Profilaxia antibiótica para prevenir complicações infecciosas em cirurgias ortognáticas
Appendices
Appendix 1. Ovid MEDLINE Search Strategy
1 exp Orthognathic Surgery/ (87)
2 exp Orthognathic Surgical Procedures/ (1052)
3 exp Osteotomy, Le Fort/ (1454)
4 (orthognathic adj5 surg*).tw. (2516)
5 (maxillary osteotom* or Le Fort osteotom* or (mandibular adj5 osteotom*) or vertical ramus osteotom* or genioplast*).tw. (2160)
6 or/1‐5 (5695)
7 exp Antibiotic Prophylaxis/ (8683)
8 exp Anti‐Bacterial Agents/ (527266)
9 (antibiotic* or cephalosporin* or cefazolin or cefuroxime or amox?cillin* or clindam?cin or penicillin* or levofloxacin).tw. (260904)
10 or/7‐9 (629847)
11 6 and 10 (65)
12 randomized controlled trial.pt. (374960)
13 controlled clinical trial.pt. (88427)
14 randomi?ed.ab. (327203)
15 placebo.ab. (146420)
16 clinical trials as topic.sh. (170264)
17 randomly.ab. (193925)
18 trial.ti. (117951)
19 or/12‐18 (878362)
20 exp animals/ not humans.sh. (3947165)
21 19 not 20 (807587)
22 11 and 21 (13)
Appendix 2. Ovid EMBASE search strategy
1 exp orthognathic surgery/ (1497)
2 exp maxilla osteotomy/ (2054)
3 (orthognathic adj5 surg*).tw. (2946)
4 (maxillary osteotom* or Le Fort osteotom* or (mandibular adj5 osteotom*) or vertical ramus osteotom* or genioplast*).tw. (2495)
5 or/1‐4 (6681)
6 exp antibiotic prophylaxis/ (20802)
7 exp antibiotic agent/ (983143)
8 (antibiotic* or cephalosporin* or cefazolin or cefuroxime or amox?cillin* or clindam?cin or penicillin* or levofloxacin).tw. (345777)
9 or/6‐8 (1089776)
10 5 and 9 (142)
11 Randomized controlled trials/ (52460)
12 Single‐Blind Method/ (18336)
13 Double‐Blind Method/ (115948)
14 Crossover Procedure/ (39087)
15 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. (1331662)
16 (doubl$ adj blind$).ti,ab. (146816)
17 (singl$ adj blind$).ti,ab. (14503)
18 or/11‐17 (1399256)
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ (20296020)
20 human/ or human cell/ (14775294)
21 and/19‐20 (14728616)
22 19 not 21 (5567404)
23 18 not 22 (1207860)
24 10 and 23 (20)
Appendix 3. EBSCO CINAHL search strategy
S23 S10 AND S22
S22 S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21
S21 MH "Quantitative Studies"
S20 TI placebo* or AB placebo*
S19 MH "Placebos"
S18 TI random* allocat* or AB random* allocat*
S17 MH "Random Assignment"
S16 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S15 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S14 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S13 TI clinic* N1 trial* or AB clinic* N1 trial*
S12 PT Clinical trial
S11 MH "Clinical Trials+"
S10 S5 and S9
S9 S6 or S7 or S8
S8 TI ( (antibiotic* or cephalosporin* or cefazolin or cefuroxime or amox?cillin* or clindam?cin or penicillin* or levofloxacin) ) OR AB ( (antibiotic* or cephalosporin* or cefazolin or cefuroxime or amox?cillin* or clindam?cin or penicillin* or levofloxacin) )
S7 (MH "Antibiotics+")
S6 (MH "Antibiotic Prophylaxis")
S5 S1 or S2 or S3 or S4
S4 TI (mandibular N5 osteotom*) OR AB (mandibular N5 osteotom*)
S3 TI ( (maxillary osteotom* or Le Fort osteotom* or vertical ramus osteotom* or genioplast*) ) OR AB ( (maxillary osteotom* or Le Fort osteotom* or vertical ramus osteotom* or genioplast*) )
S2 TI (orthognathic N5 surg*) OR AB (orthognathic N5 surg*)
S1 (MH "Orthognathic Surgery")
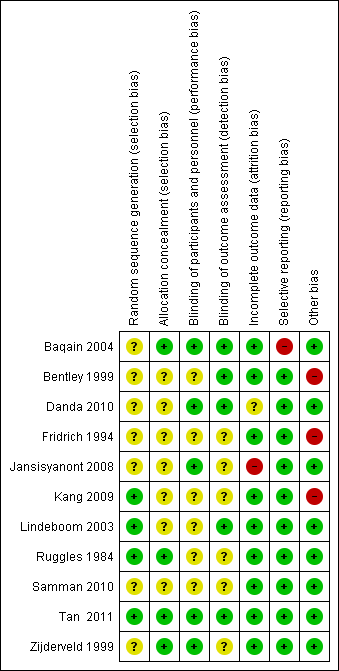
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.

Comparison 2 Sensitivity analysis: short‐term versus long‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
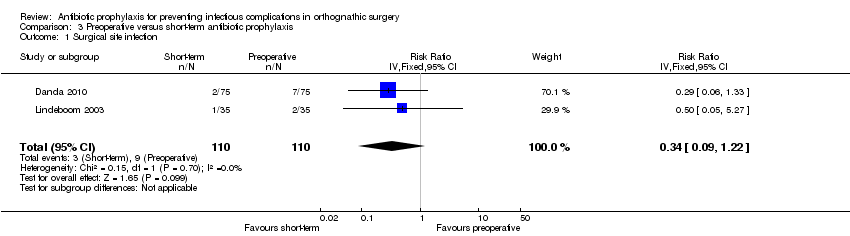
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 1 Surgical site infection.
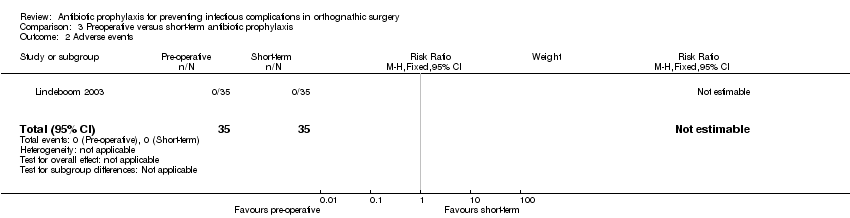
Comparison 3 Preoperative versus short‐term antibiotic prophylaxis, Outcome 2 Adverse events.

Comparison 4 Amoxicillin versus ampicillin, Outcome 1 Surgical site infection.

Comparison 4 Amoxicillin versus ampicillin, Outcome 2 Adverse events.
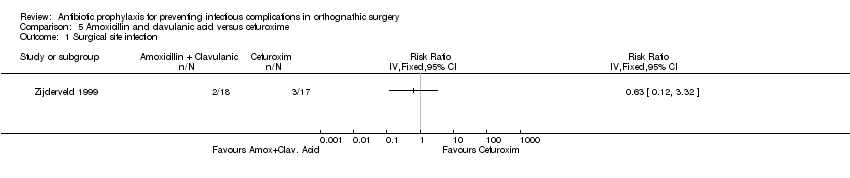
Comparison 5 Amoxicillin and clavulanic acid versus cefuroxime, Outcome 1 Surgical site infection.
| Short‐term antibiotic prophylaxis compared with long‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: short‐term antibiotic prophylaxis Comparison: long‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐term | Long‐term | |||||
| Surgical site infection Follow‐up: 2 to 36 weeks | 168 per 1000a | 71 per 1000 (41 to 125) | RR 0.42 (0.24 to 0.74) | 472 | ⊕⊕⊕⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Adverse events | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Preoperative antibiotic prophylaxis compared with short‐term antibiotic prophylaxis in patients undergoing orthognathic surgery | ||||||
| Patient or population: patients undergoing orthognathic surgery Intervention: preoperative antibiotic prophylaxis Comparison: short‐term antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Preoperative | Short‐term | |||||
| Surgical site infection Follow‐up: 4 to 12 weeks | 82 per 1000a | 28 per 1000 | RR 0.34 (0.09 to 1.22) | 220 | ⊕⊕⊝⊝ | This outcome was measured using different definitions. We accepted all authors' definitions |
| Adverse events Follow‐up: up to 12 weeks | 0 per 35 See comment | 0 per 35 See comment | Not estimable | 70 | ⊕⊕⊝⊝ | No adverse events were reported in any of arms of the trial |
| Systemic infection | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Duration of hospital stay | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| Health‐related quality of life | Not reported | ‐ | This outcome was not reported in any of the trials | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aAssumed risk based on control arms of included trials. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 7 | 472 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.24, 0.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 6 | 438 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.22, 0.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 2 | 220 | Risk Ratio (IV, Fixed, 95% CI) | 0.34 [0.09, 1.22] |
| 2 Adverse events Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Surgical site infection Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |