Profilaxis con antibióticos para la prevención de las complicaciones infecciosas en la cirugía ortognática
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010266.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 enero 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Romina Brignardello‐Petersen drafted the methods, results and discussion sections of the review, performed meta‐analysis and assessed the quality of the evidence. She also edited complete the final version of the review and directed the screening process, data abstraction and risk of bias assessment.
Alonso Carrasco‐Labra conceived of the review question and assisted with preparation of the methods section of the review. He assisted in statistical analysis, quality of evidence assessment and drafting of the final manuscript and was one of the full‐text screening review authors.
Ignacio Araya and Nicolas Yanine drafted the background section. They also performed the title and abstract screening process and data abstraction.
Luis Cordova is a context expert. All aspects of the background and methodology related to the clinical application of the results are supported by his expertise. He also acted as a review author in the full‐text screening process.
Julio Villanueva is a content expert. All aspects of the background and methodology related to clinical application of the results are supported by his expertise. He performed data abstraction and risk of bias assessments.
All authors approved the final version of the protocol and the final manuscript.
Contributions of editorial base
Nicky Cullum: edited the protocol; advised on methodology, interpretation and review content. Approved the final review before submission.
Liz McInnes, Editor: approved the final protocol before submission.
Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited and copy edited the protocol and edited the review.
Ruth Foxlee: designed the search strategy and ran the searches for the review.
Sources of support
Internal sources
-
Faculty of Dentistry, University of Chile, Chile.
External sources
-
The National Institute for Health Research (NIHR) is the sole funder of the Cochrane Wounds Group, UK.
Declarations of interest
Romina Brignardello‐Petersen: non declared.
Alonso Carrasco‐Labra: non declared.
Ignacio Araya: non declared.
Nicolas Yanine: non declared.
Luis Cordova: non declared.
Julio Villanueva: non declared.
Acknowledgements
The authors would like to acknowledge the contributions of the peer referees Anne‐Marie Glenny and Shirley Manknell and Wounds Group editors Liz McInnes and Giovanni Casazza and of the copy editor Elizabeth Royle in providing feedback on the protocol and Dolores Matthews for copy editing the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Jan 05 | Antibiotic prophylaxis for preventing infectious complications in orthognathic surgery | Review | Romina Brignardello‐Petersen, Alonso Carrasco‐Labra, Ignacio Araya, Nicolás Yanine, Luis Cordova Jara, Julio Villanueva | |
| 2012 Dec 12 | Antibiotic prophylaxis for preventing infectious complications in orthognathic surgery | Protocol | Romina Brignardello‐Petersen, Alonso Carrasco‐Labra, Ignacio Araya, Nicolás Yanine, Luis Cordova, Julio Villanueva | |
Differences between protocol and review
The following changes were made to the protocol.
-
We had planned to perform a fixed‐effect meta‐analysis if heterogeneity was lower than 40%. However, because of variability in interventions and in some of the population characteristics, we decided that it was more appropriate to perform a random‐effects meta‐analysis, which allowed us to account for this variability.
PICO
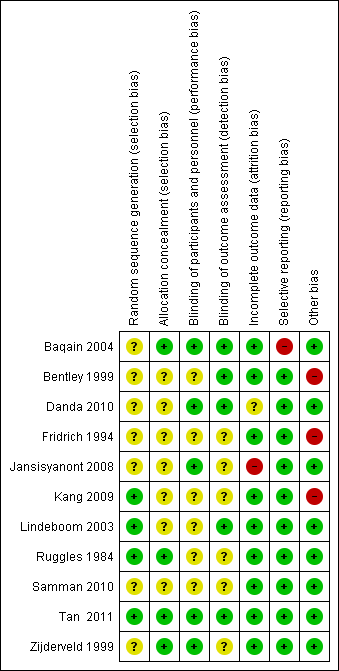
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.

Comparison 2 Sensitivity analysis: short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
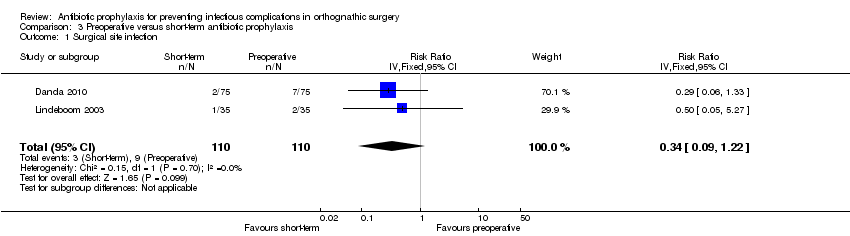
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
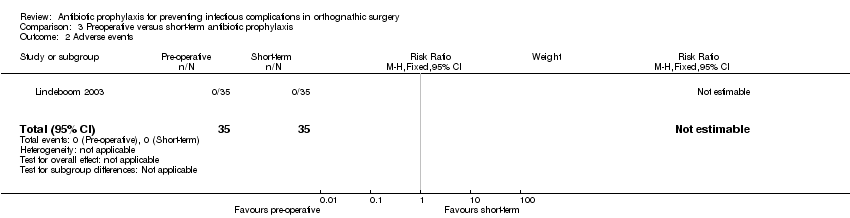
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 2 Adverse events.

Comparison 4 Amoxicillin versus ampicillin, Outcome 1 Surgical site infection.

Comparison 4 Amoxicillin versus ampicillin, Outcome 2 Adverse events.
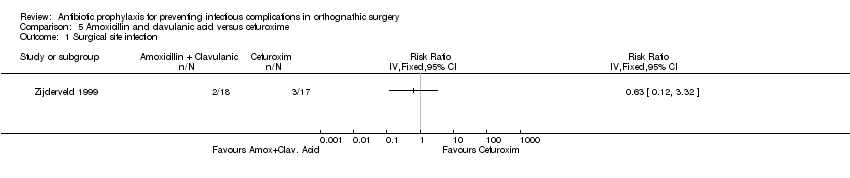
Comparison 5 Amoxicillin and clavulanic acid versus cefuroxime, Outcome 1 Surgical site infection.
| Short‐term antibiotic prophylaxis compared with long‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: short‐term antibiotic prophylaxis Comparison: long‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐term | Long‐term | |||||
| Surgical site infection Follow‐up: 2 to 36 weeks | 168 per 1000a | 71 per 1000 (41 to 125) | RR 0.42 (0.24 to 0.74) | 472 | ⊕⊕⊕⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Adverse events | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Preoperative antibiotic prophylaxis compared with short‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: preoperative antibiotic prophylaxis Comparison: short‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Preoperative | Short‐term | |||||
| Surgical site infection Follow‐up: 4 to 12 weeks | 82 per 1000a | 28 per 1000 | RR 0.34 (0.09 to 1.22) | 220 | ⊕⊕⊝⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Adverse events Follow‐up: up to 12 weeks | 0 per 35 See comment | 0 per 35 See comment | Not estimable | 70 | ⊕⊕⊝⊝ | No adverse events were reported in any of arms of the trial |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 7 | 472 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.24, 0.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 6 | 438 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.22, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 2 | 220 | Risk Ratio (IV, Fixed, 95% CI) | 0.34 [0.09, 1.22] |
| 2 Adverse events Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |