انجام دیلاتاسیون متناوب توسط خود فرد در مدیریت بیماری تنگی مجرای ادرار در مردان
چکیده
پیشینه
گاهی اوقات دیلاتاسیون متناوب مجرای ادرار توسط خود فرد (self‐dilatation) برای کاهش خطر تنگی عودکننده مجرای ادرار توصیه میشود. در مورد اینکه این یک مداخله بالینی موثر یا هزینه‐اثربخش در مدیریت این بیماری است یا خیر، اتفاقنظر وجود ندارد.
اهداف
هدف از این مرور، بررسی اثربخشی بالینی و هزینه‐اثربخشی دیلاتاسیون متناوب توسط خود فرد پس از جراحی تنگی مجرای ادرار در مردان در مقایسه با عدم انجام مداخله است. برنامههای مختلف و دستگاههای لازم را برای دیلاتاسیون متناوب توسط خود فرد نیز مقایسه کردیم.
روشهای جستوجو
پایگاه ثبت تخصصی گروه بیاختیاری در کاکرین (در 7 می 2014 جستوجو شد)، CENTRAL (سال 2014، شماره 4)؛ MEDLINE (1 ژانویه 1946 تا هفته 3 اپریل 2014)؛ PREMEDLINE (تا 29 اپریل 2014)؛ EMBASE (1 ژانویه 1947 تا هفته 17ام سال 2014)؛ CINAHL (31 دسامبر 1981 تا 30 اپریل 2014)؛ OpenGrey (در 6 می 2014 جستوجو شد)، ClinicalTrials.gov (6 می 2014)؛ پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی سازمان جهانی بهداشت (6 می 2014)، کارآزماییهای کنترلشده فعلی (Current Controlled Trials) (6 می 2014) و فهرست منابع مقالات مرتبط را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و شبه‐تصادفیسازی شدهای وارد شدند که در آنها یک بازو شامل برنامه دیلاتاسیون متناوب توسط خود فرد برای مدیریت تنگی مجرای ادرار بود. مطالعات در صورتی از مرور حذف شدند که کارآزماییهای تصادفیسازی یا شبه‐تصادفیسازی شده نبودند، یا اگر مربوط به کاتتریزاسیون متناوب تمیز توسط خود فرد برای تخلیه مثانه بودند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده رکوردها را از نظر ارتباط و کیفیت روششناسی (methodology) غربالگری کردند. استخراج دادهها بر اساس معیارهای از پیش تعیین شده و با استفاده از فرمهای استخراج اطلاعات، انجام شد. آنالیزها با استفاده از نرمافزار Review Manager کاکرین (RevMan 5) انجام شدند. پیامدهای اولیه شامل نشانههای گزارششده توسط بیمار و کیفیت زندگی مرتبط با سلامت و خطر عود بودند؛ پیامدهای ثانویه نیز عبارت بودند از عوارض جانبی، قابلیت پذیرش مداخله برای بیماران و هزینه‐اثربخشی مداخله. کیفیت شواهد با استفاده از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (Grading of Recommendations Assessment, Development and Evaluation; GRADE) ارزیابی شد.
نتایج اصلی
یازده کارآزمایی برای ورود در مرور انتخاب شدند که در مجموع شامل 776 مرد بودند. ابعاد کارآزماییها عموما کوچک بودند؛ همگی از کیفیت پائینی برخوردار بوده و با خطر بالای سوگیری (bias) در نظر گرفته شدند.
انجام دیلاتاسیون متناوب توسط خود فرد در برابر عدم انجام آن
دادههای به دست آمده از شش کارآزمایی، ناهمگون، غیردقیق و دارای خطر بالای سوگیری بودند، اما نشان دادند که احتمال عود تنگی مجرای ادرار در مردانی که دیلاتاسیون متناوب توسط خودشان انجام شد، کمتر از مردانی بود که دیلاتاسیون متناوب را انجام ندادند (RR: 0.70؛ 95% CI؛ 0.48 تا 1.00؛ شواهد با کیفیت بسیار پائین). عوارض جانبی عموما ضعیف گزارش شدند: دو کارآزمایی عوارض جانبی را گزارش نکرده و دو کارآزمایی عوارض جانبی را فقط برای گروه مداخله گزارش کردند. متاآنالیز دو کارآزمایی باقیمانده، شواهدی را مبنی بر وجود تفاوت میان انجام و عدم انجام دیلاتاسیون متناوب توسط خود فرد پیدا نکرد (RR: 0.60؛ 95% CI؛ 0.11 تا 3.26). هیچ کارآزماییای بهطور رسمی قابلیت پذیرش مداخله را ارزیابی نکرد، و در هیچ کارآزماییای نشانههای دستگاه ادراری تحتانی گزارششده توسط بیمار، کیفیت زندگی مرتبط با سلامت بر اساس گزارش بیمار یا هزینه‐اثربخشی مداخله گزارش نشدند.
یک برنامه دیلاتاسیون متناوب توسط خود فرد در برابر برنامه دیگر
دو کارآزمایی را شناسایی کردیم که مدت زمانهای مختلف دیلاتاسیون متناوب را توسط خود فرد مقایسه کردند، اما دادههای آنها با هم ترکیب نشدند. یک مطالعه به دلیل طراحی متقاطع (cross‐over)، انحراف از پروتکل، خطا در اجرا، آنالیز تعقیبی (post‐hoc) و گزارش ناقص پیامد نتوانست به نتیجهگیریهای محکمی دست یابد. مطالعه دیگر نیز هیچ شواهدی را مبنی بر تفاوت میان دیلاتاسیون متناوب توسط خود فرد به مدت شش ماه در برابر 12 ماه پس از اورتروتومی اپتیکال (optical urethrotomy) پیدا نکرد (RR: 0.67؛ 95% CI؛ 0.12 تا 3.64)، اگرچه باز هم به دلیل حجم نمونه کوچک و خطر سوگیری در مطالعه گنجاندهشده، شواهد محدود شد. عوارض جانبی به شکل نقل قول (narrative) گزارش شده و طبقهبندی بر اساس گروه صورت نگرفت. هیچ کارآزماییای بهطور رسمی قابلیت پذیرش مداخله را ارزیابی نکرد، و در هیچ کارآزماییای نشانههای دستگاه ادراری تحتانی گزارششده توسط بیمار، کیفیت زندگی مرتبط با سلامت بر اساس گزارش بیمار یا هزینه‐اثربخشی مداخله گزارش نشدند.
یک دستگاه برای انجام دیلاتاسیون متناوب توسط خود فرد در برابر دستگاه دیگر
سه کارآزمایی استفاده از یک دستگاه را برای انجام دیلاتاسیون متناوب توسط خود فرد با دستگاه دیگر مقایسه کردند. نتایج حاصل از یک کارآزمایی با خطر بالای سوگیری برای تعیین تاثیرات یک کاتتر هیدروفیلیک با اصطکاک پائین و یک کاتتر پلیوینیل کلراید استاندارد بر خطر عود تنگی مجرای ادرار بسیار نامطمئن بود (RR: 0.32؛ 95% CI؛ 0.07 تا 1.40). بهطور مشابه، یک مطالعه شواهدی را مبنی بر تفاوت میان ژل تریامسینولون (triamcinolone) یک درصد برای روان کردن کاتتر برای دیلاتاسیون متناوب توسط خود فرد در برابر ژل مبتنی بر آب از نظر خطر عود تنگی مجرای ادرار پیدا نکرد (RR: 0.68؛ 95% CI؛ 0.35 تا 1.32). در دو کارآزمایی عوارض جانبی گزارش شدند، اما یکی از آنها جزئیات کافی را برای انجام آنالیز ارائه نداد. مطالعه کوچک دیگر، موارد کمتری را از بروز پروستاتیت (prostatitis)، خونریزی مجرای ادرار یا باکتریوری (bacteriuria) با کاتتر هیدروفیلیک با اصطکاک پائین در مقایسه با کاتتر پلیوینیل کلراید استاندارد گزارش کرد (RR: 0.13؛ 95% CI؛ 0.02 تا 0.98). «رضایت از مداخله» با استفاده از یک مقیاس غیرمعتبر در یک مطالعه ارزیابی شد، اما هیچ کارآزماییای به صورت رسمی کیفیت زندگی مرتبط با سلامت را بر اساس گزارش بیمار یا قابلیت پذیرش مداخله را ارزیابی نکرد. در هیچ کارآزماییای نشانههای دستگاه ادراری تحتانی گزارششده توسط بیمار یا هزینه‐اثربخشی مداخله گزارش نشدند.
ارزیابی کیفیت GRADE
کیفیت شواهد مبنی بر اینکه دیلاتاسیون متناوب توسط خود فرد خطر عود تنگی مجرای ادرار را پس از مداخله جراحی کاهش میدهد، به سطح «بسیار پائین» تنزل داده شد، بر اساس اینکه مطالعات تشکیلدهنده این متاآنالیز دارای خطر بالای سوگیری تلقی شدند، و دادهها نیز دقیق نبوده و متناقض بودند.
شواهد ناکافی
هیچ کارآزماییای دادههای مربوط به هزینه‐اثربخشی مداخله را ارائه نداد یا از یک معیار پیامد گزارششده معتبر توسط بیمار استفاده نکرد، و عوارض جانبی نیز بهطور دقیق گزارش نشدند. قابلیت پذیرش مداخله برای بیماران از نظر کمّی یا کیفی ارزیابی نشده است.
نتیجهگیریهای نویسندگان
انجام دیلاتاسیون متناوب توسط خود فرد ممکن است خطر عود تنگی مجرای ادرار را پس از درمان آندوسکوپی کاهش دهد. به دلیل کیفیت بسیار پائین شواهد، اطمینان بسیار کمی به تخمین این تاثیر داریم. شواهد برای مقایسهها و پیامدهای دیگر محدود است. برای تعیین اینکه مزیت واضح مداخله برای ارزشمند ساختن مداخله کافی است یا خیر، و اینکه برای چه کسانی موثر است، انجام پژوهشهای بیشتر لازم است.
PICO
خلاصه به زبان ساده
درخواست از مردان برای عبور دادن کاتتر به مجرای ادرار خود برای جلوگیری از عود تنگی مجرای ادرار
پیشینه
تقریبا یک نفر از هر 300 مرد به وضعیتی به نام تنگی مجرای ادرار مبتلا میشود که در آن بخشی از مجرا دچار زخم شده و در نتیجه باریک میشود. بیشتر تنگیهای مجرای ادراری در اثر آسیب یا عفونت ایجاد میشوند. نشانه اصلی این وضعیت، مشکل در دفع ادرار است. حداقل در نیمی از بیماران، تنگی مجرای ادرار طی دو سال پس از انجام یک جراحی به نام اورتروتومی اپتیکال (optical urethrotomy) که برای گشاد کردن تنگی مجرای ادرار انجام میشود، مجددا عود میکند. به همین دلیل علاقه زیادی به یافتن راههایی برای کاهش احتمال عود تنگی مجرای ادرار وجود دارد.
دیلاتاسیون متناوب توسط خود فرد (self‐dilatation) درمانی است که برای جلوگیری از بازگشت تنگی مجرای ادرار طراحی شده است. در این روش، بیمار یک لوله کاتتر نازک و معمولا یک بار مصرف را در فواصل زمانی معینی به مجرای ادرار میفرستد تا از باریک شدن مجدد ناحیه زخم جلوگیری کند. تصور میشود که این روش از چسبیدن مجدد لبههای بریده شده مجرا پیشگیری میکند، اما با خطراتی از جمله عفونت و آسیب به مجرای ادراری همراه است.
ما نمیدانیم که دیلاتاسیون متناوب توسط خود فرد، درمان خوبی برای تنگی مجرای ادرار به حساب میآید یا خیر.
ویژگیهای مطالعه
برای این مرور 11 کارآزمایی را یافتیم که در مجموع 776 بیمار از هشت کشور در آنها شرکت داشتند.
نتایج کلیدی
ترکیب نتایج به دست آمده از شش کارآزمایی در مجموع شامل 404 شرکتکننده نشان داد که مردان مبتلا به تنگی مجرای ادرار که دیلاتاسیون متناوب را خودشان انجام میدهند، ممکن است شانس کمتری برای بازگشت تنگی مجرای ادرارشان نسبت به مردان مبتلا به تنگی مجرای ادراری که دیلاتاسیون متناوب را انجام نمیدهند، داشته باشند. با این حال، نمیتوانیم در مورد این یافته مطمئن باشیم، زیرا سطح کیفیت شواهد بسیار پائین بود.
هیچ کارآزماییای برای بررسی هزینه‐اثربخش بودن دیلاتاسیون متناوب توسط خود فرد به عنوان یک مداخله مراقبت سلامت انجام نشد، و هیچ کارآزماییای از پرسشنامههای معتبر سلامت برای ارزیابی کاهش نشانههای ادراری مردان یا بهبود کلی سلامت آنها پس از دیلاتاسیون متناوب توسط خود فرد استفاده نکرد.
در مجموع، کارآزماییها، عوارض جانبی را به گونهای گزارش نکردند که برای تخمین خطرات انجام دیلاتاسیون متناوب توسط خود فرد مفید باشند.
هنوز نمیدانیم که انواع خاصی از کاتترها برای انجام دیلاتاسیون متناوب توسط خود فرد بهتر از کاتترهای دیگر هستند یا خیر. همچنین مشخص نیست که مردان چند وقت یک بار یا برای چه مدت باید دیلاتاسیون متناوب را انجام دهند تا شانس بهتری برای رهایی از تنگی مجرای ادرار داشته باشند.
کیفیت شواهد
کارآزماییهای موجود در این مرور عموما کوچک بوده و از لحاظ طراحی، ضعیف بوده یا به خوبی توضیح داده نشده بودند. همه کارآزماییها به شیوهای انجام شدند که احتمال زیادی داشت به پاسخی منجر شود که حقیقت را نشان ندهد.
Authors' conclusions
Summary of findings
| Intermittent self‐dilatation compared to no treatment for males after urethral stricture surgery | ||||||
| Population: males after urethral stricture surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Intermittent self‐dilatation | |||||
| Recurrent urethral stricture | 618 per 1000 | 433 per 1000 | RR 0.7 | 404 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by two for risk of bias: all six trials comprising the quantitative synthesis were judged high risk of bias in two or more domains. | ||||||
Background
Urethral stricture is the most common cause of difficulty passing urine in young and middle aged men. The prevalence varies worldwide but indicative numbers from North America are 200 per 100,000 for men in their 20s rising to 900 per 100,000 for men in their 70s (Santucci 2007). In the National Health Service in the United Kingdom, urethral strictures account for approximately 16,000 hospital admissions and 12,000 operations annually, at a cost of GBP 10M (Mundy 2010). Strictures have a tendency to recur after treatment, and the concept of intermittent self‐dilatation was popularised in the 1980s as a means of reducing the risk of recurrence (Lawrence 1988).
Description of the condition
A urethral stricture is a scar of the spongy erectile tissue that surrounds the anterior urethra. Gradual contraction of this scar constricts the urethral lumen leading to progressive lower urinary tract symptoms (LUTS), the hallmark of this condition. Any process that injures the urethra can cause a urethral stricture. In developed countries one in two strictures are iatrogenic (following catheterisation or endoscopic prostate surgery, for example) and in one in three cases no cause can be identified (Lumen 2009). The pattern of aetiology is different in developing countries where sexually transmitted infection and pelvic trauma are more likely to be responsible (Ahmed 1998).
For men who present for the first time with a urethral stricture the standard treatment is an operation called endoscopic urethrotomy. A cold blade mounted on an endoscope is passed into the urethra and the stricture is incised longitudinally through to the healthy tissue underneath. This incision allows the narrow section to expand, returning the urethra to an adequate diameter. The alternatives to endoscopic urethrotomy are simple blind dilatation, where the stricture is stretched with a set of lubricated dilators or sounds; and urethroplasty, where the diseased part of the urethra is exposed through a cut in the skin behind the scrotum and then reconstructed.
Depending on the site and length of the stricture, men undergoing their first endoscopic urethrotomy have somewhere between a 25% and 89% chance of their stricture recurring (Lauritzen 2009). Some men perform intermittent self‐dilatation after an operation with the aim of delaying the onset of symptoms and recurrence.
Description of the intervention
Intermittent self‐dilatation is a treatment for urethral stricture where the patient passes a catheter tube or rod‐shaped device into their urethra at regular intervals to prevent the stricture from coming back.
The intermittent self‐dilatation device can be the same type of sterile disposable catheter used by people who perform intermittent self‐catheterisation to empty their bladder. There are cultural variations and a stainless steel chopstick was found to be safe and cost‐effective in Taiwan (Yu‐Hung Lin 2006). In principle, the device should be clean to minimise the risk of introducing infection, and it should have a low co‐efficient of friction to facilitate atraumatic passage.
It is usual for a healthcare professional to first teach the patient how to pass the intermittent self‐dilatation device safely. Once comfortable with the technique, men are given a programme of dilatation to follow at home. Men are usually advised to dilate more frequently to begin with (daily for example) and to lengthen the interval in a stepwise fashion thereafter. Intermittent self‐dilatation can continue for a fixed period or indefinitely depending on the stricture, the patient and the doctor recommending it as a treatment. There is no general consensus as to which device or programme of intermittent self‐dilatation works best.
How the intervention might work
Performing intermittent self‐dilatation regularly splints the urethra open. It might prevent the cut edges of a stricture from sticking together and contracting after an operation (Lawrence 1988).
Why it is important to do this review
Intermittent self‐dilatation is an invasive procedure with associated cost and morbidity (urinary tract infection for example). We do not know whether performing intermittent self‐dilatation after a urethral stricture operation is better than having a urethral stricture operation and then doing nothing afterwards.
This review focuses on intermittent self‐dilatation; there is another Cochrane review which focuses on dilatation, urethrotomy and urethroplasty (Wong 2012).
Objectives
The purpose of this review is to evaluate the clinical effectiveness and cost‐effectiveness of intermittent self‐dilatation after urethral stricture surgery in the management of urethral stricture disease in males.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials in which at least one arm is a programme of intermittent self‐dilatation for urethral stricture. We did not consider studies that evaluated devices for intermittent self‐catheterisation for bladder emptying.
Types of participants
Male patients of all ages with a urethral stricture. The urethral stricture may be at any site, of any length or aetiology. Intermittent self‐dilatation is a treatment that is generally only instituted after an operation to widen a urethral stricture, therefore it was expected that participants would have had at least one surgical intervention for the condition.
Types of interventions
Intermittent self‐dilatation is a programme of repeated urethral self‐dilatation using a rod‐shaped device. All permutations of device, dilation frequency and programme duration were eligible for inclusion.
One arm of any eligible trial had to involve allocation to a programme of intermittent self‐dilatation. The programme of intermittent self‐dilatation normally follows, and could be coupled to, an endoscopic intervention for urethral stricture. Where that was the case, intermittent self‐dilatation was assessed independent of the operation preceding it. Comparison interventions were therefore: no treatment; and the programmes of intermittent self‐dilatation themselves.
The following comparisons were made:
1. intermittent self‐dilatation versus no intervention;
2. one regimen of intermittent self‐dilatation (e.g. catheterisation frequency or duration of programme) versus another;
3. one device to perform intermittent self‐dilatation versus another.
Types of outcome measures
Primary outcomes
-
Risk of recurrent urethral stricture (measured as length of time to reintervention, or number of men requiring reintervention)
-
Patient‐reported lower urinary tract symptoms (validated patient‐reported outcome measures (PROMs), symptom and bother scores)
-
Patient‐reported health‐related quality of life (validated condition‐specific and generic utility measures)
Secondary outcomes
Adverse events
Rates of:
-
urinary tract infection;
-
urethral trauma;
-
hospitalisation.
Acceptability
-
Rate of withdrawal from the programme of intermittent self‐dilatation.
Cost‐effectiveness
-
Additional treatment cost;
-
Incremental cost per quality‐adjusted life year (QALY);
-
Other health economic outcomes.
Other outcomes
-
Those not specified but reported in eligible trials were considered.
Quality assessment
Two independent review authors used the Grading of Recommendations Assessment, Development and Education (GRADE) system to rate the quality of evidence (Guyatt 2008). GRADE is a systematic approach to making judgements about quality of evidence and accordingly the strength of recommendations that can be made based on meta‐analyses. It assesses methodological flaws, consistency of results, generalisability of results and how effective the treatment has been shown to be at addressing outcomes that are judged to be of utmost importance to patients. GRADE profiler 3.6.1 was used to create the Summary of Findings table. The outcome we retrospectively selected for GRADE quality assessment was "risk of risk of recurrent urethral stricture".
Search methods for identification of studies
No language or other limitations were imposed on any of the searches described below.
Electronic searches
This review drew on the search strategy developed for the Incontinence Review Group as a whole. Relevant trials were identified from the Group's Specialised Register of controlled trials, which is described under the Incontinence Group's module in The Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in Process and handsearching of journals and conference proceedings. Date of last search: 7 May 2014.
The terms used to search the Incontinence Group Specialised Register were:
({DESIGN.CCT*} OR {DESIGN.RCT*}) AND ({topic.urine.urethralStricture*})
(Searches of the keyword field of Reference Manager 2012).
Urethral stricture disease lies outside the stated remit of the Cochrane Incontinence Review Group. To ensure inclusion of all relevant trials, the electronic databases MEDLINE, MEDLINE in Process, CENTRAL, EMBASE, CINAHL, OpenGrey, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP) and Current Controlled Trials (including the UK National Research Register) were separately interrogated with terms pertaining to urethral stricture disease. MEDLINE and MEDLINE in Process were searched using the Cochrane highly sensitive search strategy (Lefebvre 2011). For details of the search terms used in MEDLINE and MEDLINE in Process please see Appendix 1 and for all other search strategies used in the databases detailed below please see Appendix 2.
The following databases were searched:
-
CENTRAL (2014, Issue 4), searched on 6 May 2014.
-
MEDLINE (January 1946 to Week 3 April 2014), searched on 30 April 2014.
-
MEDLINE In Process (29 April 2014), searched on 30 April 2014.
-
EMBASE and EMBASE Classic (January 1947 to Week 17 2014), searched on 1 May 2014.
-
CINAHL (on EBSCOhost) (31 December 1981 to 30 April 2014), searched on 1 May 2014.
-
OpenGrey, searched on 6 May 2014.
Also the following clinical trials registries/platforms were searched on 6 May 2014:
-
WHO International Clinical Trials Registry Platform (ICTRP)
-
Current Controlled Trials (including the UK National Research Register)
-
ClinicalTrials.gov
Citations and abstracts were examined by two independent review authors and reports of potentially relevant trials were retrieved in full.
Searching other resources
Reference lists of identified trials and review articles were searched to find further relevant trials not identified elsewhere.
Data collection and analysis
Selection of studies
Randomised and quasi‐randomised trials identified from the Specialised Register and electronic searches were screened for eligibility and selected for inclusion by two independent review authors.
Data extraction and management
Data relevant to the pre‐stated outcomes, characteristics of the study, interventions and participants were extracted to data collection forms by two independent review authors.
Studies were excluded from the analyses if they were non‐randomised or quasi‐randomised trials or did not meet other inclusion criteria. We did not consider studies that evaluated devices for intermittent self‐catheterisation for bladder emptying. Reasons for exclusion are stated in Characteristics of excluded studies table.
Assessment of risk of bias in included studies
Two review authors independently assessed methodological quality using the Cochrane Collaboration tool for assessing risk of bias. The quality of the trials is documented under the headings:
-
Adequate sequence generation
-
Allocation concealment
-
Blinding
-
Incomplete outcome data addressed
-
Free of selective reporting
-
Funding/conflict of interest
Studies were not necessarily excluded from the analyses on the basis of methodological quality.
The nature of intermittent self‐dilatation means that study participants cannot be blinded to the intervention.
Measures of treatment effect
Dichotomous data were presented as risk ratios (RR) with 95% confidence intervals (CI).
Dealing with missing data
We intended to seek clarification from the trialists where trial data were collected but were not fully reported, or the reported form was unsuitable for analysis in this review.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar to carry out a clinically meaningful meta‐analysis. The presence of statistical heterogeneity was assessed through visual inspection of forest plots, the χ2 test for heterogeneity (< 10%) and the I2 statistic (> 50%) (Higgins 2003). Reasons for heterogeneity were explored.
Assessment of reporting biases
There were insufficient studies per outcome to identify reporting bias by funnel plot.
Data synthesis
Trial data were handled according to the processes described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Where possible, data for each outcome was aggregated from included studies in a formal meta‐analysis of treatment effect. Analyses were carried out using the RevMan analyses software in Review Manager (RevMan 5). Data that could not be combined quantitatively were assessed qualitatively.
Subgroup analysis and investigation of heterogeneity
The data did not permit the intended subgroup analysis by type of urethral stricture (that is site, length or aetiology) or preceding operation (that is endoscopic urethrotomy or blind dilatation).
A random‐effects model was used because there was evidence of clinical and statistical heterogeneity,
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Eleven trials were selected for inclusion in this review. The studies were conducted in Pakistan, Denmark, UK, Iran, USA, Kenya, Tunisia and Finland. Collectively they included 776 male participants with urethral stricture disease randomised to a programme of intermittent self‐dilatation or no treatment, or a type of device for performing intermittent self‐dilatation, after optical urethrotomy. The number of participants in each study ranged from 49 to 146, aged between 10 and 87 years where stated. No trials evaluated intermittent self‐dilatation after urethral reconstruction.
Results of the search
The systematic literature search yielded 295 records. One further record (Khalid 2007) was identified by searching the reference lists of included studies. Twenty duplicate records were eliminated. Two hundred and sixty three records were excluded based on their title or abstract because they were not relevant to the review or were not randomised trials, including two records that were almost certainly not randomised trials and the full‐text could not be retrieved from the authors or the publishers (Khalid 2007; Suhail 2011). Therefore 13 studies were considered for inclusion in the review (Figure 1).
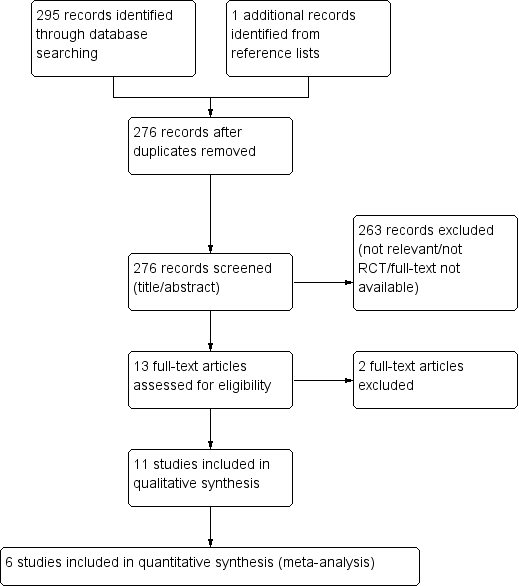
PRISMA study flow diagram
Included studies
The following interventions were included:
-
Six trials evaluated intermittent self‐dilatation versus no treatment after optical urethrotomy (Afridi 2010; Bodker 1992; Husmann 2006; Khan 2011; Kjaergaard 1994; Matanhelia 1995).
-
Two trials evaluated one programme of intermittent self‐dilatation versus another (Harriss 1994; Tammela 1993).
-
One trial evaluated intermittent self‐dilatation versus a programme of outpatient urethral dilatation by a surgeon using sounds (Ngugi 2007).
-
Two trials evaluated one type of device versus another for intermittent self‐dilatation (Hosseini 2008; Sallami 2011).
Excluded studies
Two potentially eligible trials were excluded:
-
One trial evaluated intermittent hydraulic self‐dilatation which is per se a form of urethral self‐dilatation but is not intermittent self‐dilatation using a device (Kaisary 1985);
-
One trial evaluated intermittent outpatient urethral dilatation by a surgeon, which is not intermittent self‐dilatation (Tunc 2002).
Risk of bias in included studies
We classified all 11 included studies as high risk of bias in two or more domains. A graphical display of the risk of bias assessment is presented in Figure 2 and Figure 3. None of the reports included a funding or conflict of interest statement.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequate sequence generation
Two trials were overtly quasi‐randomised because they used alternation or another predicable method of generating the randomisation sequence: (Afridi 2010; Harriss 1994). Only two trials used an adequate method (Hosseini 2008; Ngugi 2007). The remainder were judged as 'unclear' mostly due to lack of information.
Allocation concealment
Allocation concealment was globally inadequate. We judged there to be high risk of bias in this domain in all 11 trials.
Blinding
Blinding of participants is probably not possible in trials evaluating intermittent self‐dilatation versus not performing intermittent self‐dilatation owing to the self‐performed and invasive nature of the intervention. We judged blinding as high risk of bias in 10 of the 11 included trials. One trial that evaluated lubrication of the intermittent self‐dilatation catheter with triamcinolone ointment versus a water‐based gel (Hosseini 2008) packaged both medications in identical tubes, was double‐blind and deemed low risk of bias in this domain.
Incomplete outcome data
Reported outcome data was inadequate in seven (Bodker 1992; Harriss 1994; Hosseini 2008; Khan 2011; Kjaergaard 1994; Matanhelia 1995; Ngugi 2007) of the 11 included studies which were therefore judged to have high risk of attrition bias. In general, participants who did not attend follow‐up, died during the study period or discontinued intermittent self‐dilatation owing to an adverse event or outcome were excluded from the final analysis. We did not require data clarification from the study authors to undertake our pre‐stated analyses.
Selective reporting
Five (Afridi 2010; Bodker 1992; Hosseini 2008; Khan 2011; Tammela 1993) of the 11 included trials appeared free of selective reporting and were judged low risk of bias in this domain.
Other potential sources of bias
No other potential sources of bias were identified.
Conflict of interest statement
None of the reports included a conflict of interest statement.
Subgroup analysis
The data did not permit any of the pre‐stated subgroup analyses.
Effects of interventions
Comparison 1: intermittent self‐dilatation versus no treatment
Six trials addressed this comparison (Afridi 2010; Bodker 1992; Husmann 2006; Khan 2011; Kjaergaard 1994; Matanhelia 1995).
Primary outcomes
Patient‐reported lower urinary tract symptoms
No trials reported this outcome.
Patient‐reported health‐related quality of life
No trials reported this outcome.
Risk of recurrent urethral stricture
There was significant heterogeneity (I2 statistic = 72%) therefore data from all six trials and 404 recruited participants (Afridi 2010; Bodker 1992; Husmann 2006; Khan 2011; Kjaergaard 1994; Matanhelia 1995) were combined for pooled analysis using a random‐effects model. Recurrent urethral stricture was less common in men who performed intermittent self‐dilatation (85/197, 43%) than men who did not perform intermittent self‐dilatation (128/207, 62%) (RR 0.70, 95% CI 0.48 to 1.00, Analysis 1.1). Estimates using a fixed‐effect model were similar but the CI became narrower (RR 0.71, 95% CI 0.59 to 0.85). The number needed to treat to prevent one urethral stricture recurrence is 5.4. Heterogeneity was expected given that no two trials defined recurrent urethral stricture in the same way; nor employed the same programme of intermittent self‐dilatation uniformly; nor followed‐up participants for the same length of time. The six trials in this comparison defined the presence of recurrent urethral stricture variably based on urethrographic appearance, cystoscopy or maximum urinary flow rate. Only Husmann 2006 defined recurrence as the need for re‐intervention.
The GRADE approach was used to assess the quality of the evidence indicating that intermittent‐self dilatation may reduce the risk of recurrent urethral stricture (summary of findings Table for the main comparison). The recommendation was downgraded two levels on the basis that the studies comprising the meta‐analysis were deemed to have high risk of bias, and evidence of imprecision and inconsistency. Thus, our judgement informed by the GRADE approach is that the quality of the evidence is 'very low' and accordingly we have very little confidence in the estimate of the effect.
Bodker 1992 reported that the median time to recurrence was greater in men who performed intermittent self‐dilatation than men who did not perform intermittent self‐dilatation after optical urethrotomy (7 months versus 4 months respectively).
Secondary outcomes
Adverse events
No trials reported adverse events rigorously or completely. Four trials gave a narrative account of adverse events associated with performing intermittent self‐dilatation (Bodker 1992, Khan 2011, Kjaergaard 1994; Matanhelia 1995) including pain, haematuria, symptomatic and asymptomatic bacteruria and epididymitis.
-
Bodker 1992 reported that two of 28 (7.1%) patients in the intermittent self‐dilatation arm of the trial experienced urethral haemorrhage and that the remaining 26 (92.9%) patients did not experience haematuria, pain on catheter insertion or infection. Two of 28 (7.1%) patients in the treatment arm died during the study; the causes of death are not stated. The rate of adverse events in the control arm is not given for comparison.
-
Khan 2011 reported rates of urinary tract infection, defined as one or more positive urine cultures or epididymitis, of 18.1% (4/22) and 15.3% (4/26) in the intervention and control arms respectively.
-
Kjaergaard 1994 reported a lower rate of bacteruria or epididymitis in men who performed intermittent self‐dilatation compared with men who did not perform intermittent self‐dilatation [1/21 (4.7%) versus 5/22 (22.7%) respectively, P = 0.4)].
-
Matanhelia 1995 reported that no patient in the intermittent self‐dilatation arm of this trial developed urinary tract infection.
Combining the data using a random‐effects model from the two small studies that reported sufficient data (Khan 2011 and Kjaergaard 1994) gave a risk ratio of 0.60 favouring intermittent self‐dilatation but the 95% CI crossed the line of no effect (0.11 to 3.26) and the result was not statistically significant (Analysis 1.2).
Afridi 2010 and Husmann 2006 did not report adverse events.
Acceptability
No trials formally evaluated the concept of acceptability of intermittent self‐dilatation to patients.
Two studies (Khan 2011 and Kjaergaard 1994) made the identical claim that 'all of the patients who completed the prescribed CISC (intermittent self‐dilatation) program considered the method fully acceptable and all were able to perform CISC at home with no problems.' Matanhelia 1995 commented that 'patients generally found the procedure acceptable.' No trials described the means of assessment of acceptability to participants.
Cost‐effectiveness
No trials reported this outcome.
Comparison 2: One programme of intermittent self‐dilatation versus another
Two trials addressed this comparison (Harriss 1994; Tammela 1993). Both trials investigated the effect of the duration of the programme of intermittent self‐dilatation as opposed to the frequency with which intermittent self‐dilatation was performed by participants.
Patient‐reported lower urinary tract symptoms
Neither trial reported this outcome.
Patient‐reported health‐related quality of life
Neither trial reported this outcome.
Risk of recurrent urethral stricture
The stated objective of Harriss 1994 was to determine the duration of intermittent self‐dilatation required to 'stabilise' a urethral stricture. One hundred and one men were allocated by odd or even hospital number to intermittent self‐dilatation for a period of six months or a period of three years after optical urethrotomy. Participants in both arms performed intermittent self‐dilatation twice weekly for one month and then weekly. Robust conclusions cannot be drawn from the presented data owing to cross‐over, protocol deviation, administrative error, post‐hoc analysis and incomplete outcome reporting. It is notable that none of the 10 patients who performed intermittent self‐dilatation to the end of the study developed a recurrence.
Tammela 1993 randomised 49 men with recurrent urethral stricture to intermittent self‐dilatation for six months or intermittent self‐dilatation for 12 months after optical urethrotomy. The authors defined recurrence as the need for surgical re‐intervention. No patient developed a recurrence within the first six months of follow‐up. Three men who performed intermittent self‐dilatation for 12 months and two men who performed intermittent self‐dilatation for the first six months, developed recurrent urethral stricture thereafter (RR 0.67, 95% CI 0.12 to 3.64, Analysis 2.1): the numbers were too small to be reliable.
