Profilaxia antibiótica para prevenir complicações infecciosas em cirurgias ortognáticas
Resumo
Introdução
O termo cirurgia ortognática (OS) designa muitas técnicas cirúrgicas eletivas para correção de deformidades faciais e os problemas de má oclusão e distúrbios funcionais relacionados ao sistema estomatognático. Embora essa cirurgia seja classificada como "limpa‐contaminada", ainda existem controvérsias acerca da utilidade e do regime mais adequado de profilaxia antibiótica nesses pacientes.
Objetivos
Avaliar os efeitos da profilaxia antibiótica para prevenir infecção no local cirúrgico (SSI) em pessoas submetidas à cirurgia ortognática.
Métodos de busca
Em junho de 2014 fizemos buscas nas seguintes bases de dados: Cochrane Wounds Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other In‐Indexed Citations); Ovid EMBASE; e EBSCO CINAHL. Também fizemos buscas no Google Scholar, além de buscas manuais em revistas relevantes, em anais de congressos e nas listas de referências dos artigos potencialmente elegíveis para inclusão. Não houve restrições quanto ao idioma, data de publicação ou local onde o estudo foi conduzido.
Critério de seleção
Incluímos ensaios controlados randomizados (ECRs) envolvendo pessoas submetidas à cirurgia ortognática que compararam um regime de profilaxia antibiótica versus outro regime ou placebo. O desfecho primário foi SSI. Os desfechos secundários foram infecções sistêmicas, eventos adversos, duração da internação e qualidade de vida relacionada à saúde. Dois autores, trabalhando de forma independente, selecionaram os artigos.
Coleta dos dados e análises
Dois autores, trabalhando de forma independente, extraíram os dados. A concordância entre eles foi verificada. O risco de viés de cada estudo foi avaliado usando a ferramenta de risco de viés da Cochrane. Os regimes antibióticos foram classificados como profilaxia antibiótica pré‐operatória (uma dose antes da cirurgia), de curto prazo (antes ou durante a cirurgia e/ou no mesmo dia da cirurgia) e de longo prazo (antes ou durante a cirurgia e por mais de um dia após a cirurgia). Sempre que possível, fizemos metanálises com o modelo de efeitos aleatórios com o método da variância inversa. Calculamos o risco relativo (RR) e os respectivos intervalos de confiança (IC) de 95%,
Principais resultados
Incluímos nesta revisão um total de 11 estudos. A maioria dos estudos teve um risco de viés incerto o que nos levou a rebaixar a qualidade da evidência dos nossos desfechos. Combinamos os resultados dos 7 estudos que apresentaram dados para a comparação principal e para os desfechos primários. No geral, a profilaxia antibiótica de longo prazo provavelmente reduz o risco de infecção SSI. Os efeitos plausíveis da profilaxia antibiótica variaram entre uma redução relativa do risco de SSI de 76% a 0,26%: RR 0,42, IC 95% 0,24 a 0,74, 472 participantes, evidência de qualidade moderada. Existem dúvidas acerca dos efeitos do uso da profilaxia antibiótica de curto prazo versus dose única: RR 0,34, IC 95% 0,09 a 1,22, 220 participantes, evidência de baixa qualidade. Os estudos que avaliaram esse desfecho não relataram nenhum efeito adverso associado ao uso de antibióticos. Nenhum dos estudos avaliou ou apresentou dados relativos a outros desfechos.Não há dados suficientes para saber se um antibiótico específico é melhor que outro.
Conclusão dos autores
A profilaxia antibiótica de longo prazo é mais efetiva do que a profilaxia de curto prazo na redução do risco de infecção no local cirúrgico em pessoas submetidas à cirurgia ortognática. Existem dúvidas se o uso de profilaxia antibiótica de curto prazo diminui o risco de infecção no local cirúrgico em comparação com o uso de uma dose única pré‐operatória de antibióticos profiláticos.
PICO
Resumo para leigos
Antibióticos para prevenção de infecção após cirurgia maxilar
Todos os anos, muitas pessoas se submetem à cirurgia nos maxilares para corrigir malformações. Existe o risco da pessoa desenvolver uma infecção após esse tipo de cirurgia. Porém, não existe consenso quanto ao uso de antibióticos para prevenir essas infecções, nem quanto ao melhor tipo ou a dose ideal de antibiótico a ser empregada.
Realizamos uma busca abrangente para encontrar pesquisas sobre esse assunto. Coletamos dados de todos os estudos que avaliaram essa questão e resumimos os achados para saber se os antibióticos podem prevenir a infecção após a cirurgia, se esse tipo de tratamento tem efeitos adversos, se ele reduz o número de dias que o paciente precisa ficar internado no hospital e se ele melhora o estado geral de saúde das pessoas.
Encontramos 11 estudos. No geral, o uso de antibióticos por um período prolongado reduz o risco de infecção no local cirúrgico. Existem dúvidas quanto aos efeitos de usar uma dose de antibióticos imediatamente antes da cirurgia versus usar antibióticos por um curto período de tempo. Esses estudos não avaliaram os efeitos colaterais dos antibióticos. Os estudos que avaliaram isso, não encontraram efeitos colaterais. Os estudos não avaliaram nenhum dos outros efeitos de interesse para clínicos ou pacientes. Não há dados suficientes para mostrar se um determinado antibiótico é melhor que os outros.
Authors' conclusions
Summary of findings
| Short‐term antibiotic prophylaxis compared with long‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: short‐term antibiotic prophylaxis Comparison: long‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐term | Long‐term | |||||
| Surgical site infection Follow‐up: 2 to 36 weeks | 168 per 1000a | 71 per 1000 (41 to 125) | RR 0.42 (0.24 to 0.74) | 472 | ⊕⊕⊕⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Adverse events | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Preoperative antibiotic prophylaxis compared with short‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: preoperative antibiotic prophylaxis Comparison: short‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Preoperative | Short‐term | |||||
| Surgical site infection Follow‐up: 4 to 12 weeks | 82 per 1000a | 28 per 1000 | RR 0.34 (0.09 to 1.22) | 220 | ⊕⊕⊝⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Adverse events Follow‐up: up to 12 weeks | 0 per 35 See comment | 0 per 35 See comment | Not estimable | 70 | ⊕⊕⊝⊝ | No adverse events were reported in any of arms of the trial |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
Background
Description of the condition
Orthognathic surgery (OS) is the surgical correction of a deformity of the jaw (ortho meaning 'straight,' and gnath meaning 'jaws'). It is a general term that includes many elective surgical techniques to correct facial deformity, the associated malocclusion and functional disorders related to the stomatognathic system (set of anatomical features in the head that focus on the mouth, functions of which include chewing, breathing, speaking and swallowing) (Obwegeser 2007). The first orthognathic surgery was performed In 1847 by Hullihen (Hullihen 1849). This surgery involves carrying out total, or partial, osteotomies (cutting of bone) on the maxilla, the mandible and other facial bones to position the skeleton correctly and to correct functional problems (Moore 2001).
According to the American Association of Oral and Maxillofacial Surgeons (AAOMS 2008), conditions that indicate the need for orthognathic surgery include difficulty chewing or biting food; difficulty swallowing; chronic jaw or jaw joint (TMJ) pain and headache; excessive wear of the teeth; open bite; unbalanced facial appearance from the front or side; facial injury or birth defects; receding chin; protruding jaw; inability to make the lips meet without straining; chronic mouth breathing and dry mouth; and serious breathing problems while sleeping (sleep apnoea). The consequences of these conditions depend on the degree of deformity and can vary from very mild to severe. Dentofacial deformities may provoke functional problems such as physical pain, physical disability, cosmetic dissatisfaction and difficulties with speaking, breathing and chewing. Patients can improve significantly after orthognathic surgery with regard to their psychological discomfort, social disability and self‐confidence, as when functional limitations decrease, quality of life is improved (Choi 2010; Lee 2008; Rustemeyer 2011).
Although few data are available to estimate the number of OS procedures performed each year around the world, statistics from oral and maxillofacial surgery training programs reported to the American Dental Association and the American Association of Oral and Maxillofacial Surgeons show a gradual increase in the number of these procedures performed between 1996 and 2007. In 2007 it was reported that a total of 8755 OS procedures were performed in the United States of America (Sullivan 2011). The average age of patients undergoing orthognathic surgery in 2008 was 26.7 years, and a great majority of these patients were between 15 and 30 years of age (Venugoplan 2012). Female patients accounted for 56.2% of those undergoing the procedure (Venugoplan 2012). Whites constituted 71.9%, blacks 4.9%, Hispanics 12.6%, Asian/Pacific Islanders 5.6%, Native Americans 0.4% and other groups 4.6%, respectively (Venugoplan 2012).
Specific postoperative complications related to orthognathic surgery include haemorrhage, temporary or permanent sensory and motor problems affecting the face and mouth (V2 and V3 nerve dysfunction), deviation of the nasal septum, bone infection (osteomyelitis), connection of the mouth to a sinus cavity (oro‐antral fistula), sinusitis and loss or degradation of results obtained by surgery (postoperative relapse) (Chow 2007). In addition, patients can suffer more general surgical complications, such as pain, swelling and surgical site infection (SSI).
SSIs are divided into incisional SSIs and organ/space SSIs (Horan 1992). Incisional SSIs are further classified into those involving only the skin and subcutaneous tissue, and those involving deep soft tissues of the incision (called deep incisional SSIs (e.g. fascial and muscle layers)). Organ/space SSIs involve any part of the anatomy (e.g. organs, spaces) other than the incision that was opened or manipulated during the operative procedure (Horan 1992). The SSIs seen with OS are organ/space infections.
The proportion of patients developing SSI after OS is estimated to be about 7% (Alpha 2006; Barrier 2009; Chow 2007). The pathogens most commonly associated with SSI after OS are anaerobic bacteria, which have been observed in 50% of pus samples of SSI after OS, and streptococci, which have been observed in 43% of cases (Chow 2007). Studies show that risk factors that may be associated with a higher incidence of SSI after OS include longer surgery; short‐term antibiotic prophylaxis; extraction of a third molar during surgery; greater number of osteotomies performed; older age; smoking; poor oral hygiene; and a compromised immune system (Alpha 2006; Barrier 2009; Cheynet 2001; Chow 2007; Fridrich 1999; Laskin 2003; Manor 1999; Theodossy 2006). SSI following orthognathic surgery can cause localised pain, swelling, surface redness (erythema), pus formation and restricted movement. Throughout the body, these infections cause fever, swollen lymph nodes (lymphadenopathy), general discomfort, toxic reactions and an elevated white blood cell count (Topazian 2002).
A multi‐centre, retrospective study assessed the cost of and factors influencing orthognathic surgery in a single region in the UK. The average total treatment cost for people who experienced complications after orthognathic surgery, including infected bone plates, was EUR 6815.94, whereas the cost for those who had no complications was EUR 5962.61. Average ward stay costs were EUR 1421.49 and EUR 1295.64, respectively (Kumar 2008).
Description of the intervention
Surgical antibiotic prophylaxis is defined as the use of antibiotics to prevent infection at a surgical site (Munckhof 2005).
The original experiments to evaluate efficacy, which were performed 40 years ago in animal models, concluded that the most effective period for prophylaxis is within three hours of the time at which bacteria gain access to the tissues (Burke 1961). Since then, many studies have been performed on people undergoing surgery, and they have led to wide acceptance of antibiotic prophylaxis as a part of surgical practice (Dellinger 1994). A non‐systematic review of the literature indicated that intravenous antibiotics should be given ≤ 30 minutes preoperatively to patients undergoing all categories of surgery except caesarean section (Mangram 1999).
A classification system that ranks procedures according to their potential risk for infectious complications guides the administration of surgical antibiotic prophylaxis. This system ranks surgical procedures as clean, clean‐contaminated and contaminated. In clean‐contaminated surgery, the respiratory, digestive or genitourinary tract is penetrated, thus antibiotic prophylaxis is recommended. Orthognathic surgery is classified as clean‐contaminated surgery because the upper digestive tract is penetrated (Gottrup 2005; Mangram 1999).
Although researchers have investigated the effectiveness of penicillin (Jansisyanont 2008), amoxicillin (Baqain 2004), clindamycin (Baqain 2004; Lindeboom 2003), a combination of amoxicillin and clavulanic acid (Jansisyanont 2008; Zijderveld 1999) and a combination of levofloxacin and cefazolin (Yoda 2000) against placebo and/or each other, no single antibiotic regimen is currently recommended to prevent infection after OS; also, lack of agreement regarding type of antibiotic and dosing schedule is ongoing.
