نقش درمان آسپرژیلوس برونکوپولمونری آلرژیک با استفاده از آنتیایمونوگلوبین E (آنتی‐IgE) در افراد مبتلا به فیبروز سیستیک
Appendices
Appendix 1. Glossary
| Term | Explanation |
| Antigen | Any substance capable of inducing a specific immune response and of reacting with the products of that response. |
| Cɛ3 domain | One part of IgE antibody. |
| Cytokine | A non‐antibody protein released by a cell population on contact with a specific antigen. |
| Eosinophil count | Number of a type of white blood cells. |
| Eepithelialized | Where skin is present. |
| Hhyphae | Threadlike structures in a fungus. |
| Monoclonal anti‐IgE antibody | An antibody against the immunoglobulin E antibody which is produced by a single clone of cells, therefore a single pure type of antibody. |
| Peripheral blood eosinophilia | An increase in eosinophils (a type of white blood cells) circulating in the blood. |
| Quasi‐randomized trials | Where method of allocation is not completely random, e.g. alternation, date of birth, day of week or case record number etc. |
Appendix 2. Additional electronic search strategies
| Database | Date searched | Search terms |
| Clinicaltrials.gov | 29 September 2017 | 'anti IgE and cystic fibrosis' AND 'omalizumab and cystic fibrosis' |
| WHO ICTRP | 24 January 2018 | 'anti IgE and cystic fibrosis' AND 'omalizumab and cystic fibrosis' |

Study flow diagram.
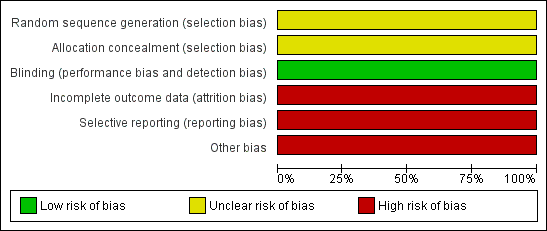
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| AEs | Omalizumab group N = 9, n (%) | Placebo group N = 5, n (%) |
| Non‐serious AEs | ||
| Participants with AE(s) | 9 (100) | 5 (100) |
| Infective pulmonary exacerbation of CF | 7 (78) | 4 (80) |
| Cough | 4 (44) | 1 (20) |
| Headache | 4 (44) | 1 (20) |
| Pyrexia | 3 (33) | 2 (40) |
| Hemoptysis (blood in sputum) | 4 (44) | 0 |
| Injection site swelling | 4 (44) | 0 |
| Injection site warmth | 4 (44) | 0 |
| Injection site erythema (redness) | 3 (33) | 0 |
| Hypokalemia | 2 (22) | 0 |
| Nasopharyngitis | 2 (22) | 0 |
| Sputum increased | 1 (11) | 1 (20) |
| Bronchopulmonary aspergillosis allergic | 2 (22) | 0 |
| Rhonchi (noisy expiratory breathing sound) | 1 (11) | 1 (20) |
| Non‐cardiac chest pain | 1 (11) | 1 (20) |
| Vomiting | 0 | 1 (20) |
| SAEs | ||
| Participants with any SAE | 6 (67) | 1 (20) |
| Infective pulmonary exacerbation of CF | 5 (56) | 1 (20) |
| Allergic Bronchopulmonary Aspergillosis | 2 (22) | 0 |
| Distal intestinal obstruction syndrome | 1 (11) | 0 |
| Hemoptysis | 1 (11) | 0 |
| Rhonchi | 1 (11) | 0 |
| AE: adverse event | ||