Adverse events
Both reports gave a narrative account of the adverse events encountered in the trials but did not stratify those adverse events by study arm. Tammela 1993 stated that 10 and two of 48 patients who completed the study developed asymptomatic and symptomatic bacteruria respectively. Harriss 1994 reported that 21 of 101 (21%) men enrolled in the trial died of unrelated diseases during follow‐up .
Acceptability
Neither trial made a formal assessment of acceptability. Harriss 1994 stated that 'most patients, even the frail and elderly, took to the procedure very easily.'
Cost‐effectiveness
Neither trial reported this outcome.
Comparison 3: One device for intermittent self‐dilatation versus another
Three trials were relevant to this comparison (Ngugi 2007; Sallami 2011; Hosseini 2008).
-
One trial evaluated triamcinolone gel (a synthetic corticosteroid) for lubricating the intermittent self‐dilatation catheter compared to a water‐based gel (Hosseini 2008).
-
One trial evaluated a low friction hydrophilic catheter (LoFric™) for performing intermittent self‐dilatation compared to a standard Nelaton polyvinyl chloride (PVC) catheter (Sallami 2011).
-
One trial evaluated intermittent self‐dilatation versus regular outpatient urethral dilatation by a surgeon with Clutton sounds (Ngugi 2007).
Patient‐reported lower urinary tract symptoms
No trials assessed this outcome.
Patient‐reported health‐related quality of life
No trials used a psychometrically robust patient‐reported outcome measure to assess health‐related quality of life.
Ngugi 2007 employed a novel, non‐validated questionnaire to compare regular outpatient urethral dilatation with sounds by a surgeon with intermittent self‐dilatation. Participants were asked to say how happy they were with the intervention one, three and six months after enrolment in the trial using a Likert‐type happiness scale. At each time point a greater proportion of men who were randomised to intermittent self‐dilatation reported being happier with their intervention than those men who were randomised to outpatient urethral dilatation with sounds (100% versus 73% at one month, 88% versus 12% at three months and 85% versus 20% six months post‐enrolment).
Risk of recurrent urethral stricture
Sallami 2011 reported a lower rate of recurrent urethral stricture in men randomised to a low‐friction hydrophilic catheter (LoFric™) versus a standard Nelaton PVC catheter for performing intermittent self‐dilatation (RR 0.32, 95% CI 0.07 to 1.40, Analysis 3.1.1). Hosseini 2008 reported a lower rate of recurrent urethral stricture in men randomised to 1% triamcinolone gel versus water‐based gel for lubrication of the intermittent self‐dilatation catheter (RR 0.68, 95% CI 0.35 to 1.32, Analysis 3.1.2,). In both cases the 95% confidence interval of the effect size crosses the line of no effect and the trials were too small for significance.
Adverse events
Using a LoFric™ catheter conferred a lower rate of adverse events than using a standard Nelaton PVC catheter in one study (Sallami 2011). In a cohort of 31 men performing intermittent self‐dilatation with a LoFric™ catheter there were no instances of prostatitis or urethral bleeding and one instance of bacteriuria versus one, two and four instances respectively in a cohort of 28 men performing intermittent self‐dilatation with a PVC catheter (RR 0.13, 95% CI 0.02 to 0.98, Analysis 3.3) . Ngugi 2007 commented that there was a higher rate of urinary tract infection in the group receiving regular outpatient urethral dilatation with sounds versus the group who performed intermittent self‐dilatation. No further details are given.
Acceptability
No trials made a formal assessment of acceptability.
Sallami 2011 commented that 30 of 31 men performing intermittent self‐dilatation using a LoFric™ catheter considered the device fully acceptable versus seven of 28 men using a standard PVC catheter, with the caveat that only 10 of 31 men who performed intermittent self‐dilatation with the LoFric™ catheter were able to do so at home without any difficulty.
Cost‐effectiveness
No trials assessed the cost‐effectiveness of one device for performing intermittent self‐dilatation compared with another.
Discussion
Summary of main results
Repeated transurethral intervention for urethral stricture can lead to a chronic disease state necessitating regular treatment throughout the course of a man's life. In the assessment of therapeutic interventions for urethral stricture the outcome measure of single greatest importance is arguably therefore the rate of, or interval to, recurrence, as a proxy for the length of the time a man will spend with symptomatic disease and thus the number of quality‐adjusted life years they will lose as a result. Unfortunately there is no consensus as to the definition of recurrent urethral stricture across trials, case series or clinical guidelines. The trials included in this review variably employed cystoscopic and urethrographic appearance, maximum urinary flow rate and re‐intervention.
In practice it is usually a patient's symptoms or level of bother that dictate the need for intervention but the use of psychometrically validated patient‐reported outcome measures in the management of this disease is a relatively recent development (Jackson 2011). Pooled analysis of six trials indicated that men who perform intermittent self‐dilatation may have a lower chance of developing recurrent urethral stricture than men who do not perform intermittent self‐dilatation. This finding is tempered by the absence of reliable evidence regarding the intervention in the following areas:
-
a quantitative or qualitative evaluation of acceptability of intermittent self‐dilatation to patients;
-
the comparative rate and nature of adverse events versus no treatment;
-
cost‐effectiveness;
-
effect on feasibility of future urethral reconstruction.
The programme of intermittent self‐dilatation varied across the studies in this review and the optimum frequency and timing of this technique, or whether indeed there is a generalisable optimum programme or protocol, cannot be determined from the available body of evidence. The effect of the duration of intermittent self‐dilatation on risk of recurrence was compared in two trials (Harriss 1994, Tammela 1993). These data suggest that increasing the duration for which intermittent self‐dilatation is performed delays the onset of recurrence. This is in some respects axiomatic and there is no evidence that performing intermittent self‐dilatation has any enduring preventative benefit once the treatment has been discontinued.
Most of the available devices for performing intermittent self‐dilatation have not been robustly compared. Sallami 2011 reported a reduction in recurrence rate associated with the use of a low‐friction hydrophilic catheter versus a standard PVC catheter. Hosseini 2008 found that one percent triamcinolone gel is superior to conventional water‐based gel in terms of prevention of urethral stricture recurrence.
Overall completeness and applicability of evidence
Most of the trials in this review were undertaken before the proliferation of urethral reconstruction which many urethral surgeons, accepting the absence of high level evidence, would regard as the standard of care for recurrent urethral stricture in men who are fit enough to have the procedure.
Quality of the evidence
This systematic review has highlighted a paucity of reliable data on the subject of intermittent self‐dilatation for urethral stricture disease in men. Relevant randomised trials were small, few in number and were generally of low methodological quality or poorly reported, with a high risk of bias. The evidence that performing intermittent self‐dilatation reduces the risk of recurrent urethral stricture is 'very low' quality on the basis of the GRADE approach such that we have very little confidence in the effect estimate.
Potential biases in the review process
The method for assessing the quality of evidence was not specified at the time of protocol writing and was selected while conducting the review. Selection of GRADE at this stage could be a potential source of bias.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews of intermittent self‐dilatation for the management of urethral stricture disease in males.
PRISMA study flow diagram
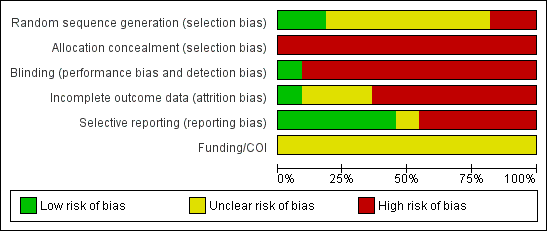
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
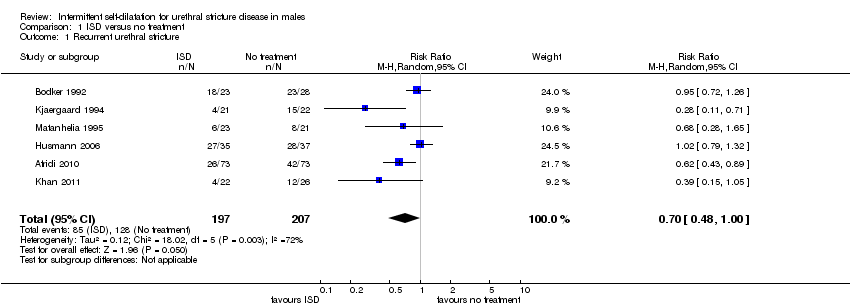
Comparison 1 ISD versus no treatment, Outcome 1 Recurrent urethral stricture.
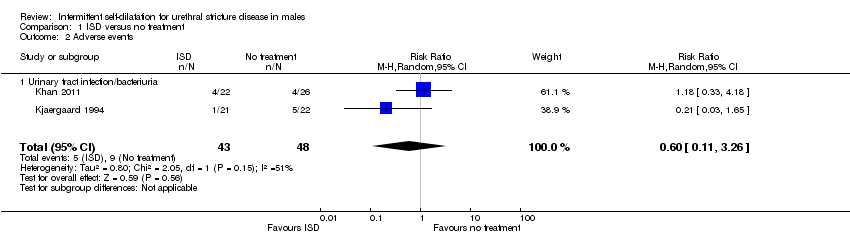
Comparison 1 ISD versus no treatment, Outcome 2 Adverse events.
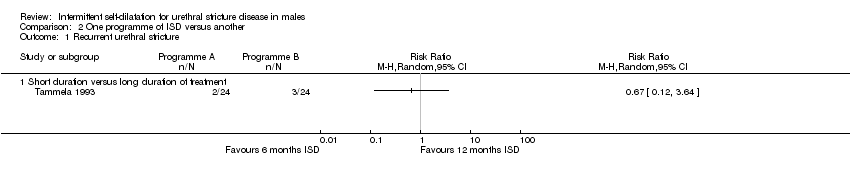
Comparison 2 One programme of ISD versus another, Outcome 1 Recurrent urethral stricture.
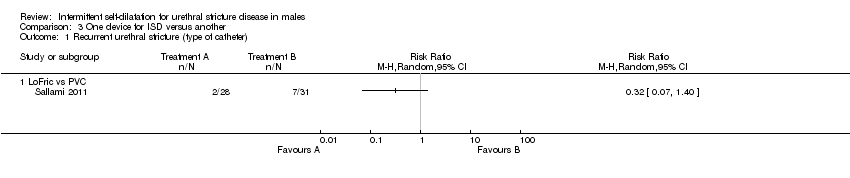
Comparison 3 One device for ISD versus another, Outcome 1 Recurrent urethral stricture (type of catheter).

Comparison 3 One device for ISD versus another, Outcome 2 Recurrent urethral stricture (catheter lubrication).
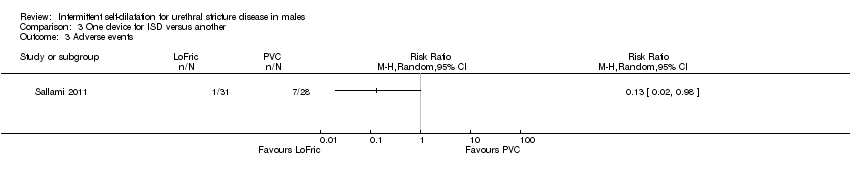
Comparison 3 One device for ISD versus another, Outcome 3 Adverse events.
| Intermittent self‐dilatation compared to no treatment for males after urethral stricture surgery | ||||||
| Population: males after urethral stricture surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Intermittent self‐dilatation | |||||
| Recurrent urethral stricture | 618 per 1000 | 433 per 1000 | RR 0.7 | 404 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by two for risk of bias: all six trials comprising the quantitative synthesis were judged high risk of bias in two or more domains. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent urethral stricture Show forest plot | 6 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.48, 1.00] |
| 2 Adverse events Show forest plot | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.26] |
| 2.1 Urinary tract infection/bacteriuria | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent urethral stricture Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Short duration versus long duration of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent urethral stricture (type of catheter) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 LoFric vs PVC | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Recurrent urethral stricture (catheter lubrication) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Triamcinolone gel versus water‐based lubricant | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

