نقش درمان آسپرژیلوس برونکوپولمونری آلرژیک با استفاده از آنتیایمونوگلوبین E (آنتی‐IgE) در افراد مبتلا به فیبروز سیستیک
چکیده
پیشینه
فیبروز سیستیک نوعی اختلال چند‐ارگانی اتوزومی مغلوب است که شیوعی تقریبا 1 مورد در هر 3500 تولد زنده دارد. آسپرژیلوس برونکوپولمونری آلرژیک (allergic bronchopulmonary aspergillosis) نوعی بیماری ریوی است که به دنبال بروز افزایش حساسیت ناشی از قارچ آسپرژیلوس رخ میدهد و شیوع آن 2% تا 15% از افراد مبتلا به فیبروز سیستیک است. اصلیترین راه درمان این بیماری استفاده از کورتیکواستروئیدها و ایتراکونازول (itraconazole) است. درمان با کورتیکواستروئیدها برای یک بازه زمانی طولانی یا استفاده مکرر از آنها در مواقع تشدید آسپرژیلوس برونکوپولمونری آلرژیک، ممکن است عوارض جانبی قابل توجهی به همراه داشته باشد. درمان با نوعی آنتیبادی مونوکلونال آنتی‐IgE، مانند اومالیزوماب (omalizumab)، در چندین مورد مبتلا به آسم آلرژیک شدید منجر به بهبود کنترل آسم شده است. استعمال دارو به صورت تزریق زیر‐جلدی هر دو تا چهار هفته یکبار بوده است. از آنجایی که آسپرژیلوس برونکوپولمونری آلرژیک هم مانند آسم، در نتیجه حساسیت به انواع خاصی از آلرژنها ایجاد میشود، برای درمان این بیماری هم شاید بتوان از روش درمان با آنتیبادیهای آنتی‐IgE استفاده کرد. بنابراین درمان با آنتی‐IgE، با استفاده از عواملی چون اومالیزوماب (omalizumab)، ممکن است بهعنوان یک درمان بالقوه در درمان این بیماری در افراد مبتلا به فیبروز سیستیک به حساب آید. این یک نسخه بهروز شده از مرور است.
اهداف
ارزیابی اثربخشی و عوارض جانبی درمان آسپرژیلوس برونکوپولمونری آلرژیک با استفاده از درمان با آنتی‐IgE در افراد مبتلا به فیبروز سیستیک.
روشهای جستوجو
پایگاه ثبت کارآزماییهای مربوط به فیبروز سیستیک در کاکرین که در نتیجه جستوجو در بانکهای اطلاعاتی الکترونیکی و جستوجوی دستی در مجلات و چکیده کتب کنفرانسها به دست آمدهاند، را جستوجو کردیم. همچنین فهرست منابع مقالات مرتبط و مرورها را جستوجو کردیم. آخرین جستوجو: 29 سپتامبر 2017.
دو پایگاه ثبت کارآزماییهای در حال انجام را جستوجو کردیم (Clinicaltrials.gov و پلتفرم کارآزماییهای WHO). تاریخ آخرین جستوجو: 24 ژانویه 2018.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و شبه‐تصادفیسازی و کنترل شده که به مقایسه درمان با آنتی‐IgE با دارونما (placebo) یا سایر روشهای درمان آسپرژیلوس برونکوپولمونری آلرژیک در افراد مبتلا به فیبروز سیستیک پرداختهاند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم به استخراج دادهها و ارزیابی خطر سوگیری (bias) در مطالعه وارد شده پرداختند. آنها برای انجام تجزیهوتحلیل دادهها با استفاده از نرمافزار Review Manager برنامهریزی کردند.
نتایج اصلی
فقط یک مطالعه با حضور 14 شرکتکننده، واجد شرایط برای ورود به مرور بود. این مطالعه دوسو‐کور به مقایسه دریافت روزانه 600 میلیگرم اومالیزوماب یا دارونما همراه با دریافت دو بار در روز ایتراکونازول و کورتیکواستروئیدهای خوراکی، حداکثر به میزان 400 میلیگرم در روز پرداخته بود. دوره درمان شش ماه به طول انجامید اما مطالعه قبل از موعد مقرر منقطع شده و دادههای کامل شده در دسترس نبودند. با محققین مطالعه تماس گرفتیم و به ما گفته شد که مطالعه به دلیل عدم امکان بهکارگیری شرکتکنندگان در مطالعه، علیرغم همه تلاشهای مستدل به عمل آمده، منقطع شده بود. شش نفر از هر نه شرکتکننده (66.67%) و یک مورد از هر پنج شرکتکننده (20%) به ترتیب در گروه تحت درمان با اومالیزوماب و گروه تحت درمان با دارونما، با یک یا چند مورد عوارض جانبی شدید مواجه شدند.