How the intervention might work
The aim of surgical antibiotic prophylaxis is to prevent SSI (Salmeron‐Escobar 2006) in patients at greatest risk of infection and/or when a clean‐contaminated surgery and implant insertions are being performed (Munckhof 2005).
The risk of infection is increased in orthognathic surgery because of the use of titanium plates and screws to fix bones together. Bacteria and other micro‐organisms organise in thin, but robust, layers of mucilage that adhere to the surface of implants, such as plates and screws. Consequently, implants stimulate the adherence and multiplication of micro‐organisms and increase infection rates (Mangram 1999).
The role of bacterial biofilms from the surface of implants in the development of SSI is well recognised (Costerton 1999; Deacon 1996; Lee 2010; Mombelli 2011; Murdoch 2001; Peel 2011; Southwood 1985). Many experimental studies have confirmed the pro inflammatory and bone‐remodeling effects of toxins present on orthopaedic implant surfaces, which are also capable of causing osteolytic (dissolving of bone) and immune responses (Bi 2002; Greenfield 2005; Gristina 1985; Ragab 1999; Xing 2006). Therefore, it seems reasonable to believe that the oral biofilm and its toxins, adhered to the surface of titanium plates and screws used for stabilisation of maxillary osteotomy segments, could be a source of local or regional infectious complications. In consequence, antibiotic prophylaxis could be useful for preventing these infections.
Many studies show that antibiotic prophylaxis may reduce the risk of infection in orthognathic surgery, but the best route of administration is still not clear (Baqain 2004; Bentley 1999; Danda 2010; Fridrich 1994; Jansisyanont 2008; Lindeboom 2003). Nonetheless, two main types of regimens can be differentiated: first, short‐term antibiotic prophylaxis administered any time before or after surgery for up to 24 hours after the surgical intervention; and second, long‐term antibiotic prophylaxis that is continued for longer than 24 hours (SIGN 2008). In patients undergoing OS, prophylaxis with broad‐spectrum antibiotics has been recommended (Baqain 2004; Bentley 1999; Fridrich 1994; Zijderveld 1999).
Why it is important to do this review
The usefulness of antibiotic prophylaxis in orthognathic surgery is still debated, as is the most appropriate regimen. Some study authors advocate that perioperative morbidity can be kept to a minimum with adherence to general surgical principles (Fridrich 1994; Laskin 2003; Waddell 1994), that prophylactic antibiotics have questionable value in preventing infection and that their deployment could lead to the development of super‐infections (i.e. infections resistant to antibiotics) (Kunitake 1986; Peterson 1976). On the other hand, it has been reported that the use of prophylactic antibiotics may significantly reduce the postoperative infection rate after orthognathic surgery (Zijderveld 1999). Many attempts have been made to determine the effects of antibiotic prophylaxis in patients undergoing orthognathic surgery (Baqain 2004; Danda 2010; Jansisyanont 2008; Zallen 1971; Zijderveld 1999); therefore the literature identified in a systematic review must be summarised so that the beneficial and adverse effects of antibiotic prophylaxis for orthognathic surgery can be determined and the best evidence provided to clinicians.
Objectives
To assess the effects of antibiotic prophylaxis for preventing SSI in people undergoing OS.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) conducted in people undergoing OS. Quasi‐randomised and non‐randomised trials, observational studies, narrative reviews, commentaries and letters to editors were excluded.
Types of participants
People of any age undergoing OS in any setting.
Types of interventions
-
Intervention: any type of antibiotic (penicillin and its derivatives, cephalosporins, etc.), with any regimen or mode of administration (short‐term or long term; oral, endovenous or intramuscular; preoperative or perioperative regimen).
-
Comparison: placebo, or another antibiotic, or another regimen of antibiotic.
Types of outcome measures
Studies had to report any of the following outcomes to be included:
Primary outcomes
Occurence of postoperative SSI (i.e. infection of organs/spaces in relation with OS) as defined by Centers for Disease Control and Prevention (CDC) criteria (Horan 1992), or the authors' definition of SSI. We did not differentiate between superficial and deep‐incisional infection.
Secondary outcomes
-
Systemic infection: defined as a systemic inflammatory response syndrome associated with a postoperative SSI consecutive to OS. It is a secondary, not a primary, outcome because it is very unlikely to occur. OS is a scheduled procedure, and surgeons do not perform it in patients at high risk of infection.
-
Length of hospital stay (LOS): defined as the number of days from the day of admission to the day of discharge of participants undergoing OS.
-
Participant health‐related quality of life (HRQoL): measured using a standardised generic questionnaire such as EQ‐5D (standardised measure of health outcomes) (EuroQol 1990), Short Form (SF)‐36 (Ware 1992), SF‐12 (Müller‐Nordhorn 2004) or SF‐6 (Brazier 2002) or wound‐specific questionnaires such as the Cardiff wound impact schedule (Price 2004). We did not include ad hoc measures of quality of life, which are unlikely to be validated and are not common to multiple trials.
-
Adverse events (e.g. gastrointestinal complications, allergic reactions due to antibiotic administration): Gastrointestinal adverse events were defined as any abnormal or harmful effects in the gastrointestinal tract related to the use of antibiotic prophylaxis. Allergic reactions were defined as any hypersensitive reactions of the immune system related to the use of antibiotic prophylaxis. Information regarding any other adverse events reported by trial authors was collected.
RCTs that evaluate any of these outcomes were included, irrespective of the scale used for assessment. If possible, outcomes were evaluated at one week, at one month and up to three months after surgery.
Search methods for identification of studies
Electronic searches
In June 2014 we searched the following electronic databases to identify reports of relevant randomised clinical trials.
-
The Cochrane Wounds Group Specialised Register (up to 05 June 2014);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 5);
-
Ovid MEDLINE (1946 to May Week 4 2014);
-
Ovid MEDLINE (in‐Process & Other Non‐Indexed Citations, June 04 2014);
-
Ovid EMBASE (1980 to 2014 June 04);
-
EBSCO CINAHL (1982 to 05 June 2014).
We used the following search strategy in Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Orthognathic Surgery] explode all trees 8
#2 MeSH descriptor: [Orthognathic Surgical Procedures] explode all trees 61
#3 MeSH descriptor: [Osteotomy, Le Fort] explode all trees 63
#4 (orthognathic near/5 surg*):ti,ab,kw 197
#5 ((maxillary next osteotom*) or "Le Fort" or (mandibular near/5 osteotom*) or (vertical next ramus next osteotom*) or genioplast*):ti,ab,kw 210
#6 #1 or #2 or #3 or #4 or #5 343
#7 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees 1226
#8 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees 9253
#9 (antibiotic* or cephalosporin* or cefazolin or cefuroxime or amoxicillin* or amoxycillin* or clindamicin or clindamycin or penicillin* or levofloxacin):ti,ab,kw 20956
#10 #7 or #8 or #9 24784
#11 #6 and #10 22
We adapted this strategy to search Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL. The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 1, Appendix 2 and Appendix 3 respectively. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). We did not restrict the search and study selection with respect to language, date of publication or study setting. If an article published in a language other than English was identified, relevant data were extracted by a translator.
We searched the following ongoing trials databases up to February 2013.
-
Current Controlled Trials (http://www.controlled‐trials.com/).
-
ClinicalTrials.gov (http://www.clinicaltrials.gov/).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/).
-
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/)
Searching other resources
We examined reference lists of relevant articles that were identified by the electronic searches for other pertinent articles to include in the review. We also searched in Google Scholar to detect unpublished or grey literature.
To account for any delay in indexing in the electronic databases mentioned above, we also searched the last six months of the following journals, up to February 2013.
• Journal of Oral and Maxillofacial Surgery.
• International Journal of Oral and Maxillofacial Surgery.
• British Journal of Oral and Maxillofacial Surgery.
• Journal of Craniofacial Surgery.
• Head & Neck: Journal for the Sciences & Specialties of the Head and Neck.
Finally, we handsearched the last five years of the online abstract indexes of conference proceedings of the annual meetings of the American Association of Oral and Maxillofacial Surgeons and the International Association for Dental Research up to February 2013.
Data collection and analysis
Selection of studies
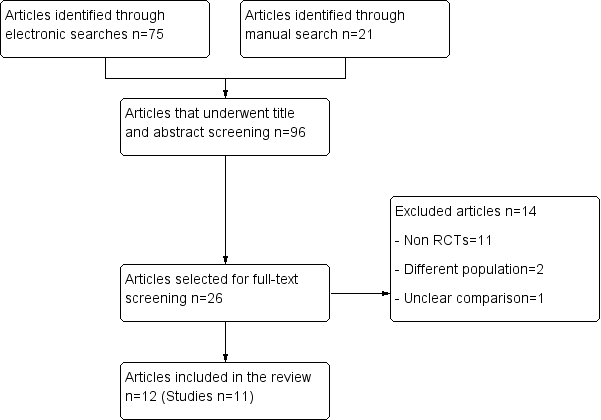
In a first screening, the title and the abstract of all potentially relevant articles were listed and were evaluated using a pre established selection criteria form. This process was done independently by two review authors who followed instructions especially designed for this stage, which were widely inclusive. All articles selected for full‐text screening by either review author were retrieved. The full text of all articles that potentially met the eligibility criteria were assessed independently by two review authors. Disagreements were resolved by discussion, and, if no consensus was reached, a third review author acted as arbiter. We include a study flow diagram, as recommended by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement (Liberati 2009), to illustrate the results of all searching activity and the process of screening and selecting studies for inclusion in the review (Figure 1). Articles that were published in a different language were evaluated by reviewers fluent in that language, with previous experience in systematic reviews.
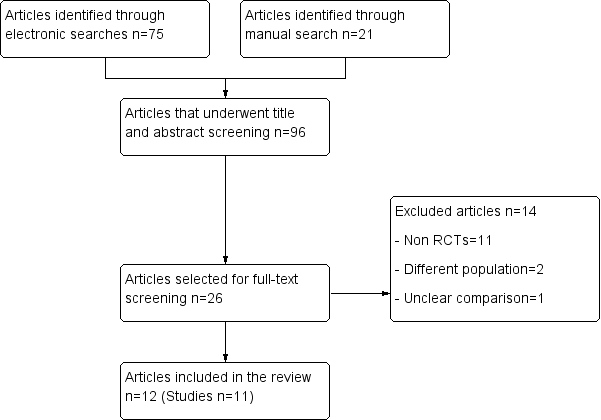
Flow diagram.
Data extraction and management
Two review authors extracted the data from all selected studies independently, using a standardised form of Microsoft Excel Office for Mac 2011 created for this purpose. We extracted data regarding the characteristics of the studies relevant for this review, such as the characteristics of the population (sex, age, and selection criteria for enrolment in the trial), surgery (type and number of osteotomies, if reported), intervention and comparison (antibiotic type and regimen), and outcomes (definitions and results of the trial). Discrepancies between the data were reviewed by the two review authors, and, if needed, a third review author acted as arbiter. If data for the outcomes of interest were missing, we contacted the trial authors to obtain the information. A standard form was to be designed to request trial authors to provide specific data needed for the review, and the fields required were highlighted in each case. We planned to liaise with the Wounds Group should any translations be required.
Assessment of risk of bias in included studies
The risk of bias of the included studies was evaluated using the Cochrane risk of bias tool (Higgins 2011). All domains of this tool were used (sequence generation, allocation concealment, blinding of participants, personnel and outcome, incomplete outcome data, selective outcome reporting and other sources of bias), and the instructions published in the Cochrane Handbook for Systematic Reviews of Interventions were followed in assessing each domain and in performing the evaluation of the overall risk of bias. This evaluation was performed by two review authors independently, based on the full text of the trials. Disagreements between the review authors were discussed, and consensus had to be reached for a study to be classified as having low, high or unclear risk of bias. As allocation concealment was judged to be the most critical risk of bias domain for this review, a trial would have been classified as having overall HIGH risk of bias if either the allocation concealment domain or two or more other domains were judged to have been at high risk of bias. The same criteria were applied to classify a trial as having unclear risk of bias. Otherwise, the trial was to be classified as having low risk of bias. As it has been shown that blinding to study author and/or affiliation of the study is not associated with the overall results of evaluations (Moher 1999), the review authors were not blinded to these characteristics of the trials.
Measures of treatment effect
The measures of treatment effect that were to be used for evaluating the outcome in each trial include the following.
-
SSI: Rate of infection at the surgical site was analysed as dichotomous, and risk ratios (RRs) and 95% confidence intervals (CIs) were calculated. We expected studies to report how the presence of SSI was judged and two review authors with clinical expertise determined whether this definition was consistent with the CDC definition.
-
Systemic infection: We planned to analyse data as dichotomous, and RRs and 95% CIs would have been calculated; however, no data were available that would permit the review authors to do this.
-
Length of hospital stay (days): We planned to analyse data as continuous, using mean difference (MD) with 95% CI. If the data were presented as median and were likely skewed, we would not have pooled the data. Data were insufficient for evaluation of this outcome.
-
Adverse events: When trials reported adverse events in sufficient detail (e.g. the number of participants who experienced at least one adverse event), we analysed these data dichotomously.
Unit of analysis issues
We used the participant as the unit of analysis. No unit of analysis issues were anticipated, nor were any found.
Dealing with missing data
Each trial was analysed with regards to the presence and proportion of missing data, and it was judged that the amount of missing data was not substantial enough to change the direction of the results. Therefore, it was not necessary to impute missing data, and only complete case data were used.
Assessment of heterogeneity
The Chi2 test was used to determine the presence of statistical heterogeneity, using a level of significance of 0.1. Quantification of inconsistency across studies was done using the I2 statistic, and its interpretation was based on recommendations of The Cochrane Collaboration (Deeks 2011), that is, an I2 between 0% and 40% might be considered as unimportant heterogeneity amongst the trials; 30% to 60% might represent moderate heterogeneity; 50% to 90% might represent substantial heterogeneity; and 75% to 100% might represent considerable heterogeneity. Clinical heterogeneity was assessed qualitatively by considering participants, setting and intervention characteristics with the help of experts. Methodological heterogeneity was evaluated using the domains of the risk of bias tool (Higgins 2011). Exploration of heterogeneity was based on subgroup analyses (detailed below).
Assessment of reporting biases
Efforts were made to detect reporting biases, if possible, in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). Outcome reporting biases were explored by looking for published protocols of the trials included in the systematic review (where available). If a sufficient number of studies had been identified, publication bias was to have been explored using funnel plots for all outcomes and tested using the Egger test (Egger 1997).
Data synthesis
We present a narrative overview of the included trials. When appropriate, we present meta‐analyses of outcome data using RevMan 5.3. The decision to pool data in a meta‐analysis depended on the availability of outcome data and assessment of between‐trial heterogeneity. Because of anticipated and observed clinical heterogeneity between primary studies, random‐effects meta‐analysis was planned and performed. Participants were analysed in the arms to which they were randomly assigned, in accordance with the intention‐to‐treat principle.
For analytical purposes, we classified the antibiotic regimens according to time and duration of antibiotic prophylaxis as follows.
-
Preoperative dose only: antibiotic was administered only once before the OS.
-
Short‐term prophylaxis: antibiotic was administered before and during surgery, and during the same day of surgery.
-
Long‐term prophylaxis: antibiotic was administered before, during and longer than one day after surgery.
In studies that included more than one arm of any of the categories, data from those arms were combined. The main findings are presented in a 'Summary of findings' table (Rosenbaum 2010).
Subgroup analysis and investigation of heterogeneity
We anticipated that two factors could cause heterogeneity across results of the trials, and these would be explored through subgroup analyses. The number of osteotomies was the predictor of greatest interest. Based on previous findings (Chow 2007), the a priori hypothesis for this factor is that trials in which participants had a lower number of osteotomies would show greater treatment effect in favour of antibiotic prophylaxis than trials in which participants underwent a greater number of osteotomies. Other sources of heterogeneity that would have been investigated, if possible, include mode of administration of the antibiotic and duration of surgery.
However, as statistical heterogeneity between studies was low and we detected no important clinical source of heterogeneity; we did not perform any subgroup analysis.
Sensitivity analysis
We had planned to perform a sensitivity analysis by excluding trials with high risk of bias. As the allocation concealment was judged to be the most critical risk of bias domain for this review, a trial would have been classified as having high risk of bias if either the allocation concealment domain or two or more other domains were judged to have high risk of bias. The same criteria were applied to classify a trial as having unclear risk of bias. Otherwise, the trial was to be classified as having low risk of bias. None of the trials was classified as having high risk of bias, thus no sensitivity analysis was performed.
We also planned to perform sensitivity analyses if data imputation was needed, if a meta‐analysis showed a trial with an effect that was different from the others in an important way (outlier) and if this trial had clinical features that made it different from the others. None of these scenarios were faced, thus no sensitivity analyses were performed.
Assessment of the quality of the evidence
After this entire process was performed, we graded the quality of the evidence (confidence in the estimates) for each outcome (postoperative SSI; LOS; HRQoL; adverse events) using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) approach. We used the five criteria of this approach: limitations in study design of studies that contribute to the outcome, inconsistency, imprecision, indirectness and publication bias (Guyatt 2008). As mentioned above, we included a GRADE summary of findings table in the results section.
Results
Description of studies
We included a total of 11 studies. See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search resulted in 96 references that underwent screening. After two screening stages, a total of 11 studies (Baqain 2004; Bentley 1999; Danda 2010; Fridrich 1994; Jansisyanont 2008; Kang 2009; Lindeboom 2003; Ruggles 1984; Samman 2010; Tan 2011; Zijderveld 1999), reported in 12 articles, were included in this review. Details of the results of each search and screening stage can be found in Figure 1.
Included studies
See Characteristics of included studies for further details.
Setting
Of the 11 studies included, six were undertaken in Asia (Baqain 2004; Danda 2010; Jansisyanont 2008; Kang 2009; Samman 2010; Tan 2011), three in North America (Bentley 1999; Fridrich 1994; Ruggles 1984) and two in Europe (Lindeboom 2003; Zijderveld 1999). Six of the trials were performed in academic settings (e.g. universities) (Baqain 2004; Danda 2010; Jansisyanont 2008; Lindeboom 2003; Ruggles 1984; Samman 2010), one was performed in a hospital setting (Bentley 1999), two were performed in collaboration between a university and a hospital (Fridrich 1994; Kang 2009) and two were performed at a University Hospital (Tan 2011; Zijderveld 1999).
Participants
The trials recruited between 30 (Bentley 1999; Fridrich 1994) to 160 (Samman 2010) participants. Most trials recruited a higher proportion of females than males; only two studies recruited more males (Fridrich 1994; Ruggles 1984). Most study participants were young adults. In all but one study (Jansisyanont 2008), the mean age of participants was younger than 30 years. In two studies, neither the sex nor the age distribution of recruited participants was reported (Bentley 1999; Samman 2010).
