/60 to 70 mmHg) was associated with increased risk of future cardiovascular events.
A recent cohort study explored the association between achieved blood pressure and cardiovascular events in people with hypertension and a history of coronary disease (Vidal‐Petiot 2016). These investigators concluded that when a goal < 120/70 mmHg was reached, an association with more cardiovascular adverse events was detected, supporting the J‐curve hypothesis (Vidal‐Petiot 2016).
Uncertainty remains regarding many aspects of this controversial topic, leading to differing opinions (Mancia 2014; Verdecchia 2014).
Why it is important to do this review
The arterial pressure threshold above which benefits of treatment outweigh harms in people with hypertension and cardiovascular disease is unclear.
Some, but not all, clinical guidelines have recommended blood pressure targets lower than standard targets. Following are recommendations for blood pressure targets in people with hypertension and cardiovascular disease as stated in recently published guidelines.
The Joint National Committee‐7 Report recommended blood pressure targets < 140/90 mmHg for people with uncomplicated hypertension, and blood pressure targets < 130/80 mmHg for people with hypertension and either diabetes or kidney disease (JNC‐7 2003). However, an updated statement in 2014 reflects some changes in the goals policy (JNC‐8 2014). JNC‐8 2014 suggests treating to goals of SBP < 150 mmHg and DBP < 90 mmHg in the general population aged 60 years and older. In the general population aged up to 60 years, the guideline maintains the recommendation of treating to goals of SBP < 140 mmHg and DBP < 90 mmHg. In people with diabetes or kidney disease, new targets are similar to those for the general population. JNC‐8 2014 provides no direct recommendation for those with previous cardiovascular disease, although this is acknowledged as a relevant question to be assessed and answered. The last update recommends a blood pressure target < 130/80 mmHg for adults with confirmed hypertension and known cardiovascular disease (ACC‐AHA 2017).
The 2007 European Society of Hypertension and European Society of Cardiovascular Guidelines for Management of Arterial Hypertension recommended that blood pressure should be reduced to < 140/90 mmHg (systolic/diastolic) and to lower values, if tolerated, in all people with hypertension (ESH‐ESC 2007). The blood pressure goal was < 130/80 mmHg for people with diabetes and others at high risk, such as those with associated clinical conditions (stroke, myocardial infarction, kidney dysfunction, proteinuria). Reappraisal of European guidelines on hypertension management remarks that the recommendation to lower blood pressure to ≤ 130/80 mmHg for people with diabetes or a history of cardiovascular disease is not supported by incontrovertible trial evidence (ESH 2009). The most recent update proposed an SBP goal < 140 mmHg for those at low to moderate cardiovascular risk, or with diabetes, previous stroke, CHD, or kidney disease (ESH‐ESC 2013). For older people with hypertension, good evidence is considered to recommend reducing SBP to between 150 and 140 mmHg, regardless of age, provided individuals are in good physical and mental health. A DBP target of 90 mmHg is always recommended, except for those with diabetes, for whom values < 85 mmHg are suggested.
The 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice indicate that evidence was sufficient to recommend a blood pressure target < 140/90 mmHg for all people who are hypertensive (except older people, for whom the benefit has not been tested in randomized trials) (Joint ESC 2016). According to Joint ESC 2016, the recommendation to aim for a lower systolic blood pressure goal < 130 mmHg in people with diabetes and those at very high cardiovascular risk (previous cardiovascular events) is not consistently supported by trial evidence. Thus, it would be prudent to recommend lowering blood pressure to values within the range from 130 to 139/80 to 85 mmHg, and possibly closer to lower values in this range for all people with hypertension.
In its Recommendations for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension, the 2015 Canadian Hypertension Education Program made a proposal to reach blood pressure targets < 140/90 mmHg in most situations, including for people with previous cardiovascular disease (CHEP 2015). Nevertheless, the last update of this guideline is prone to an intensive intervention in some people with high cardiovascular risk, including those with cardiovascular disease (CHEP 2018). Specifically, the guideline calls for consideration of a < 120 mmHg target, taking into account the SPRINT results (SPRINT 2015).
A Cochrane Review found that treating hypertension to lower than standard blood pressure target ≤ 140 to 160/90 to 100 mmHg was not proven to reduce mortality or morbidity in the overall population (Arguedas 2009). Another Cochrane Review analyzing the same question in people with diabetes found a reduction in the incidence of stroke with the lower goal but a significant increase in the number of serious adverse events (Arguedas 2013).
Two non‐Cochrane reviews on this issue have also been published (Ettehad 2016; Xie 2016). Ettehad 2016 combined data from all relevant clinical trials published on blood pressure reduction. Review authors estimated effects of a blood pressure decrease in terms of mortality or cardiovascular morbidity, and according to different basal characteristics, such as established cardiovascular disease. A decrease in mortality and in other cardiovascular events was identified as blood pressure was reduced. The Review found inconsistent results on safety issues. Xie 2016 focused on the efficacy and safety of a blood pressure decrease for intensive strategies, including clinical trials with at least six months' follow‐up that randomized participants to more intensive versus less intensive blood pressure targets, different blood pressure targets, or different blood pressure changes from baseline. Participants in the intensive group showed decreased risk in terms of less ictus and fewer relevant cardiovascular events.
Several guidelines that directly focus on the main objective of this Cochrane Review ‐ cardiovascular secondary prevention ‐ have been published. The 2007 guidelines for Treatment of Hypertension in the Prevention and Management of Ischemic Heart Disease from the American Heart Association (AHA 2007) recommended blood pressure targets < 130/80 mmHg for people with demonstrated coronary artery disease or risk equivalents (carotid artery disease, peripheral arterial disease, abdominal aortic aneurysm) and for high‐risk people. Subsequently, when performance measures based on these recommendations were proposed, limitations were admitted because of lack of clinical trials that directly compared clinical outcomes of large populations of people with coronary disease randomized to different blood pressure targets (Drozda 2011). This guideline was updated in 2015 (Rosendorff 2015). The update concluded that < 140/90 mmHg would seem a reasonable target for the secondary prevention of cardiovascular events in people with hypertension and coronary artery disease.
Conversely, with less supportive evidence, a lower blood pressure target (< 130/80 mmHg) could be appropriate for some people with coronary artery disease, previous myocardial infarction, stroke, or coronary artery disease equivalents (carotid artery disease, peripheral artery disease, abdominal aortic aneurysm).
Limited data specifically assess the optimal blood pressure target in relation to secondary stroke prevention. American guidelines note that goals for target blood pressure level or reduction from pretreatment baseline are uncertain and should be individualized (Kernan 2014). For people who have had a recent lacunar stroke, a systolic blood pressure < 130 mmHg is accepted as reasonable; for people who have had other types of stroke, < 140/90 mmHg is recommended.
Lowering blood pressure too much also may cause adverse cardiovascular events (Filippone 2011). Some observations have suggested that excessive lowering of blood pressure through drug treatment is associated with an increased number of deaths due to coronary heart disease (Farnett 1991), particularly among those with coronary artery disease (Bangalore 2010; Messerli 2006). Given that controversy over a potential J‐curve phenomenon continues (Mancia 2014; Verdecchia 2014), additional studies are expected to clarify the dilemma.
Therefore, at present the optimal blood pressure target for reducing morbidity and mortality in people with hypertension and history of cardiovascular disease is unknown. This Review aimed to establish if a more strict blood pressure target should be recommended for these people.
Objectives
To determine if lower blood pressure targets (≤ 135/85 mmHg) are associated with reduction in mortality and morbidity as compared with standard blood pressure targets (≤ 140 to 160/90 to 100 mmHg) in the treatment of people with hypertension and a history of cardiovascular disease (myocardial infarction, angina, stroke, peripheral vascular occlusive disease).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) with more than 50 participants per group and at least six months' follow‐up. In addition, no less than 70% of participants had to meet all cited criteria in the 'Types of participants' section (see below). Studies could also be included if individual patient data were available, or if data from relevant participants were provided separately, enabling specific inclusion of this population as defined. Blinding was not possible. To be eligible for inclusion, trial reports had to present data for at least one primary outcome.
We excluded trials that used anything other than accepted randomized allocation methods such as alternate allocation, week of presentation, or retrospective controls. We placed no restrictions on publication language.
Types of participants
Participants had to be at least 18 years of age with hypertension documented in a standard way, or had to be receiving treatment for hypertension, with a positive cardiovascular history of myocardial infarction, stroke (not including transient ischemic attack (TIA)), chronic peripheral vascular occlusive disease, or angina pectoris.
Trials were not limited by any other factor nor by baseline risk.
Types of interventions
Intervention: lower blood pressure treatment target: systolic/diastolic ≤ 135/85 mmHg; mean blood pressure ≤ 102 mmHg.
Control: standard blood pressure treatment target: systolic/diastolic ≤ 140 to 160/90 to 100 mmHg; mean blood pressure ≤ 107 to 120 mmHg.
Mean blood pressure (MBP) was also accepted as a valid way of measuring interventions, while prespecified targets are taken into account and according to the following equation: MBP = [(2 × diastolic) + systolic]/3.
Types of outcome measures
Primary outcomes
-
Total mortality
-
Total serious adverse events
-
Total cardiovascular events including myocardial infarction, stroke, sudden death, hospitalization or death from congestive heart failure, and other significant vascular events such as ruptured aneurysms (excluding angina, transient ischemic attack, surgical or other procedures, or accelerated hypertension). In practice, this was measured as total number of participants with at least one cardiovascular event, including fatal and non‐fatal cardiovascular events
-
Cardiovascular mortality
We defined serious adverse events according to the International Conference on Harmonisation Guidelines as any event that leads to death, was life‐threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability, or was a congenital anomaly or birth defect (ICH 1995).
If a study used a different definition for serious adverse events, review authors resolved this inclusion of data by consensus.
We included all four primary outcomes in 'Summary of findings' tables.
Secondary outcomes
-
Participant withdrawals due to adverse effects
-
Systolic blood pressure and the difference from baseline at one year, or both
-
Diastolic blood pressure and the difference from baseline at one year, or both
-
Proportion of participants reaching the target blood pressure level
-
Number of antihypertensive drugs that each participant needed at the end of the study
We considered participant withdrawals due to adverse effects to be an important outcome and included these data in the 'Summary of findings' table.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches of the following databases for randomized controlled trials without language, publication year, or publication status restrictions.
-
Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 13 February 2018).
-
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (searched 13 February 2018).
-
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 13 February 2018).
-
Embase Ovid (from 1974 onwards) (searched 13 February 2018).
-
Latin American and Caribbean Health Sciences Literature (LILACS) Bireme (from 1982 onwards) (searched 13 February 2018).
-
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 13 February 2018).
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched 13 February 2018).
The Information Specialist modeled subject strategies for databases on the search strategy designed for MEDLINE. When appropriate, subject strategies were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomized controlled trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). We have provided search strategies for databases in Appendix 1. We did not apply a language restriction to the database searches.
Searching other resources
-
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches of CAB Abstracts & Global Health, Cumulative Index to Nursing and Allied Health Literature (CINAHL), ProQuest Dissertations & Theses, and Web of Science.
-
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
-
When necessary, we contacted the authors of key papers and abstracts to request additional information about their trials.
-
We searched Trip Database (www.tripdatabase.com/), updated to April 2018.
-
We attempted to identify additional trials by searching the reference lists of included trials and (systematic) reviews, meta‐analyses, and health technology assessment reports (Appendix 2). We contacted authors of trials reporting incomplete information to request the missing information.
Duplicate publications
When we identified more than one publication of an original trial, we assessed these articles together to maximize data collection. In the case of substantial disagreements between articles, we contacted study authors.
References from published studies
We examined the references of included and excluded studies to identify further references linked to potentially eligible RCTs.
Language
We applied no language restrictions. Any study not published in English, French, or Spanish was translated.
Correspondence
We contacted trial investigators to request data from subgroups of participants with cardiovascular disease or missing data, or to clarify study details.
Data collection and analysis
Review authors worked in pairs to assess search results independently. One review author (LCS) reviewed all results. We used Early Review Organizing Software version 2.0 (www.eros‐systematic‐review.org) and Covidence (www.covidence.org) when screening and classifying references.
Selection of studies
Two independent review authors carried out the selection of papers, excluding records when title, keywords, and abstract showed that they were not RCTs, groups had fewer than 50 participants, follow‐up was less than six months, no review primary outcomes were addressed, participants did not match prespecified criteria, blood pressure targets were not the only intervention, or specific targets were different from those prespecified. We obtained the full text of all remaining articles considered for inclusion and excluded these if inclusion criteria were not met. We obtained the full text of papers that could not be assessed by information presented in the abstract. We provisionally included studies that were likely to include subgroups of participants who met our criteria, and we contacted study authors to request data for those subgroups.
We resolved discrepancies by discussion or by consultation with a third review author, if necessary. When we considered an issue to be a highly significant point, we scheduled a plenary discussion.
We constructed a PRISMA flow diagram depicting the study selection process (Figure 1).

Results of the search.
Data extraction and management
Two review authors independently extracted data from included trials using a previously prepared data extraction form, including basic information, verification of study eligibility, assessment of risk of bias, baseline study characteristics, results in outcomes, and subgroup analyses. Another review author cross‐checked extracted data.
We resolved differences between review authors by discussion and by involvement of a third review author, when necessary. We used Review Manager 2014 for data analyses. We based quantitative analyses of outcomes on the intention‐to‐treat principle.
We used Microsoft Access and Microsoft Excel when organizing and analyzing individual participant data.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each study using the six domains of the Cochrane 'Risk of bias' tool, according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any differences in opinion by discussion among all review authors.
We tried to find study protocols for comparison with published study reports.
Review authors reported the overall risk of bias for all included studies according to the following.
-
Low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met.
-
Unclear risk of bias (plausible bias that raises some doubt about the results) if we assessed one or more criteria as unclear.
-
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
We performed sensitivity analyses while excluding trials with high or unclear risk of bias.
Measures of treatment effect
We used Review Manager 2014 for analyses. We based quantitative analyses of outcomes on intention‐to‐treat results. We used risk ratios (RRs) and a fixed‐effect model, if appropriate, to combine dichotomous outcomes across trials. We calculated absolute risk reduction (ARR) or absolute risk increase (ARI) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for total mortality, total serious adverse events, and total cardiovascular events. We estimated 95% confidence intervals (CIs). We recorded combined outcomes and analyzed participants with at least one event in the outcome.
We combined data for blood pressure reached and the difference from baseline using a weighted mean difference (WMD) method. This combines weight based on the number of participants in the trial and within‐study variance. If the trial did not report within‐study variance for decrease in blood pressure, we imputed the standard deviation (SD) from the average standard deviation provided by other trials. This imputation is a limitation, and to overcome it, we reported the 99% CI instead of the standard 95% CI as reported for all other data. We carried out sensitivity analyses to assess the impact of changing the assumptions made.
Unit of analysis issues
We based the analysis of outcomes on randomized participants, but if cluster‐randomized trials were included, we planned to conduct appropriate analyses. We have taken special care to identify if data presented signified the total number of events or the total number of participants with a first event. We contacted study authors for clarification when necessary.
We selected data for the longest follow‐up of the trial.
Dealing with missing data
We contacted study authors to obtain additional information not provided in published articles.
Assessment of heterogeneity
We used Chi² and I² statistics to test for heterogeneity of treatment effect among trials. We consider a Chi² P < 0.05 or I² > 50% as indicative of heterogeneity. We used a random‐effects model to test for statistical significance when significant heterogeneity existed and 'random' distribution of intervention effects could be justified.
We planned to investigate possible reasons for data showing more than moderate heterogeneity (I² > 60%). If we could not identify sources of heterogeneity, we excluded studies from meta‐analysis.
Assessment of reporting biases
We planned to construct a funnel plot to test for asymmetry if we included 10 or more studies in the meta‐analysis.
Data synthesis
Two review authors analyzed data using RevMan (Review Manager 2014) and reported data in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If meta‐analysis was not appropriate, we planned to provide a narrative description of the results.
Subgroup analysis and investigation of heterogeneity
If possible, we planned subgroup analysis for:
-
participants with diabetes;
-
male and female participants; and
-
people aged ≥ 75 years.
We aimed to investigate clinical heterogeneity by examining differences in achieved blood pressure among trials, trial duration, different interventions used for hypertension, and history of stroke or coronary heart disease as inclusion criteria.
Sensitivity analysis
We tested the robustness of results using several sensitivity analyses including:
-
risk of bias of trials; and
-
industry‐sponsored versus non‐industry‐sponsored trials.
We also tested the robustness of results by repeating the analysis using different measures of effect size (e.g. odds ratio) and different statistical models.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
Through the search, we identified 19785 records. After removal of duplicates and partial screening, 6338 records remained; we assessed them on the basis of title and abstract and excluded 6112 records. We obtained the full text of 226 study reports; after exclusions, 13 reports remained. We contacted the authors of these 13 studies for further information and subsequently excluded seven studies based on the information obtained.
Six studies in this update met the review inclusion criteria (Figure 1).
Included studies
We included six studies (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPRINT 2015; SPS3 2013).
Four trials compared two different systolic blood pressure targets that met our inclusion criteria (SPS3 2013; and subgroups of participants with basal cardiovascular disease in ACCORD BP 2010; Past BP 2016; and SPRINT 2015). One trial compared two different diastolic blood pressure targets within our criteria for lower and standard targets in a subgroup of participants with secondary cardiovascular prevention (HOT 1998). Another trial compared two mean blood pressure targets in a subgroup of participants who met our predefined inclusion criteria (AASK 2002). We have described comparative basal characteristics of these six studies in Table 1.
| Mean (SD) unless otherwise stated | ||||||
| Number of participants | 155 | 1531 | 3232 | 295 | 1562 | 2709 |
| Sex (% male) | 68% | 63% | 53% | 64% | 76% | 62% |
| Age in years | 57 (9) | 62 (8) | 62 (‐) | 71 (9) | 70 (9) | 63 (11) |
| Ethnic group (% Caucasian) | 0% | 62% | 92% | 98% | 71% | 53% |
| Diabetes | 0% | 100% | 12% | 10% | 0% | 36% |
| Current smoker | 31% | 13% | 16% | 13% | 14% | 20% |
| Systolic blood pressure | 149 (28) | 138 (16) | 174 (15) | 143 (14) | 138 (16) | 146 (18) |
| Diastolic blood pressure | 93 (16) | 74 (11) | 106 (3) | 80 (10) | 74 (12) | 79 (11) |
| Ischemic heart disease (IHD) | 25% | 86% | 95% | 22% | ‐‐‐ | 11% |
| Stroke | 69% | 20% | 7% | 85% | 0% | 99% |
| Peripheral vascular disease | 23% | ‐‐‐ | ‐‐‐ | 7% | ‐‐‐ | ‐‐‐ |
| Thiazides | ‐‐‐ | 51% | ‐‐‐ | 35% | ‐‐‐ | 35% |
| ACEI/ARB | ‐‐‐ | 84% | ‐‐‐ | 65% | ‐‐‐ | 71% |
| Calcium channel blocker | ‐‐‐ | 26% | ‐‐‐ | 43% | ‐‐‐ | 28% |
| Beta blocker | ‐‐‐ | 57% | ‐‐‐ | 20% | ‐‐‐ | 27% |
| Other antihypertensive drugs | ‐‐‐ | 28% | ‐‐‐ | 11% | ‐‐‐ | 8% |
| Number of antihypertensive drugs | ‐‐‐ | 3.0 (1.4) | 1.0 (‐‐) | 1.1 (0.8) | 2.1 (1.0) | 1.7 (1.1) |
(‐‐) no information is available. Ischemic heart disease, stroke, and peripheral vascular disease percentages are totally independent of each other because participants can have more than one cardiovascular event at the same time. A similar explanation can be offered with respect to percentages in the different classes of antihypertensive drugs.
Abbreviations: ACEI: angiotensin‐converting enzyme inhibitor; ARB: angiotensin receptor blocker; IHD: ischemic heart disease; SD: standard deviation.
Methods
All included trials were randomized and open with blinded end point design. In AASK 2002, participants were also randomly assigned (in a 3 × 2 factorial design) to metoprolol, ramipril, or amlodipine treatment. In ACCORD BP 2010, participants were randomized to intensive or standard glycemic control according to a 2 × 2 factorial design. HOT 1998 also used a 3 × 2 factorial design and randomized participants to receive acetylsalicylic acid (aspirin) or placebo. SPS3 2013 had a 2 × 2 factorial design with additional randomization to aspirin + placebo or aspirin + clopidogrel.
Mean follow‐up duration was 3.7 years (range 1.0 to 4.7 years).
Participants
The total number of participants included in the review was 9484 (lower target, 5301; standard target, 4183). AASK 2002 included 155 participants (14% of total AASK study); ACCORD BP 2010 1531 participants (32% of total ACCORD study); HOT 1998 3232 participants (17% of total HOT study); Past BP 2016 295 participants (56% of total Past BP trial); SPRINT 2015 1562 participants (17% of total SPRINT study); and SPS3 2013 2709 participants (90% of total SPS3 study).
AASK 2002 and SPRINT 2015 were conducted in the USA: ACCORD BP 2010 in the USA and Canada; Past BP 2016 in the UK; SPS3 2013 in eight countries in the Americas and Europe; and HOT 1998 in over 20 countries in Asia, the Americas, and Europe.
Basal participant characteristics differed among trials (Table 1).
For participants' basal cardiovascular condition, we accepted the following participant profiles as valid secondary prevention.
-
AASK 2002: participants with ischemic heart disease (IHD), stroke, or peripheral vascular disease (PVD).
-
ACCORD BP 2010: participants with myocardial infarction, stroke, or angina.
-
HOT 1998: participants with myocardial infarction, stroke, or angina.
-
Past BP 2016: participants had stroke or, less frequently, IHD.
-
SPRINT 2015: all included participants had IHD or PVD.
-
SPS3 2013: some participants had IHD, but all had recent lacunar stroke.
We considered myocardial infarction and angina identified by electrocardiogram (ECG) or coronary revascularization, and silent events, as meeting the inclusion criteria. In general, stroke was the prevalent condition in AASK 2002, Past BP 2016, and SPS3 2013, whereas ischemic heart attack was the most prevalent condition in ACCORD BP 2010, HOT 1998, and SPRINT 2015.
AASK 2002 and SPRINT 2015 excluded people with history of diabetes, but HOT 1998, Past BP 2016, and SPS3 2013 included some people with diabetes; all ACCORD BP 2010 participants had this cardiovascular risk factor.
All studies included more men than women with mean age from 57 to 71 years.
Ethnicity varied from all or mostly Caucasian (HOT 1998; Past BP 2016), to mixed populations (ACCORD BP 2010;SPRINT 2015;SPS3 2013), to African American participants (AASK 2002).
Trials included participants with reduced kidney function (AASK 2002), additional cardiovascular risk factors (ACCORD BP 2010; SPRINT 2015), previous stroke (Past BP 2016; SPS3 2013), or general hypertension (HOT 1998).
The baseline blood pressure required for inclusion also varied. AASK 2002 and HOT 1998 required diastolic blood pressure (DBP) ≥ 95 mmHg and DBP 100 to 115 mmHg, respectively, whereas ACCORD BP 2010 and SPRINT 2015 required systolic blood pressure (SBP) 130 to 180 mmHg, Past BP 2016 sought SBP 125 mmHg, and SPS3 2013 participants had SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg or a history of hypertension with blood pressure lowering medication at randomization.
HOT 1998 was fully industry funded, and AASK 2002 was partially industry funded. ACCORD BP 2010, Past BP 2016, SPRINT 2015, and SPS3 2013 were fully publicly funded. ACCORD BP 2010, SPRINT 2015, and SPS3 2013 were supported by the National Institutes of Health in the USA. Past BP 2016 was funded by the National Institute for Health Research (NIHR) in the UK.
Interventions
Participants in AASK 2002 were randomized to MBP 102 to 107 mmHg (standard target) or MBP < 92 mmHg (lower target). ACCORD BP 2010 and SPRINT 2015 randomized participants to SBP < 140 mmHg (standard target) or SBP < 120 mmHg (lower target). Participants in Past BP 2016 were randomized to SBP < 140 mmHg (standard target) or < 130 mmHg (lower target); those in SPS3 2013 to SBP 130 to 149 mmHg (standard target) or SBP < 130 mmHg (lower target); and those in HOT 1998 to DBP ≤ 90 mmHg (standard target) or to DBP ≤ 85 mmHg or ≤ 80 mmHg (lower target).
In AASK 2002, if the blood pressure goal could not be achieved by the drug initially randomized (metoprolol, ramipril, or amlodipine), researchers added open‐labelled antihypertensives (furosemide, doxazosin, clonidine, hydralazine, or minoxidil) sequentially. Felodipine was proposed as basal therapy in HOT 1998, with other drugs added according to a five‐step regimen. In SPRINT 2015, the protocol encouraged the use of drug classes with strongest evidence for reduction in cardiovascular outcomes, including thiazide‐type diuretics (chlorthalidone encouraged as the first‐line agent), loop diuretics (for participants with advanced chronic kidney disease), and beta‐adrenergic blockers (for those with coronary artery disease). ACCORD BP 2010, Past BP 2016, and SPS3 2013 provided no specific drug instructions.
Outcomes
The primary analysis in AASK 2002 focused on change in glomerular filtration rate, with relevant cardiovascular events measured as secondary outcomes. In ACCORD BP 2010, HOT 1998, and SPRINT 2015, the main outcome was occurrence of several types of cardiovascular events. The primary outcome in Past BP 2016 was change in systolic blood pressure between baseline and one year. Time to recurrent stroke was the main analysis in SPS3 2013.
Additional notes
AASK 2002 was conducted between February 1995 and September 2001; ACCORD BP 2010 between January 2001 and June 2009; HOT 1998 between October 1992 and August 1997; Past BP 2016 between July 2008 and July 2012: SPRINT 2015 between November 2010 and March 2013; and SPS3 2013 between February 2003 and April 2012.
Excluded studies
We excluded 33 records following assessment of full‐text reports (Figure 1). Among them, we considered it useful to provide more detailed information about seven excluded studies (HOT 1998; MDRD 1994; NCT01230216; PODCAST 2013; PRESERVE 2018; REIN‐2 2005; RESTART‐AP 2013).
HOSP 2006 randomized participants up to five years and intended to assess two home blood pressure target strategies. Unfortunately, the number of recruited participants was much smaller than intended and was not sufficient for analysis of the effects of different levels of target home blood pressure.
MDRD 1994 focused mainly on effects of dietary protein restriction and blood pressure control on progression of chronic kidney disease. The National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK, USA) provided individual patient data. However, after a first analysis, we excluded this study because researchers included fewer than 50 participants per group (an inclusion criterion) (lower target (N = 56), eight total deaths; standard target (N = 47), three total deaths).
NCT01230216 was designed to assess whether an intensive blood pressure target could reduce the per cent of atheroma volume measured by intravascular ultrasound in hypertensive patients with coronary artery disease. This study was terminated early owing to slow patient enrolment.
The primary outcome for PODCAST 2013 was Addenbrooke’s Cognitive Examination. Secondary outcomes included vascular events, quality of life, functional outcome, depression, and death. The trial recruited 83 participants during the pilot phase. Low recruitment meant that the trial did not proceed and did not meet the 50 participants per arm inclusion criterion of this review.
PRESERVE 2018 was designed to assess potential differences between two strategies for lowering blood pressure in terms of a composite cognitive score and other secondary outcomes. Only 62 analyzable patients were recruited, and this did not meet the 50 participants per arm inclusion criterion.
REIN‐2 2005 was designed to establish whether further blood pressure‐lowering therapy in addition to angiotensin‐converting enzyme inhibitors (ACE‐Is) could benefit people with chronic kidney disease. Accordingly, the primary objective assessed the effect of intensified versus conventional blood pressure control on progression to end‐stage kidney disease. The Istituto di Ricerche Farmacologiche Mario Negri (Bergamo, Italy) provided individual patient data. It was confirmed that the trial included fewer than 50 participants per arm, so this study did not meet this review inclusion criterion (lower target (N = 34), two deaths; standard target (N = 39), two deaths).
RESTART‐AP 2013 was designed to determine whether restarting antithrombotic agents had an impact on the number of new‐onset cerebral microbleeds, and if intensive blood pressure lowering reduced their numbers. Study authors confirmed that insufficient funding was available, and the study was terminated early.
Studies awaiting classification
Three studies await classification (ABCD‐H 1998; BBB 1994; Cardio‐Sis 2014). These studies did not report data for participants with cardiovascular disease at baseline. We have requested these data from study authors but had not received these data before publication of this review.
Ongoing studies
We identified four ongoing studies (ESH‐CHL‐SHOT 2014; INFINITY 2013; NCT01198496; NCT03015311). We will evaluate these studies when complete for possible inclusion in future updates of this review.
Risk of bias in included studies
The summary of the risk of bias assessment of each trial is shown in Figure 2. Assessment of risk of bias was based on both published and unpublished data.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Except for SPRINT 2015, which provided no specific information on allocation method, all other included studies used a computerized system for randomization. We judged methods used for allocation as having low risk of bias for five studies (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPS3 2013). We judged SPRINT 2015 as having unclear risk of bias. The allocation concealment domain was judged as low risk of bias for all included trials.
Blinding
None of the included studies was blinded to participants or clinicians because of the need to titrate antihypertensives to reach a specific blood pressure goal. However, an independent committee blinded to group allocation assessed clinical events in all trials. Hence, we assessed all trials as having low risk of performance and detection bias.
Incomplete outcome data
Available information (both published and unpublished) for five trials did not suggest a significant imbalance between arms for withdrawals or dropouts (AASK 2002; ACCORD BP 2010; Past BP 2016; SPRINT 2015; SPS3 2013); we assessed these trials as having low risk of attrition bias.
In HOT 1998, 14% of total ECGs could not be obtained, leading to some uncertainty on silent myocardial infarctions. We decided to assume a conservative perspective and consider this trial to have unclear risk of bias.
Selective reporting
We assessed protocols and published articles for AASK 2002, ACCORD BP 2010, HOT 1998, Past BP 2016, and SPRINT 2015 and confirmed no sign of reporting bias. We assessed these trials as having low risk of reporting bias.
Serious adverse effects reporting in SPS3 2013 was related to hypotension and blood pressure management only. We contacted study authors for clarification but received no response. Finally, the National Institute of Neurological Disorders and Stroke (NINDS) provided individual patient data. After reviewing all data, we assessed this study as having low risk of selective reporting bias.
Other potential sources of bias
All data used in this Cochrane Review came from subgroups of participants not predefined in the original study protocols, and this constitutes a potential source of bias.
Some studies were partially (e.g. AASK 2002) or fully (e.g. HOT 1998) funded by pharmaceutical industry sources, which constitutes another potential source of bias.
We also considered early termination of SPRINT 2015 as a potential source of bias.
Effects of interventions
Lower versus standard blood pressure targets
Six randomized controlled trials (RCTs) met inclusion criteria (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPRINT 2015; SPS3 2013). We obtained data from published and unpublished sources. We assumed that silent myocardial infarction complied with the definition of cardiovascular event when provided.
Primary outcomes
Total mortality
Results show no difference in total mortality between lower and standard blood pressure target groups (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.91 to 1.23; P = 0.47; six studies; Analysis 1.1). When the absolute effect was measured, results show four additional total deaths per 1000 participants identified in the lower target (95% CI 6 fewer to 16 more total deaths per 1000 participants). Researchers reported a total of 366 deaths (of 5301 participants) in the lower target group and 285 deaths (of 4183 participants) in the standard target group (Figure 3).

Forest plot of comparison: 1 Lower versus standard, outcome: 1.1 Total mortality.
Serious adverse events
All included studies provided data for analysis of serious adverse events. We adopted a broad definition of serious adverse event, according to the ICH 1995 definition. We included participants with any cause of death, any cardiovascular event (as predefined in our protocol), or any other serious adverse event as defined by trial authors, while avoiding double‐counting of participants. When all data were pooled, they showed no difference in serious adverse events between lower and standard blood pressure target groups (RR 1.01, 95% CI 0.94 to 1.08; P = 0.86; Analysis 1.2). When measuring the absolute effect, researchers identified three additional serious adverse events per 1000 participants in the lower target group (95% CI 15 fewer to 20 more serious adverse events per 1000 participants). Results show 1197 (of 5301 participants) with at least one serious adverse event in the lower target group and 1053 (of 4183 participants) in the standard target group (Figure 4). We considered SPRINT 2015 to report the full range of serious adverse events (Analysis 1.2.1), and five studies reported subsets of events (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPS3 2013; Analysis 1.2.2).

Forest plot of comparison: 1 Lower versus standard, outcome: 1.2 Serious adverse events.
Cardiovascular events
AASK 2002 analyzed data from 27 participants in relation to individual cardiovascular events for myocardial infarction, stroke, and heart failure hospitalization; as well as data from seven further participants from a direct cardiovascular mortality diagnosis.
Five included studies provided data by means of well‐defined categories. The total number of cardiovascular events was not significantly reduced in the lower blood pressure target group compared with the standard group (RR 0.89, 95% CI 0.80 to 1.00; P = 0.044; six studies; Analysis 1.3). When measuring the absolute effect, researchers in these studies identified 14 fewer cardiovascular events per 1000 participants in the lower blood pressure target group (95% CI 0 to 25 fewer cardiovascular events per 1000 participants). Results show 562 (of 5301 participants) with cardiovascular events in the lower target group and 532 (of 4183 participants) with cardiovascular events in the standard target group (Figure 5).

Forest plot of comparison: 1 Lower versus standard, outcome: 1.3 Cardiovascular events.
Cardiovascular mortality
We need to make some comments related to AASK 2002 before we report analysis results. AASK 2002 researchers used two different documents to register causes of death (CARDIO_REVW Form #38 and CC_DEATH Form #48). We noted no complete overlap between forms. After discussion, we considered there to be valid cardiovascular mortality when the researcher answered 'yes' to question 4 on Form #38: "Was there a cardiovascular death?" This indicated 11 deaths. Two clinicians (a cardiologist and a general practitioner) analyzed data from Form #48 case‐by‐case and identified two additional deaths after completing a careful validation process.
Five trials provided data by means of well‐defined categories. Results show no difference in cardiovascular mortality between lower and standard blood pressure target groups (RR 1.03, 95% CI 0.82 to 1.29; P = 0.83; Analysis 1.4). Researchers reported 172 cardiovascular deaths (among 5301 participants) in the lower target group and 131 (among 4183 participants) in the standard target group (Figure 6).

Forest plot of comparison: 1 Lower versus standard, outcome: 1.4 Cardiovascular mortality.
Secondary outcomes
Withdrawals due to adverse effects
Four trials provided no information about withdrawals due to adverse effects among participants with basal cardiovascular disease (AASK 2002; ACCORD BP 2010; SPRINT 2015; SPS3 2013).
Review authors extracted data from free text notes only for HOT 1998; Past BP 2016 provided data of better quality. Despite limited information, results show a difference in withdrawals due to adverse effects between lower and standard blood pressure target groups (RR 8.16, 95% CI 2.06 to 32.28; P = 0.003; Analysis 1.5). Researchers reported 22 withdrawals due to adverse effects (among 420 participants) in the lower target group and only two (among 270 participants) in the standard target group.
Blood pressure target achieved at one year
Results show that 3073 of 4820 participants (64%) reached the target in the lower target group and 2817 of 3768 (75%) in the standard target group (six trials; Analysis 1.6). Therefore, more people in the standard group achieved particular blood pressure targets.
Systolic blood pressure change from baseline at the end of one year
After the first year of therapy, the average systolic blood pressure achieved was significantly lower in the lower blood pressure target group (mean difference (MD) ‐8.90 mmHg, 95% CI ‐4.56 mmHg to ‐13.24 mmHg, P = 0.000058; six trials; Analysis 1.7). Heterogeneity among trials was high, so we preferred a random‐effects model for this analysis. We considered the different targets and specific basal characteristics for each trial as the most likely causes of this heterogeneity.
Diastolic blood pressure change from baseline at the end of one year
After the first year of therapy, the average diastolic blood pressure achieved was significantly lower in the lower blood pressure target group (MD ‐4.50 mmHg, 95% CI ‐2.65 mmHg to ‐6.35 mmHg; P < 0.00001; six trials; Analysis 1.8). Heterogeneity between trials for this outcome was high, so we chose a random‐effects model for this analysis. We considered the different targets and specific basal characteristics for each trial as the most likely causes of this heterogeneity.
Number of antihypertensive drugs needed at the end of the study
At the end of the study, the number of antihypertensive drugs needed was significantly lower in the standard blood pressure target group (average 1.9 drugs) than in the lower blood pressure target group (average 2.4 drugs) (MD 0.56, 95% CI 0.16 to 0.96; P = 0.0066; five trials; Analysis 1.9). Heterogeneity between trials for this outcome was high, so we chose a random‐effects model for this analysis. We considered the different targets and specific basal characteristics for each trial as the most likely causes of this heterogeneity.
Discussion
Pharmacological treatment of high blood pressure aims to reduce morbidity and mortality. Specific blood pressure targets have been proposed in guidelines for people with hypertension who have established cardiovascular disease, but optimal thresholds remain uncertain because the benefit‐to‐harm ratio of more intensive treatment has not been established.
This Cochrane Review explored current evidence from randomized controlled trials (RCTs) and assessed relevant outcomes linked to two alternative strategies: standard blood pressure target (≤ 140 to 160/90 to 100 mmHg) and lower blood pressure target (≤ 135/85 mmHg).
We included six RCTs with a total of 9484 participants and a mean follow‐up of 3.7 years (range 1.0 to 4.7 years). Four studies compared systolic blood pressure targets, one compared diastolic blood pressure targets, and one compared mean blood pressure targets. Individual patient data were available for all six trials (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPRINT 2015; SPS3 2013).
Two previous Cochrane Reviews adopted different strategies for analysis. Arguedas 2009 pooled trial data for the main analysis, but Arguedas 2013 considered each target (systolic or diastolic) separately. Both approaches are suitable and relevant, and our Cochrane Protocol did not specify any particular strategy (Gorricho 2013). For this Cochrane Review, we decided to use pooled data as the main analysis, but we also tested whether results were consistent when blood pressure targets were considered separately. To avoid misclassification problems, we added a third category (mean blood pressure) to systolic/diastolic.
Summary of main results
Evidence from the six included trials indicates that blood pressure targets were more frequently achieved in the standard blood pressure target arm (2817/3768; 75%) than in the lower target arm (3073/4820; 64%).
Researchers used more antihypertensive drugs in the lower blood pressure target group (average 2.4 drugs) than in the standard arm (average 1.9 drugs).
Results show broad differences for systolic (8.9 mmHg) and diastolic (4.5 mmHg) blood pressure changes from baseline in the lower target arm.
We detected no benefits for total mortality (RR 1.06, 95% CI 0.91 to 1.23) or for cardiovascular mortality (RR 1.03, 95% CI 0.82 to 1.29). Subsequent analyses separating trials by systolic, diastolic, or mean blood pressure targets did not change these results. We also found no difference with regard to total cardiovascular events (including myocardial infarction, stroke, sudden death, hospitalization or death from congestive heart failure) (RR 0.89, 95% CI 0.80 to 1.00) and total serious adverse events (RR 1.01, 95% CI 0.94 to 1.08) in favour of the lower blood pressure target. When we considered systolic target trials separately, we identified no significant changes in the main results.
Most withdrawals due to adverse effects occurred in the lower target arm (RR 8.16, 95% CI 2.06 to 32.28). However, little evidence was available, making establishment of a trustworthy global assessment of benefits and harms very challenging.
It is important to note that we detected no significant heterogeneity for any primary outcome. Therefore, at present there does not seem to be sufficient sound evidence to justify more strict blood pressure targets (≤ 135/85 mmHg) than the standard range (≤ 140 to 160/90 to 100 mmHg) for people with hypertension and established cardiovascular disease.
We detected significant heterogeneity for two outcomes ‐ blood pressure difference from baseline at one year and number of hypertensive drugs that each participants needed at the end of study. We considered the different targets and the specific basal characteristics for each trial as the most likely causes for this heterogeneity. Subgroup analysis indicated significant heterogeneity in the male subgroup for cardiovascular mortality. The source of heterogeneity could be linked to a decrease in the numbers of participants and events and differences in trial design between HOT 1998 and ACCORD BP 2010/SPRINT 2015.
The minimum 5‐mmHg difference in systolic or diastolic blood pressure targets predefined as clinically significant in our protocol is consistent with previous guideline decisions (NICE 2016). Nonetheless, as Arguedas 2009 and Arguedas 2013 reported, it could be argued that this difference is not large enough to show significant changes in relevant outcomes. To test this hypothesis, we conducted an additional sensitivity analysis of participants with diabetes, while excluding the intermediate < 85 mmHg target in HOT 1998; results were very similar between the main analysis in participants with cardiovascular disease and the subgroup analysis in participants with diabetes and show large differences in targets (Table 2).
| Outcome | Studies | Participants | Statistical Method | Effect Estimate |
| Total mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.91,1.45] | |
| Cardiovascular mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69,1.39] | |
| Cardiovascular events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74,1.03] | |
| Serious adverse events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88,1.15] |
We specified in our Cochrane Protocol four subgroup analyses (people with diabetes, participants by sex, and people aged ≥ 75 years) designed to explore potential differences in specific populations. Despite the large amount of information retrieved from individual participant data for this review, data available for people aged ≥ 75 years were too few to permit any definitive conclusions. When participant data were split according to sex, and when only participants with diabetes were considered, we found magnitudes of effect similar to those described in the main analysis. People with diabetes and established cardiovascular disease could be seen at first as being in a higher risk category than people who do not have diabetes (Mancia 2011). However, estimates for people with diabetes were similar to estimates for the general population with basal cardiovascular disease: no differences in total mortality, cardiovascular mortality, or total cardiovascular events associated with lower target; and no differences in both target strategies for serious adverse events. Evidence was insufficient to reveal greater effect from a lower blood pressure target in these subgroups, although sample sizes were not large enough to exclude a significant effect.
We planned two sensitivity analyses to test the robustness of results: risk of bias of the included trials; and industry‐sponsored versus non‐industry‐sponsored trials.
Because we rated overall risk of bias as high, we could not perform sensitivity analysis. We found no difference in any main outcome favouring the lower blood pressure target in industry‐sponsored or non‐industry‐sponsored trials (ACCORD BP 2010; Past BP 2016; SPRINT 2015; SPS3 2013).
Overall completeness and applicability of evidence
Cardiovascular diseases are prevalent, and high blood pressure is an added risk factor commonly treated in this population. Evidence‐based guidelines focused on this issue are needed. Unfortunately, data derived from randomized controlled trials designed to clarify this uncertainty remain insufficient.
All six studies contributed individual patient data for subgroups of participants (AASK 2002, 155 participants; ACCORD BP 2010, 1531 participants; HOT 1998, 3232 participants, Past BP 2016, 295 participants; SPRINT 2015, 1562 participants; SPS3 2013, 2709 participants).
Although this review analyzed a significant body of evidence and results are considered to be robust, we cannot state these results as conclusive. Two ongoing trials have been designed to explicitly answer relevant questions for people with established cardiovascular disease (ESH‐CHL‐SHOT 2014; NCT01198496); it is anticipated that these studies will yield additional evidence.
Over 6000 participants provided data on systolic targets, and over 3000 on diastolic targets. Neither subanalysis substantially changed overall results in primary outcomes when all target strategies were considered together. From this perspective, results of this review can be generalized for physicians prescribing antihypertensive drugs, no matter the specific target strategy (systolic, diastolic, or both) chosen.
As identified by Arguedas 2009, and probably fueled by the intention‐to‐treat approach, this review did not find real differences as wide as expected between arms in achieved systolic and diastolic blood pressures, according to the predefined targets for each study. All six included trials achieved the standard target, but only ACCORD BP 2010, Past BP 2016, and SPS3 2013 achieved the required blood pressure in the lower target group (in HOT 1998, the ≤ 80 mmHg target was not achieved). This underlines the difficulty of putting the intervention into practice, as often happens in real life. Accordingly, this aspect could be seen as both a limitation and a strength.
Quality of the evidence
We downgraded the quality of the evidence for total mortality and cardiovascular mortality to moderate owing to imprecision and lack of data. In our opinion, other potential limitations (e.g. cardiovascular disease subgroups were not predefined in several studies) are unlikely to lower confidence in the estimate, given the large sample sizes, the design of SPS3 2013 (29% of total participants), the sensitivity analysis performed about potential risk of bias, and the strength of the individual patient data analysis.
We also downgraded the quality of evidence for other outcomes: we assessed total cardiovascular events and serious adverse events as providing low‐quality evidence, and withdrawals due to adverse effects as providing very low‐quality evidence. Total cardiovascular events data were affected by high risk of bias. Furthermore, available data on drug side effects were insufficient, and as for withdrawals, imprecision was especially marked, leading to further downgrading of evidence quality. (See summary of findings Table for the main comparison.)
Potential biases in the review process
Because of study requirements, none of the included studies were blinded to participants or clinical researchers. However, all studies implemented mechanisms for assessment of outcomes by independent blinded committees. Consequently, we considered potential performance bias as high and detection bias as low.
Another potential source of bias came from the fact that all included participants were also included in subgroup studies. Also, to adapt study interventions to those defined in our review, HOT 1998 participants in two different target groups (< 85 mmHg and < 80 mmHg) were pooled only for the lower blood pressure target.
Additionally, primary outcomes in AASK 2002 were not aligned with the interests of our review. It must be stressed that most subgroups included a large number of participants, and all findings were analyzed as individual patient data.
Differences between trials in types and definitions of outcomes could also be a source of bias (see Outcomes in Characteristics of included studies tables). For example, not all studies provided adequate information about the ways silent myocardial infarctions were dealt with, revealing differences among studies that included heart failure hospitalization as an outcome.
We observed no homogeneous information among trials for serious adverse events ‐ the most comprehensive outcome on safety. Only SPRINT 2015 was deemed to report the total number of serious adverse events according to its international standardized definition (ICH 1995). Other included trials provided an unreliably low number of serious adverse events (HOT 1998); reported only events judged by researchers as probably related to the interventions (ACCORD BP 2010); considered serious adverse events from a extremely narrow perspective (Past BP 2016); or did not offer any specific information on this outcome (AASK 2002). Deaths, major cardiovascular events, and serious adverse effects reported by trialists were included as serious adverse events in analyses when only partial or disaggregated information was available, as in SPS3 2013. Because of these concerns, we strongly suspect reporting bias for certain outcomes such as serious adverse events and withdrawals due to adverse effects, for which few data were reported.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review with meta‐analysis that assessed blood pressure targets in people with established cardiovascular disease from randomized controlled trials that directly compared different target strategies.
We found no evidence of additional benefit from a lower blood pressure target compared to a standard blood pressure target in terms of total mortality, cardiovascular mortality, total cardiovascular events, or total serious adverse events.
Some prominent hypertension guidelines have not issued direct recommendations on blood pressure targets for people with previous cardiovascular disease (JNC‐8 2014; NICE 2016). Those reviews or guidelines that include explicit recommendations obtained them from observational data or post hoc analyses of achieved blood pressure in trials designed for various purposes (Bangalore 2013). This perspective could easily lead to selection bias, favouring lower risk of experiencing a cardiovascular event in participants with lower achieved blood pressure. Only one study directly compared clinical outcomes in people who had stroke and were treated to different blood pressure targets (SPS3 2013); no studies have been conducted in people with cardiovascular disease.
Our results do not seem to support widespread implementation of an intensive target strategy (≤ 135/85 mmHg) for cardiovascular secondary prevention. The conservative approach is also recommended by ESH‐ESC 2013 and CHEP 2015, both of which recommend a < 140/90 mmHg target in most patient situations, including previous cardiovascular disease. In addition, a similar systematic review on chronic kidney disease did not show that a blood pressure target < 125/75 to 130/80 mmHg is more beneficial than a target < 140/90 mmHg (Upadhyay 2011).
However, based on SPRINT 2015 data, CHEP 2018 recommends consideration of lower targets in some patients at high cardiovascular risk. Similarly, ACC‐AHA 2017 suggests lower goals for patients with established cardiovascular disease, according to SPRINT 2015 data and the conclusions of several meta‐analyses. However, no specific analysis was performed on this population. Other guidelines, such as Joint ESC 2016, Rosendorff 2015, and Kernan 2014, only partially agree with our view. Joint ESC 2016 recommends a 130 to 139 mmHg systolic target but supports a more intensive effort (80 to 85 mmHg) as the diastolic goal. No specific supportive evidence was provided on this statement. Two American guidelines focusing on coronary and stroke patients are available. Rosendorff 2015 suggests < 140/90 mmHg as a reasonable target for secondary prevention of cardiovascular events in coronary patients but considers a lower target (< 130/80 mmHg) as useful for some individuals; researchers admit that this is not supported by evidence and offer no additional details of potential benefit profiles. Kernan 2014 recommends a < 140/90 mmHg target strategy as a general rule for stroke patients but points out that 130/80 mmHg could be reasonable for patients with a recent lacunar stroke, based mainly on SPS3 2013 results. However, the SPS3 2013 study did not achieve a statistically significant difference between lower and standard targets for any of the primary or secondary outcomes measured. In SPS3 2013, the difference detected in intracerebral hemorrhages (a subtype of intracranial hemorrhages not pre‐planned even as a secondary outcome) could well have been due to chance. It is surprising that despite no evidence of substantial benefit confirmed with the lower target, the SPS3 2013 authors concluded that, based on their results, use of a systolic blood pressure target < 130 mmHg was likely to be beneficial in patients with recent lacunar stroke.
Ettehad 2016, a systematic review, identified large‐scale blood pressure‐lowering trials to quantify the effects of reducing systolic blood pressure by 10 mmHg in terms of mortality and cardiovascular outcomes. This analysis was conducted for the main comparison and for several subgroups, one of them including patients with established cardiovascular disease. Results showed benefit for this subgroup in terms of mortality and cardiovascular events when blood pressure was reduced but inconsistent results for safety outcomes. The review authors concluded that lowering current normotensive levels is supported by their review, provided there is a relevant absolute risk. In this regard, relevant limitations must be taken into account. First, heterogeneity was extremely high in Ettehad 2016, including large differences among populations, basal comorbidities, and comparisons between treatment groups. In fact, some included studies compared the effects of different blood pressure targets, the effects of different drugs, or even the effects of drugs versus placebo. Second, the review did not consider individual patient data, leading to particularly low accuracy when conclusions are assumed about participants with or without basal cardiovascular disease. Finally, among the included studies comparing different blood pressure targets, researchers mixed strategies that were too diverse, from < 120 mmHg to < 150 mmHg systolic blood pressure targets. Certainly this review gathered a large amount of information, but, at the same time, a careful approach should be demanded to avoid misleading conclusions.
Another systematic review has paid attention to clinical trials comparing only blood pressure targets (Xie 2016). Although this design seems to be more appropriate than that used in the previous case, review authors established inclusion criteria with high laxity. Limits are not well defined with regard to what is considered an intensive or standard target. Because of this, two studies can share the same target while simultaneously assigning treatment to different groups ‐ standard and intensive (Brunström 2016). Participants with a wide range of blood pressure targets were mixed, leading to few informative results, even when data from a large number of patients were collected. The review authors declare that, with high cardiovascular risk, benefits from intensive treatment clearly overcome potential harms, even in patients with targets < 140 mmHg, calling for changes to current guidelines.
In contrast, our systematic review does not support this view. We have not identified any advantages after taking into account more appropriate inclusion criteria, all individual patient data, and informative outcomes such as 'serious adverse events'. Furthermore, even if SPRINT 2015 mortality results show a trend favouring the lower target strategy, we detected no overall benefits for these outcomes and noted that adverse events were poorly informed by all concerned clinical trials. In our opinion, whereas the scientific community is dealing with this key lack of information, recommendations on blood pressure targets for hypertensive patients with cardiovascular disease should give priority to caution.

Results of the search.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.1 Total mortality.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.2 Serious adverse events.

Forest plot of comparison: 1 Lower versus standard, outcome: 1.3 Cardiovascular events.
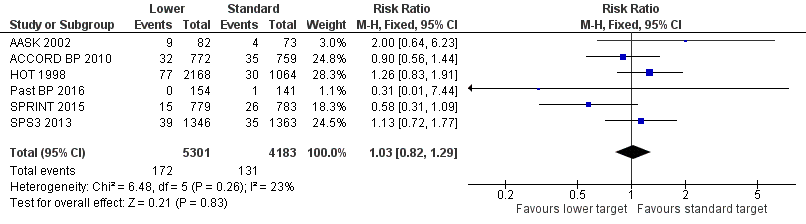
Forest plot of comparison: 1 Lower versus standard, outcome: 1.4 Cardiovascular mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 1 Total mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 2 Serious adverse events.
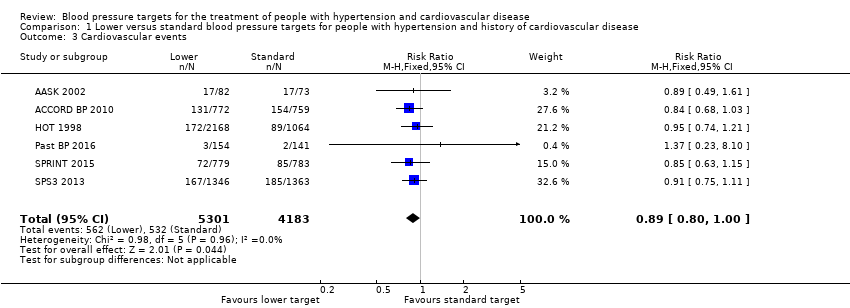
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 3 Cardiovascular events.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 4 Cardiovascular mortality.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 5 Withdrawals due to adverse effects.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 6 Blood pressure target achieved at 1 year.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 7 Systolic blood pressure change from baseline at end of 1 year.

Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 8 Diastolic blood pressure change from baseline at end of 1 year.
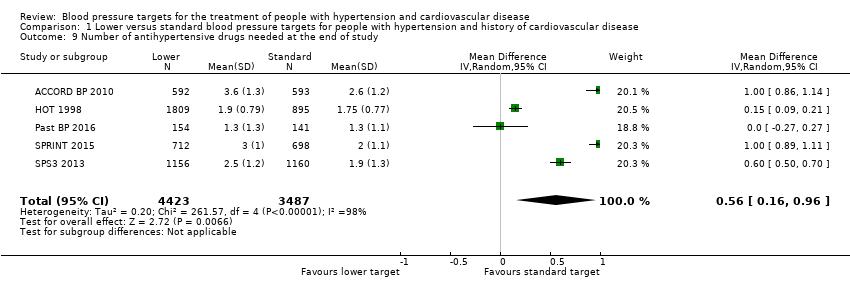
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 9 Number of antihypertensive drugs needed at the end of study.
| Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity | ||||||
| Patient or population: cardiovascular disease with high blood pressure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with standard blood pressure target | Risk with lower blood pressure target | |||||
| Total mortality | Study population | RR 1.06 | 9484 | ⊕⊕⊕⊝ | ||
| 68 per 1000 | 72 per 1000 | |||||
| Serious adverse events | Study population | RR 1.01 | 9484 | ⊕⊕⊝⊝ | ||
| 252 per 1000 | 255 per 1000 | |||||
| Total cardiovascular events | Study population | RR 0.89 | 9484 | ⊕⊕⊝⊝ | ||
| 127 per 1000 | 113 per 1000 | |||||
| Cardiovascular mortality | Study population | RR 1.03 (0.82 to 1.29) | 9484 (6 RCTs) | ⊕⊕⊕⊝ | ||
| 31 per 1000 | 32 per 1000 (25 to 40) | |||||
| Withdrawals due to adverse effects | Study population | RR 8.16 | 690 | ⊕⊝⊝⊝ | ||
| 7 per 1000 | 60 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level owing to serious imprecision (95% CI is wider than the minimal important difference). bDowngraded one level owing to incomplete available data. cDowngraded one level owing to high risk of bias. dDowngraded two levels because only two of the smaller studies reported this outcome. | ||||||
| Mean (SD) unless otherwise stated | ||||||
| Number of participants | 155 | 1531 | 3232 | 295 | 1562 | 2709 |
| Sex (% male) | 68% | 63% | 53% | 64% | 76% | 62% |
| Age in years | 57 (9) | 62 (8) | 62 (‐) | 71 (9) | 70 (9) | 63 (11) |
| Ethnic group (% Caucasian) | 0% | 62% | 92% | 98% | 71% | 53% |
| Diabetes | 0% | 100% | 12% | 10% | 0% | 36% |
| Current smoker | 31% | 13% | 16% | 13% | 14% | 20% |
| Systolic blood pressure | 149 (28) | 138 (16) | 174 (15) | 143 (14) | 138 (16) | 146 (18) |
| Diastolic blood pressure | 93 (16) | 74 (11) | 106 (3) | 80 (10) | 74 (12) | 79 (11) |
| Ischemic heart disease (IHD) | 25% | 86% | 95% | 22% | ‐‐‐ | 11% |
| Stroke | 69% | 20% | 7% | 85% | 0% | 99% |
| Peripheral vascular disease | 23% | ‐‐‐ | ‐‐‐ | 7% | ‐‐‐ | ‐‐‐ |
| Thiazides | ‐‐‐ | 51% | ‐‐‐ | 35% | ‐‐‐ | 35% |
| ACEI/ARB | ‐‐‐ | 84% | ‐‐‐ | 65% | ‐‐‐ | 71% |
| Calcium channel blocker | ‐‐‐ | 26% | ‐‐‐ | 43% | ‐‐‐ | 28% |
| Beta blocker | ‐‐‐ | 57% | ‐‐‐ | 20% | ‐‐‐ | 27% |
| Other antihypertensive drugs | ‐‐‐ | 28% | ‐‐‐ | 11% | ‐‐‐ | 8% |
| Number of antihypertensive drugs | ‐‐‐ | 3.0 (1.4) | 1.0 (‐‐) | 1.1 (0.8) | 2.1 (1.0) | 1.7 (1.1) |
| (‐‐) no information is available. Ischemic heart disease, stroke, and peripheral vascular disease percentages are totally independent of each other because participants can have more than one cardiovascular event at the same time. A similar explanation can be offered with respect to percentages in the different classes of antihypertensive drugs. Abbreviations: ACEI: angiotensin‐converting enzyme inhibitor; ARB: angiotensin receptor blocker; IHD: ischemic heart disease; SD: standard deviation. | ||||||
| Outcome | Studies | Participants | Statistical Method | Effect Estimate |
| Total mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.91,1.45] | |
| Cardiovascular mortality | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69,1.39] | |
| Cardiovascular events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74,1.03] | |
| Serious adverse events | 2773 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88,1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total mortality Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 2 Serious adverse events Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.08] |
| 2.1 Total serious adverse events | 1 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.09] |
| 2.2 Subset of total serious adverse events | 5 | 7922 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
| 3 Cardiovascular events Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 4 Cardiovascular mortality Show forest plot | 6 | 9484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 5 Withdrawals due to adverse effects Show forest plot | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.16 [2.06, 32.28] |
| 6 Blood pressure target achieved at 1 year Show forest plot | 6 | 8588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.17, 1.24] |
| 7 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 6 | 8546 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐13.24, ‐4.56] |
| 8 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 6 | 8546 | Mean Difference (IV, Random, 95% CI) | ‐4.50 [‐6.35, ‐2.65] |
| 9 Number of antihypertensive drugs needed at the end of study Show forest plot | 5 | 7910 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.16, 0.96] |