نتیجهگیریهای نویسندگان
شواهد کافی در رابطه با اثربخشی و ایمنی درمان با آنتی‐IgE (اومالیزوماب) در افراد مبتلا به فیبروز سیستیک و آسپرژیلوس برونکوپولمونری آلرژیک وجود ندارد. اجرای مطالعات تصادفیسازی و کنترل شده با مقیاس بزرگ و آیندهنگر در رابطه با درمان با آنتی‐IgE در افراد مبتلا به فیبروز سیستیک و آسپرژیلوس برونکوپولمونری آلرژیک که در آنها به اندازهگیری پیامدهای آزمایشگاهی و بالینی همانند نیاز به استروئید، تشدید آسپرژیلوس برونکوپولمونری آلرژیک و عفونت ریه پرداخته شود، نیاز است.
PICO
خلاصه به زبان ساده
استفاده از درمان با آنتی‐IgE برای آسپرژیلوس برونکوپولمونری آلرژیک در افراد مبتلا به فیبروز سیستیک
سوال مطالعه مروری
شواهد موجود درباره تاثیر استفاده از درمان آنتی‐IgE برای درمان آسپرژیلوس برونکوپولمونری آلرژیک (allergic bronchopulmonary aspergillosis) را در افراد مبتلا به فیبروز سیستیک مرور کردیم.
پیشینه
فیبروز سیستیک یک بیماری ژنتیکی وراثتی است که در دنیای غرب غیر‐معمول نیست. آسپرژیلوس برونکوپولمونری آلرژیک یک بیماری ریوی است که در نتیجه حساسیت بالا به آسپرژیلوس (نوعی قارچ) رخ داده و ممکن است در 2% تا 15% از افراد مبتلا به فیبروز سیستیک بروز کند. درمان با کورتیکواستروئیدها و داروهای ضد‐قارچ، روشهای اصلی در درمان این نوع قارچ هستند اما استفاده طولانیمدت یا استفاده مکرر از کورتیکواستروئیدها ممکن است عوارض جانبی جدی به همراه داشته باشد. آسپرژیلوس برونکوپولمونری آلرژیک در نتیجه عمل آنتیبادیهای IgE (نوعی پروتئین) بروز میکند. یک دارو که علیه این آنتیبادیهای IgE (درمان با آنتی‐IgE)، مانند اومالیزوماب (omalizumab) عمل کند، ممکن است بهعنوان یک شیوه درمانی در درمان این بیماری در افراد مبتلا به فیبروز سیستیک به حساب آید. این دارو بهصورت تزریق زیر‐جلدی، هر دو تا چهار هفته یکبار تجویز میشود. هدف این مرور این است که نشان دهد درمان با آنتی‐IgE در رابطه با این بیماری در افراد مبتلا به فیبروز سیستیک اثربخش است یا خیر؛ و هرگونه عوارض جانبی احتمالی را مشخص کند.
تاریخ جستوجو
شواهد تا تاریخ زیر بهروز است: 29 سپتامبر 2017.
ویژگیهای مطالعه
ما فقط توانستیم یک مطالعه با مقیاس کوچک (شامل 14 شرکتکننده) را وارد مرور کنیم و این مطالعه به دلیل فراهم نشدن امکان همکاری افراد به تعداد کافی و بهصورت داوطلبانه در مراحل اولیه متوقف شد. مطالعه مذکور شش ماه به طول انجامید و در آن تزریق زیر‐جلدی اومالیزوماب (با نام تجاری Xolair) در ناحیه بازو یا ران با تزریق دارونما (placebo) (درمان ساختگی فاقد ماده موثر) مقایسه شدند. به داوطلبان روزانه 600 میلیگرم اومالیزوماب یا دارونما همراه با حداکثر 400 میلیگرم روزانه ایتراکونازول (itraconazole) (نوعی داروی ضد‐قارچ) و کورتیکواستروئید دو مرتبه در روز داده شد.
نتایج کلیدی
نتایج کامل مطالعه منتشر نشد. فقط نتایج محدودی از مطالعه در رابطه با عوارض جانبی درمان به صورت آنلاین منتشر شدند. شش مورد از هر نه داوطلب شرکتکننده در گروه تحت درمان با اومالیزوماب (66.67%) و یک مورد از هر پنج داوطلب شرکتکننده در گروه تحت درمان با دارونما (20%) یک یا چند مورد عوارض جانبی جدی را گزارش کردند.
به دلیل ناکافی بودن شواهد، نمیتوانیم در تایید یا رد استفاده از آنتی‐IgE (اومالیزوماب) در درمان فیبروز سیستیک در افراد مبتلا به آسپرژیلوس برونکوپولمونری آلرژیک توصیههایی ارائه دهیم. پژوهش بیشتری در رابطه با این نوع درمان مورد نیاز است.
Authors' conclusions
Background
Please refer to the glossary for the explanation of clinical terms (Appendix 1).
Description of the condition
Cystic fibrosis (CF), an autosomal recessive multisystem disorder, is characterized by the obstruction and infection of respiratory airways, malabsorption, and various other manifestations and complications (Rowe 2005). With an approximate prevalence of 1 in 3500 live births, CF is more frequent in northern Europe, North America, Australia and New Zealand (Rowe 2005). The mutation in the CF transmembrane regulator (CFTR) gene, with resulting dysfunction of epithelialized surfaces, is the predominant pathogenetic feature in CF. Pulmonary lesions and complications are a major cause of morbidity and mortality in people with CF. Allergic bronchopulmonary aspergillosis (ABPA) is a lung disease caused by aspergillus‐induced hypersensitivity, which usually occurs in susceptible people with bronchial asthma and CF (Agarwal 2009; Greenberger 2002; Tillie‐Leblond 2005). The prevalence of ABPA in people with CF has been described as ranging from 2% to 15% (Becker 1996; Carneiro 2008; Geller 1999; Mastella 2000; Skov 2005; Stevens 2003; Taccetti 2000). These prevalence studies included either just adults or both adults and children. Although, sensitization to aspergillus is not uncommon in people with CF (31% to 59% of people with CF), only a fraction of these individuals develop ABPA (Hemmann 1998; Valletta 1993). The pathophysiology of ABPA is not yet clear. The aspergillus spores attach and penetrate the pre‐activated epithelium in genetically susceptible individuals with CF and form hyphae (Knutsen 2011). The aspergillus antigens from hyphae activate the body’s immune response with a release of inflammatory cytokines (especially IL‐4) which cause bronchial or bronchiolar inflammation and destruction (Knutsen 2003; Knutsen 2011).
The Cystic Fibrosis Foundation Consensus Conference laid down diagnostic criteria for ABPA in CF as well as criteria for screening for ABPA in people with CF (Stevens 2003). Clinical features of ABPA in CF include an acute exacerbation of symptoms, weight loss and a marked increase in productive coughing (Stevens 2003). The presence of ABPA in people with CF has been associated with a decline in lung function (Nepomuceno 1999). If untreated, ABPA may progress to bronchiectasis or pulmonary fibrosis, or both, with significant morbidity and mortality. The mainstay of treatment for ABPA includes corticosteroids and itraconazole. The treatment with corticosteroids may be required for prolonged periods or repeatedly for exacerbations of ABPA, which may lead to many adverse effects. A Cochrane review found no evidence for the use of itraconazole or other antifungal agents for ABPA in people with CF (Elphick 2014).
Description of the intervention
The monoclonal anti‐IgE antibody, omalizumab, has produced a new approach to intervene in the allergic pathway; this new approach is directed to an epitope expressed on the Cɛ3 domain of IgE that binds to high‐ and low‐affinity receptors (Shields 1995). Omalizumab has been shown to decrease circulating free IgE levels by the formation of trimeric and hexameric complexes. These complexes are then cleared by the reticuloendothelial system and there is no activation of a complement system (Corne 1997). In people with asthma, omalizumab inhibits early and late phases of bronchoconstriction induced by allergens, hyper‐responsiveness, and skin‐prick test results (Fahy 1997). In a randomized controlled study, omalizumab treatment improved asthma control in severely allergic asthmatics, reducing inhaled corticosteroid requirements without a worsening of symptom control or an increase in rescue medication use (Holgate 2004). It has been shown to decrease eosinophil counts and also levels of interleukin (IL)‐2+ and IL‐13+ T‐lymphocytes in people with allergic asthma (Noga 2006). Omalizumab is usually given subcutaneously every two to four weeks and the dose is calculated by body weight and baseline total serum IgE levels (Holgate 2004; van der Ent 2007). In a Cochrane review, omalizumab had been found to be effective and safe in allergic asthma (Normansell 2014).
How the intervention might work
Allergic bronchopulmonary aspergillosis is a pulmonary hypersensitivity disease induced by Aspergillus fumigatus (A. fumigatus) (Tillie‐Leblond 2005). In people with CF and ABPA, a number of immunological responses to antigens of A. fumigatus can be observed, such as peripheral blood eosinophilia, immediate cutaneous reactivity, increased levels of total serum IgE, the presence of precipitating antibodies and increased specific serum IgE and IgG antibodies to A. fumigatus (Stevens 2003). Thus, immunomodulatory drugs may be effective for treating ABPA. Omalizumab, an anti‐IgE antibody has been found to be effective in treating asthma with a strong allergy component. Since ABPA is also a condition resulting from hypersensitivity to specific allergens, it may be a candidate for therapy using anti‐IgE antibodies.
Why it is important to do this review
Currently, corticosteroids and antifungal therapy are the mainstay of treatment for ABPA. Corticosteroids may lead to serious side‐effects when used for prolonged periods or repeatedly for exacerbations of ABPA. Therefore, it is prudent to have a therapy for ABPA with a steroid‐sparing effect. Anti‐IgE therapy, using agents such as omalizumab, may be such a potential therapy for ABPA in people with CF.
This is an updated version of a previously published review (Jat 2013).
Objectives
To evaluate the efficacy and adverse effects of anti‐IgE therapy for ABPA in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials were eligible for inclusion. Cross‐over and cluster‐randomized trials were not eligible for inclusion.
Types of participants
People with CF and ABPA. Diagnosis of CF should be in accordance with the criteria laid down by the Cystic Fibrosis Foundation Consensus Report (Farrell 2008); ABPA should be diagnosed using the Rosenberg‐Patterson criteria (Rosenberg 1977), Nelson’s criteria (Nelson 1979), Greenberger’s criteria (Greenberger 2002) or the Cystic Fibrosis Foundation Consensus Criteria (Stevens 2003). There was no limit to age or disease severity for participants included in the review.
Types of interventions
Anti‐IgE therapy compared to placebo or other therapies for ABPA in people with CF. We considered all doses of anti‐IgE therapy in the review.
Types of outcome measures
Primary outcomes
-
Number of participants responding to anti‐IgE therapy (as defined by a decrease in oral corticosteroid dose by 50% or more in comparison to baseline)
-
Number of participants requiring rescue therapy with corticosteroids
-
Adverse effects
-
mild (do not lead to discontinuation of treatment)
-
moderate (lead to a change in treatment)
-
severe (lead to hospitalisation or are life‐threatening)
-
Secondary outcomes
-
Lung function
-
forced expiratory volume in one second (FEV₁)
-
peak expiratory flow rate (PEFR)
-
forced vital capacity (FVC)
-
ratio of FEV1/FVC
-
-
Time until steroid use ceases
-
Number of ABPA exacerbations
-
Pulmonary exacerbations requiring treatment (oral or nebulised or intravenous (IV) or combination)
-
Hospitalisation
-
number of admissions
-
number of days in hospital
-
Search methods for identification of studies
There were no restrictions regarding language or publication status.
Electronic searches
We identified relevant studies from the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's CF Trials Register using the term: allergic bronchopulmonary aspergillosis.
The CF Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group's website.
Date of last search of CF Trials Register: 29 September 2017.
We searched two ongoing trial registries (apps.who.int/trialsearch/; clinicaltrials.gov); details of these searches are provided in the appendices (Appendix 2).
Searching other resources
We also checked the reference lists of any identified studies for additional potentially relevant studies. We contacted authors of the included study to find any ongoing or unpublished relevant studies.
Data collection and analysis
Selection of studies
All three authors independently assessed the results of the searches for any potentially relevant studies. The authors then retrieved and independently assessed the full text of the single study for inclusion, as per the criteria listed above. The authors did not identify any further studies and therefore did not list any studies in any other sections (Excluded studies; Studies awaiting classification; Ongoing studies). There were no disagreements to resolve by discussion.
Data extraction and management
Two review authors (KRJ and AK) independently extracted as much data as possible using a standardized data collection form in accordance with Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), which included the following data: source; eligibility; study methods; participants and settings; interventions and comparisons; outcomes; results; adverse outcomes; and miscellaneous details (e.g. funding source of the study, or potential conflicts of interest). We resolved any disagreement which arose by discussion.
The authors planned to assess all primary and secondary outcomes at 'up to three months', 'over three and up to six months’ and 'over six and up to 12 months' of treatment and to consider any other time‐points reported. There are no efficacy study data available, but there are some adverse event data available on the website clinicaltrials.gov that were reported after six months of treatment.
Assessment of risk of bias in included studies
Two review authors (KRJ and AK) independently assessed the risk of bias in the included study using the criteria described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The authors assessed the risk of bias for the domains listed below.
-
Allocation sequence generation
-
Concealment of allocation
-
Blinding of participants and investigators
-
Incomplete outcome data
-
Selective outcome reporting
-
Any other potential risk of bias
The authors judged each of these criteria to have a low, high or unclear risk of bias and resolved any disagreement by discussion. The authors produced figures for a 'Risk of bias graph' and 'Risk of bias summary' using Review Manager 5.1 (RevMan 2014).
Measures of treatment effect
The authors planned to analyse dichotomous variables by calculating the risk ratio (RR). They planned to express continuous variables as a mean difference (MD). They will express continuous variables as standardized mean difference (SMD) if different scales are used for an outcome in different studies in future updates of review. The authors planned to analyse time‐to‐event data using the hazard ratio (HR). They intended to express the overall results with 95% confidence intervals (CI). Finally, the authors planned to report the numbers of ABPA exacerbations and hospital admissions as a rate ratio (ratio of events per person years) in experimental and control group (Deeks 2011).
Unit of analysis issues
The authors planned to include only randomized and quasi‐randomized controlled parallel trials in the review; the included trial was randomized. They planned to exclude both cross‐over and cluster‐randomized trials.
Dealing with missing data
One author (KRJ) contacted study investigators via email to request some missing outcome data; in response, the authors received a small amount of additional information other than that available on website and have added this to the review. The authors also considered taking the following steps to deal with any missing data in future.
-
Perform sensitivity analyses to assess the sensitivity of any assumptions, if made in future updates.
-
Address the potential impact of missing data on the findings of the review in the 'Discussion' section.
-
For missing standard deviations (SDs) of continuous outcome data, to calculate the SD from study statistics (e.g. CIs, standard errors, T values, P values or F values); if it was still not possible to calculate the SD, then they planned to impute it from other studies in the meta‐analysis as per details given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If data are still not available, the authors will generate analyses making assumptions to create the best and worst case scenarios. In this case, the authors planned to undertake a sensitivity analysis (see below).
-
If the review authors include more studies in future, they plan to explore the impact of including studies with missing outcome data in the overall assessment of results by a sensitivity analysis.
Where possible, the authors planned to extract data to allow an intention‐to‐treat (ITT) analysis, which aims to include all participants randomized into a trial irrespective of what happened subsequently.
Assessment of heterogeneity
The current review included only one study. in future updates of review if additional studies become available, the authors will assess clinical and methodological heterogeneity before pooling data in a meta‐analysis. We will carry out an assessment for statistical heterogeneity initially visually and then using a Chi² test and also using the I² statistic. Using the Chi² test, a low P value (P < 0.1) or a large Chi² statistic relative to its degree of freedom will provide evidence of heterogeneity of intervention effects (i.e. the variation in effect estimates beyond chance). The authors will interpret the value of the I² statistic as follows:
-
0% to 40% ‐ might not be important;
-
40% to 60% ‐ may represent moderate heterogeneity;
-
60% to 75% ‐ may represent substantial heterogeneity; and
-
75% to 100% ‐ considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
One author (KRJ) contacted study investigators through email to provide missing outcome data, but no additional missing data were available as the trial was stopped prematurely.
If sufficient numbers of included studies are available in future updates of review, the authors will assess publication bias, by using funnel plots in Review Manager (RevMan 2014).
Data synthesis
If a sufficient number of included studies are available in future updates of review, the authors will perform meta‐analyses using Review Manager (RevMan 2014). We plan to use a fixed‐effect model for pooled data analysis; however, if there is important statistical heterogeneity identified (I² statistic is more than 50%) we will also use a random‐effects model in a sensitivity analysis among studies.
Subgroup analysis and investigation of heterogeneity
If a sufficient number of included studies (at least 10) are available in future updates of review and if the authors identify substantial heterogeneity (over 50%), we will explore it by performing the following subgroup analyses:
-
children (up to and including 18 years of age) versus adults (over 18 years of age);
-
different stages of ABPA (five stages according to Patterson (Patterson 1982): (1) acute; (2) remission; (3) exacerbation; (4) corticosteroid‐dependent asthma; and (5) fibrosis (end stage));
-
colonization of participants with Staphylococcus aureus or Pseudomonas aeruginosa or both or neither.
Sensitivity analysis
Further, the authors will perform a sensitivity analysis to explore whether the heterogeneity is a result of different risk of bias. If sufficient numbers of trials are available (at least 10) they will undertake sensitivity analyses as follows in future updates of review:
-
repeating meta‐analysis after exclusion of studies with a high risk of bias due to concealment of allocation;
-
repeating meta‐analysis after exclusion of studies in which the outcome evaluation was not blinded;
-
repeating meta‐analysis after imputing missing data as best possible and worst possible outcome; and
-
comparing the difference of pooling analysis results by using a fixed‐effect model and a random‐effects model.
Results
Description of studies
Results of the search
Only one study for inclusion in the review was found through the search strategy (Figure 1).
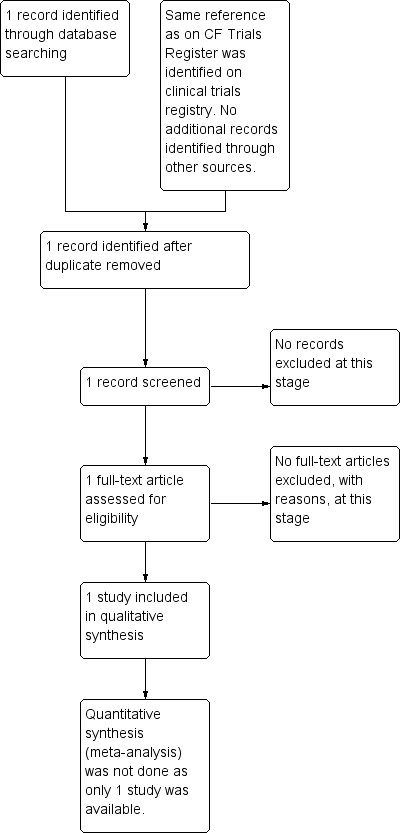
Study flow diagram.
Included studies
Ony one study which evaluated the safety and efficacy of omalizumab for the treatment of ABPA in people with CF was included in the review (Novartis 2008). The study details are available at www.clinicaltrials.gov only and the characteristics of the study are shown in Characteristics of included studies table.
Trial design
The study was double‐blinded, randomized and placebo‐controlled. Assignment was of parallel design. The study was multicentre, conducted in five countries (Belgium, Germany, Italy, Netherlands, and United Kingdom) (Novartis 2008). The double‐blind phase of the study was followed by an open‐label period of six months for those participants who completed the double‐blinded phase and continued the same regimen of omalizumab they had received in the double‐blinded phase. No placebo was used in the open‐label period, hence the data from this period are not eligible for inclusion in the review.
Participants
Study participants were individuals with CF complicated by ABPA and aged 12 years and older (except for Italy where participants were up to and including 18 years of age). Both males and females with oral corticosteroid use for ABPA flare and total serum IgE levels greater than or equal to 500 IU/mL were eligible for enrolment. The study enrolled nine (five females) participants in the intervention group and five (four females) participants in the placebo group. The mean (SD) age of participants in the intervention group was 21 (4.1) years and in the placebo group 28 (9.5) years (Novartis 2008).
Interventions
In the double‐blind phase of the study, the participants in the experimental arm received omalizumab (Xolair®) in the form of subcutaneous injections (into the upper arm in the area of the deltoid or to the thigh) of 600 mg daily for six months along with itraconazole twice daily, while receiving oral corticosteroids, with a maximum daily dose of 400 mg. The participants in the control arm received placebo in place of omalizumab in the same regimen as the experimental arm.