The mean duration of OS ranged from 2.5 hours (Lindeboom 2003) to 7.5 hours (Tan 2011); no information regarding duration of surgery was provided in five trials (Danda 2010; Fridrich 1994; Ruggles 1984; Samman 2010; Zijderveld 1999). The type and number of osteotomies varied according to the details reported. Some study authors described the bone in which it was performed (i.e. mandibular/ maxillary osteotomy) (Danda 2010, Fridrich 1994; Kang 2009; Ruggles 1984; Tan 2011), whereas others specifically described the type of surgery performed (i.e. LeFort I, bilateral sagittal split osteotomy, etc ) (Baqain 2004; Bentley 1999; Jansisyanont 2008; Lindeboom 2003; Zijderveld 1999). Seven studies reported different combinations of osteotomies performed and the total number of participants who received these combinations, without distinguishing the arm to which the participants belonged (Danda 2010; Fridrich 1994; Jansisyanont 2008; Kang 2009; Lindeboom 2003; Tan 2011; Zijderveld 1999). Two studies reported the combination of osteotomies and the numbers of participants who received these combinations per arm (Baqain 2004; Bentley 1999), and one study reported the number of each single type of osteotomy performed, without considering its combinations and the arms in which each was performed (Ruggles 1984). One study did not report any information regarding the type or number of osteotomies performed (Samman 2010).
Interventions
Most of the trials were parallel two‐arm studies (Baqain 2004; Bentley 1999; Danda 2010; Fridrich 1994; Kang 2009; Lindeboom 2003; Ruggles 1984; Tan 2011). One study had three arms (Zijderveld 1999), and two studies had four arms (Jansisyanont 2008; Samman 2010). Six trials assessed the effects of preoperative and perioperative antibiotic prophylaxis versus the effects of preoperative, perioperative and postoperative prophylaxis (Baqain 2004; Bentley 1999; Fridrich 1994; Jansisyanont 2008; Kang 2009; Ruggles 1984); two trials assessed the effects of a single preoperative dose of antibiotic prophylaxis versus preoperative and perioperative antibiotic prophylaxis (Danda 2010; Lindeboom 2003); one trial assessed the effects of preoperative antibiotic prophylaxis versus no prophylaxis (Zijderveld 1999); one trial compared the effects of two different antibiotics up to the second day postoperatively (Tan 2011); and one trial compared four different regimens of the same antibiotic (Samman 2010).
In five trials, participants received penicillin (Bentley 1999; Fridrich 1994; Jansisyanont 2008; Ruggles 1984; Samman 2010). Other antibiotics administered were amoxicillin (Baqain 2004; Jansisyanont 2008; Tan 2011), ampicillin (Danda 2010; Tan 2011), amoxicillin plus clavulanic acid (Jansisyanont 2008; Zijderveld 1999), clindamycin (Lindeboom 2003), cefpiramide (Kang 2009) and cefuroxime (Zijderveld 1999).
Outcomes
All studies measured the outcome SSI. Three studies used the CDC criteria to diagnose a postoperative infection (Baqain 2004; Jansisyanont 2008; Kang 2009); one study used the CDC criteria but modified them by eliminating one criterion (Danda 2010); in five studies, a definition and criteria determined by the authors were used (Baqain 2004; Lindeboom 2003; Ruggles 1984; Tan 2011; Zijderveld 1999; see details of measurement criteria in Characteristics of included studies); and two studies did not provide details on how this outcome was measured (Fridrich 1994; Samman 2010).
Only two studies measured the occurrence of adverse reactions to antibiotics (Lindeboom 2003; Tan 2011), and none of the studies measured systemic infection, duration of hospital stay or HRQoL.
Follow‐up
In four studies, participants were followed‐up for up to one month (Baqain 2004; Bentley 1999; Danda 2010; Zijderveld 1999). The shortest follow‐up was two weeks (Kang 2009), and the longest follow‐up was six months (Samman 2010).
Excluded studies
A total of 14 studies that underwent full‐text screening were excluded from this review (see Characteristics of excluded studies). Most of these studies were excluded because they were not RCTs (Bystedt 1987; Danda 2011; Fenner 1950; Fridrich 1999; Martis 1984; Paterson 1970; Peterson 1976; Schubert 1990; Spaey 2005; Tan 2011; Yrastorza 1976). Two studies were excluded because although the participants underwent maxillofacial surgery the results were not available separately for participants who underwent OS (Dumbach 1987; Sixou 2006). One study was excluded because no description of what participants in the control arm received was reported (Xiaoyi 1996).
Risk of bias in included studies
The risk of bias assessment is illustrated in Figure 2 and Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 2 shows the assessment of risk of bias per study and domain. In only one study all domains were judged as having low risk of bias (Tan 2011). In many of the studies, the information reported did not allow a judgement as to whether the risk of bias related to sequence generation, allocation concealment and/or blinding was high or low (Bentley 1999; Fridrich 1994; Kang 2009; Lindeboom 2003; Samman 2010; Tan 2011). Five studies had one domain judged to have high risk of bias (Baqain 2004; Bentley 1999; Fridrich 1994; Jansisyanont 2008; Kang 2009).
Figure 3 shows the risk of bias assessment per domain, across studies. The domain judged to have the lowest risk of bias was selective reporting, whereas the domain judged to have the highest risk of bias was other bias.
Random sequence generation
The generation of the randomisation sequence was described on only four of the studies (Kang 2009; Lindeboom 2003; Ruggles 1984; Tan 2011). Although the other seven studies described allocating patients to the intervention and comparison groups randomly, they authors did not provide details regarding the sequence generation, and therefore were judged as having an unclear risk of bias.
Allocation
Allocation concealment was described in only four of the 11 studies (Baqain 2004; Ruggles 1984; Tan 2011; Zijderveld 1999). In all other studies, no information regarding this design feature was provided, thus those studies were judged as having an unclear risk of bias.
Blinding
Blinding in relation to performance bias and detection bias was assessed separately. With respect to performance bias, blinding was described in five of the studies (Baqain 2004; Danda 2010; Jansisyanont 2008; Tan 2011; Zijderveld 1999). In relation to detection bias, blinding was described in five studies (Baqain 2004; Bentley 1999; Danda 2010; Lindeboom 2003; Tan 2011). The other studies did not provide any information and thus were judged as having an unclear risk of bias.