Outcomes measured
Primary outcome measures were:
-
the change from baseline in the percentage of participants requiring rescue with corticosteroids; and
-
the time to deviation from the protocol‐prescribed steroid‐tapering regimen.
Secondary outcome measures were:
-
change in ABPA exacerbation rates;
-
change in FEV1 from baseline;
-
time to steroid‐free state;
-
change from baseline in average oral corticosteroid use;
-
percentage of participants responding to omalizumab, as defined by a reduction in oral corticosteroid dose use of 50% or more as compared to baseline.
Most of the outcome measures were assessed at six and 12 months of study, except for FEV1 which was assessed at three and six months (Novartis 2008).
Excluded studies
The search strategy revealed only one relevant study that was included in the review. No studies were excluded.
Risk of bias in included studies
The risk of bias for the only included study (Novartis 2008) is shown in Characteristics of included studies, risk of bias graph (Figure 2) and risk of bias summary (Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
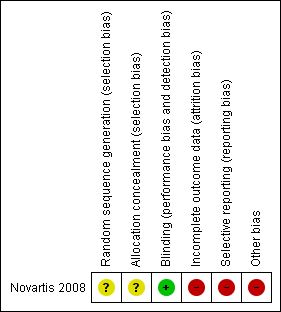
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
There was unclear risk of bias for sequence generation and allocation concealment for the study as information was not available (Novartis 2008).
Blinding
There was low risk bias as the study was described as double‐blinded; where participant, caregiver, investigator, and outcome assessor were masked to treatment assignment (Novartis 2008).
Incomplete outcome data
There was a high risk of bias for incomplete outcome data because a large proportion of enrolled participants dropped out. Of the nine participants enrolled in the intervention group only four completed the double‐blind phase of the study, and of the five participants in the placebo group only three completed this phase. The five participants who dropped out of the intervention group did so for the following reasons: one due to adverse events; one due to lack of efficacy; and three due to administrative problems. Both participants who dropped out of the placebo group did so due to administrative problems.
Selective reporting
There is a high risk of selective reporting since data related to all the outcome measures were not reported on the website, even for those participants who completed the trial before it was terminated.
Other potential sources of bias
There was high risk of bias as the study was terminated early and results were not published in any journal.
Effects of interventions
Details of the included study are available at www.clinicaltrials.gov only (Novartis 2008). It was terminated early and results for any outcome measures except for adverse effects are not available online (www.clinicaltrials.gov).
The study reported one or more non‐serious side effects in all participants in the double‐blinded phase (Table 1; Novartis 2008). One or more serious side effects were encountered in six out of nine (66.67%) and one out of five (20.00%) participants in omalizumab group and placebo group respectively (Table 1).
| AEs | Omalizumab group N = 9, n (%) | Placebo group N = 5, n (%) |
| Non‐serious AEs | ||
| Participants with AE(s) | 9 (100) | 5 (100) |
| Infective pulmonary exacerbation of CF | 7 (78) | 4 (80) |
| Cough | 4 (44) | 1 (20) |
| Headache | 4 (44) | 1 (20) |
| Pyrexia | 3 (33) | 2 (40) |
| Hemoptysis (blood in sputum) | 4 (44) | 0 |
| Injection site swelling | 4 (44) | 0 |
| Injection site warmth | 4 (44) | 0 |
| Injection site erythema (redness) | 3 (33) | 0 |
| Hypokalemia | 2 (22) | 0 |
| Nasopharyngitis | 2 (22) | 0 |
| Sputum increased | 1 (11) | 1 (20) |
| Bronchopulmonary aspergillosis allergic | 2 (22) | 0 |
| Rhonchi (noisy expiratory breathing sound) | 1 (11) | 1 (20) |
| Non‐cardiac chest pain | 1 (11) | 1 (20) |
| Vomiting | 0 | 1 (20) |
| SAEs | ||
| Participants with any SAE | 6 (67) | 1 (20) |
| Infective pulmonary exacerbation of CF | 5 (56) | 1 (20) |
| Allergic Bronchopulmonary Aspergillosis | 2 (22) | 0 |
| Distal intestinal obstruction syndrome | 1 (11) | 0 |
| Hemoptysis | 1 (11) | 0 |
| Rhonchi | 1 (11) | 0 |
AE: adverse event
CF: cystic fibrosis
SAE: serious adverse event
Discussion
Summary of main results
Only one study was eligible for inclusion in the review, but that was terminated prematurely (Novartis 2008).The study details are available at www.clinicaltrials.gov only and full results were not published. Only results relating to adverse effects were available; and serious side effects were encountered more frequently in the omalizumab group compared to the placebo group.
Overall completeness and applicability of evidence
There is lack of evidence for the efficacy and safety of anti‐IgE therapy for ABPA in people with CF.
Quality of the evidence
It is difficult to comment on the quality of the only included study as there is not sufficient information available. A summary of findings table could not be created because of the lack of included studies.
Potential biases in the review process
The search strategy was broad enough to include all relevant studies. However, only one study with incomplete information was eligible for inclusion in the review (Novartis 2008).
Agreements and disagreements with other studies or reviews
A few case reports have described the effectiveness of omalizumab for ABPA in people with CF. In 2007, for the first time, van der Ent reported the use of a single dose of omalizumab in a 12‐year‐old girl with CF and ABPA who showed a rapid and good improvement of respiratory symptoms and lung functions (van der Ent 2007). Zirbes reported the use of omalizumab in three children with CF and ABPA (three males aged 12.9 years, 12.8 years and 17 years) who were steroid‐dependent with significant side effects from chronic steroid therapy (Zirbes 2008). After the start of omalizumab (300 mg to 375 mg subcutaneously every two weeks), these children showed significant and sustained clinical improvements and all were able to discontinue steroids. In a third study, Kanu reported on a girl aged 13 years with CF and ABPA who was poorly controlled on steroids; she received 300 mg omalizumab subcutaneously every two weeks and her clinical symptoms and lung function improved markedly to a level that she had not been able to achieve with steroids or antibiotics (Kanu 2008). In a final study, two teenagers with CF and ABPA exacerbations who were reluctant to undertake a further course of oral steroids, started subcutaneous injections of 375 mg omalizumab twice monthly (Lebecque 2009). Both respiratory symptoms and lung functions improved rapidly and the therapy was gradually withdrawn without any recurrence after 20 weeks of follow up.
In contrast to the above case reports, in a study by Brinkman, the use of 300 mg omalizumab for four weeks in a 15‐year‐old with CF and steroid‐dependent ABPA was not successful and the individual deteriorated again after an initial improvement of lung function, remaining steroid dependent over 12 months of omalizumab treatment (Brinkmann 2010).

Study flow diagram.
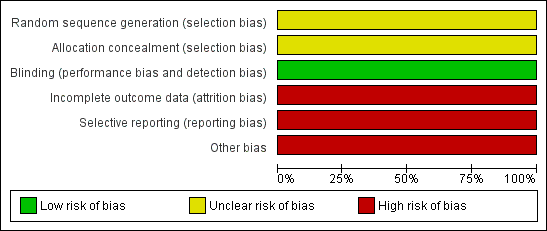
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| AEs | Omalizumab group N = 9, n (%) | Placebo group N = 5, n (%) |
| Non‐serious AEs | ||
| Participants with AE(s) | 9 (100) | 5 (100) |
| Infective pulmonary exacerbation of CF | 7 (78) | 4 (80) |
| Cough | 4 (44) | 1 (20) |
| Headache | 4 (44) | 1 (20) |
| Pyrexia | 3 (33) | 2 (40) |
| Hemoptysis (blood in sputum) | 4 (44) | 0 |
| Injection site swelling | 4 (44) | 0 |
| Injection site warmth | 4 (44) | 0 |
| Injection site erythema (redness) | 3 (33) | 0 |
| Hypokalemia | 2 (22) | 0 |
| Nasopharyngitis | 2 (22) | 0 |
| Sputum increased | 1 (11) | 1 (20) |
| Bronchopulmonary aspergillosis allergic | 2 (22) | 0 |
| Rhonchi (noisy expiratory breathing sound) | 1 (11) | 1 (20) |
| Non‐cardiac chest pain | 1 (11) | 1 (20) |
| Vomiting | 0 | 1 (20) |
| SAEs | ||
| Participants with any SAE | 6 (67) | 1 (20) |
| Infective pulmonary exacerbation of CF | 5 (56) | 1 (20) |
| Allergic Bronchopulmonary Aspergillosis | 2 (22) | 0 |
| Distal intestinal obstruction syndrome | 1 (11) | 0 |
| Hemoptysis | 1 (11) | 0 |
| Rhonchi | 1 (11) | 0 |
| AE: adverse event | ||