Incomplete outcome data
One study had high risk of bias with regard to this domain (Jansisyanont 2008), as 15 participants were excluded from the analysis, and this is a high number compared with the overall number of events. One study had an unclear risk of bias because no information regarding losses to follow‐up was provided, nor were details given regarding whether the number of participants accounted for in the results section was the same as the number of participants enrolled (Danda 2010). All other studies were judged as having low risk of bias.
Selective reporting
Most of the studies were judged to have low risk of bias. Even though the only outcome measured in most of them was SSI, it is uncommon to find trials in this area measuring duration of hospital stay or quality of life, thus selective reporting was not suspected. One trial was judged as having high risk of bias because the study authors did not report explicitly in the results section the number of participants with postoperative infection, and instead reported in the discussion section the number of participants who required extra antibiotics (Baqain 2004).
Other potential sources of bias
We detected other potential sources of bias in three studies. In two of the trials (Fridrich 1994; Kang 2009) a co‐intervention applied to patients in both arms after the surgery (drainage tubes) could have increased the risk of SSI in patients receiving shorter term regimens of antibiotics. One trial was stopped early (Bentley 1999). More details can be found in the risk of bias table for each of these studies, provided in Characteristics of included studies.
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2
Comparison 1. Short‐term compared with long‐term antibiotic prophylaxis
Surgical site infection
A total of seven studies (472 participants) provided data for this comparison and outcome (Baqain 2004; Bentley 1999; Fridrich 1994; Jansisyanont 2008; Kang 2009; Ruggles 1984; Samman 2010). Participants who received long‐term antibiotic prophylaxis were less likely to develop an SSI than those who received short‐term prophylaxis (risk ratio (RR) 0.42, 95% confidence interval (CI) 0.24 to 0.74; Analysis 1.1). Heterogeneity was low (I2 0%), with all of the trials individually showing the same direction of the effect (or no effect) and with overlapping confidence intervals.
We performed a post hoc sensitivity analysis by excluding one study (Baqain 2004) from the analysis. The authors of this study reported the number of participants who required additional antibiotics, which, according to clinical experts, could be considered as a proxy for postoperative infection. However, as no explicit report of infection was provided, we explored whether the results changed when this study was left out of the meta‐analysis. Exclusion of Baqain 2004 from the meta analysis did not change the result (RR 0.41, 95% CI 0.22 to 0.75) (Analysis 2.1).
Other outcomes
The trials did not report on systemic infection, adverse events, duration of hospital stay or HRQoL.
Comparison 2. Preoperative compared with short‐term antibiotic prophylaxis
Surgical site infection
In all, two studies (220 participants) provided data for this comparison and outcome (Danda 2010; Lindeboom 2003) and the results of these trials were pooled (I2 0%). Whilst participants who received short‐term antibiotic prophylaxis were less likely to develop an SSI than those who received a single, preoperative dose, this result is rather uncertain and no difference is also a plausible result (RR 0.24, 95% CI 0.09 to 1.22; Analysis 3.1). No evidence of statistical heterogeneity was found.
Adverse events
One study (70 participants) reported on this outcome (Lindeboom 2003). None of the participants suffered any types of adverse events
Other outcomes
No trials for this comparison reported on systemic infection, duration of hospital stay or HRQoL.
Comparison 3. Amoxicillin compared with ampicillin
Surgical site infection
One study (42 participants) reported on this comparison (Tan 2011). In this study, both antibiotics were administered in a long‐term regimen. Only nine people in this small study developed an SSI and therefore the results are highly uncertain (RR 0.5, 95% CI 0.14 to 1.74; Analysis 4.1).
Adverse events
Tan 2011 reported that none of the participants suffered any types of adverse events.
Other outcomes
No trials for this comparison reported on systemic infection, duration of hospital stay or HRQoL.
Comparison 4. Amoxicillin and clavulanic acid compared with cefuroxime
Surgical site infection
One small study (35 participants; 5 SSIs) reported on this comparison (Zijderveld 1999) and therefore the results are highly uncertain (RR of SSI with amoxicillin/clavulanic acid relative to cefuroxime 0.63, 95% CI 0.12 to 3.32; Analysis 5.1). Both antibiotics were administered preoperatively.
Other outcomes
No trials for this comparison reported on systemic infection, adverse events, duration of hospital stay or HRQoL.
Discussion
Summary of main results
The results of this review suggest that patients who receive long‐term antibiotic prophylaxis (compared with short‐term prophylaxis) experience a reduction in the absolute risk of developing a SSI (from 168 SSIs per 1000 surgeries with short‐term antibiotics to 71 SSIs per 1000 surgeries with long‐term antibiotics). The quality of the evidence was downgraded due to risk of bias of included studies and overall the evidence is of moderate quality (see the Summary of Findings Table). There was no evidence of a difference between all antibiotic comparisons and insufficient evidence to show if one antibiotic was any better than another or whether rates of adverse effects differed. Furthermore the results were uncertain for people who receive short‐term antibiotic prophylaxis (compared with a single pre‐operative dose). Whilst participants who received short‐term antibiotic prophylaxis were less likely to develop an SSI than those who received a single, preoperative dose, no difference is also a plausible result. The evidence for this outcome was low quality (downgraded for risk of bias of included studies and imprecision).”
Overall completeness and applicability of evidence
The studies included in this review provided information regarding only two of the five outcomes of interest. All studies reported on SSI, and two studies reported on adverse events. No data were provided on the incidence of systemic infection, duration of hospital stay and HRQoL.
The studies included a broad sample of participants (given the characteristics of patients who undergo OS) who were treated in different settings and with different regimens of antibiotic prophylaxis. This and the fact that the results were very similar among trials favour the applicability of the evidence to many populations and settings.
Quality of the evidence
We found moderate‐quality evidence for SSIs in the comparison of short‐term versus long‐term antibiotic prophylaxis. We downgraded the quality of the evidence because of serious limitations in risk of bias, as most of the trials were judged to have an unclear risk of bias in the domains of allocation concealment and blinding, and this decreases our confidence in the estimates of effects.
We found low‐quality evidence for the comparison of preoperative versus short‐term antibiotic prophylaxis. For both outcomes—SSIs and adverse events—the quality of the evidence was downgraded because of serious limitations in risk of bias and imprecision. For the outcome SSI, the confidence interval of the pooled estimate of effect suggests uncertainty over the possibility of harm or benefit. For the outcome adverse events, it was not possible to pool an estimate of effect, because although investigators collected data on adverse events, no adverse events were observed. However, the number of participants included for this outcome was low.
Potential biases in the review process
This systematic review adhered in a rigorous manner to all of the steps recommended in the Cochrane Handbook for Systematic Reviews of Interventions.
We were not able to assess publication bias using a funnel plot. Our broad searching strategy, however, minimises the likelihood of publication bias for the other comparisons and outcomes.
We were not able to perform the subgroup analyses that we had planned because the number of trials in each comparison were insufficient. However, the low statistical heterogeneity makes it unnecessary to perform such analyses.
Finally, we would have liked to confirm some information with the authors of the included trials; however, contact efforts were unfruitful in all cases. The main issue we would have liked to clarify was the number of participants who were reported to receive additional antibiotics versus the number of participants diagnosed with SSI in the trial by Baqain 2004; a sensitivity analysis excluding this trial did not result in a change in the pooled estimate.
Agreements and disagreements with other studies or reviews
Two systematic reviews on this topic were published in the literature (Danda 2011; Tan 2011). Some differences are evident between those reviews and ours, particularly differences regarding the criteria used to classify the interventions. Danda 2011 classified studies based on the presence of a placebo comparator and blinding in three categories. They performed a meta‐analysis in which arms were classified as short‐term and extended‐term, with the shorter‐term regimen considered the 'control.' This led to short‐term prophylaxis classified as short‐term when the comparison was long‐term but classified as extended‐term when the comparison was preoperative prophylaxis. In addition, review authors identified a smaller number of trials than were included in our review. The systematic review performed by Tan 2011 excluded some of the trials that we included for lack of details regarding the medical conditions of study participants, and, according to the review authors, lack of specified criteria used to define the outcomes. However, the findings of both studies tend to agree with ours, as the review authors observed that participants who received antibiotic prophylaxis for a longer time were less likely to develop postoperative infection. This finding was determined to be statistically significant in one review (Danda 2010) and non‐significant in the other (Tan 2011).
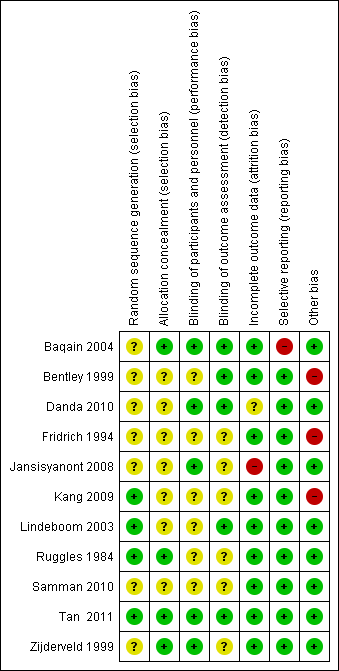
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.

Comparison 2 Sensitivity analysis: short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
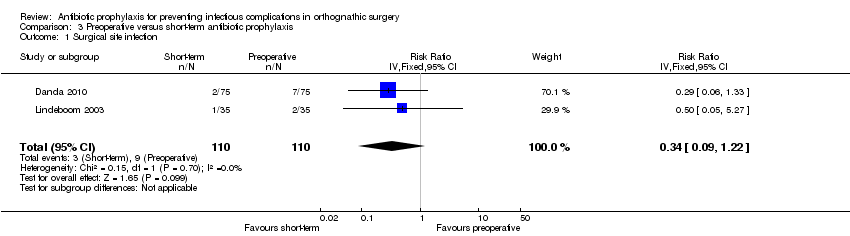
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
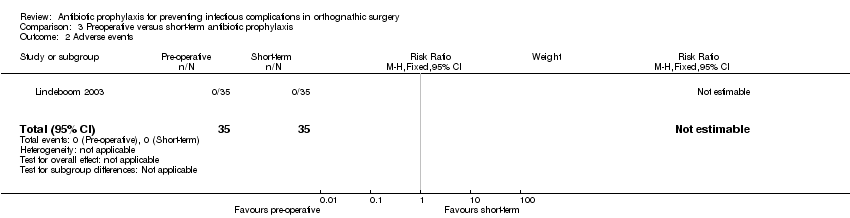
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 2 Adverse events.

Comparison 4 Amoxicillin versus ampicillin, Outcome 1 Surgical site infection.

Comparison 4 Amoxicillin versus ampicillin, Outcome 2 Adverse events.
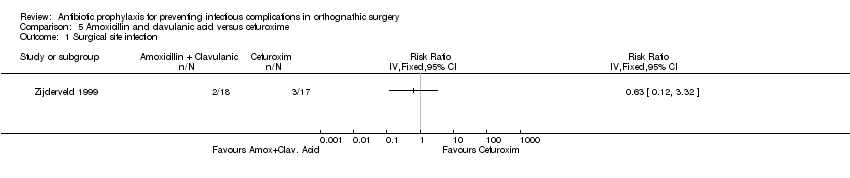
Comparison 5 Amoxicillin and clavulanic acid versus cefuroxime, Outcome 1 Surgical site infection.
| Short‐term antibiotic prophylaxis compared with long‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: short‐term antibiotic prophylaxis Comparison: long‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐term | Long‐term | |||||
| Surgical site infection Follow‐up: 2 to 36 weeks | 168 per 1000a | 71 per 1000 (41 to 125) | RR 0.42 (0.24 to 0.74) | 472 | ⊕⊕⊕⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Adverse events | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Preoperative antibiotic prophylaxis compared with short‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: preoperative antibiotic prophylaxis Comparison: short‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Preoperative | Short‐term | |||||
| Surgical site infection Follow‐up: 4 to 12 weeks | 82 per 1000a | 28 per 1000 | RR 0.34 (0.09 to 1.22) | 220 | ⊕⊕⊝⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Adverse events Follow‐up: up to 12 weeks | 0 per 35 See comment | 0 per 35 See comment | Not estimable | 70 | ⊕⊕⊝⊝ | No adverse events were reported in any of arms of the trial |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 7 | 472 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.24, 0.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 6 | 438 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.22, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 2 | 220 | Risk Ratio (IV, Fixed, 95% CI) | 0.34 [0.09, 1.22] |
| 2 Adverse events Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |