Antibióticos para las exacerbaciones de la enfermedad pulmonar obstructiva crónica
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | Participants: outpatients seen by chest physicians received antibiotic or placebo for moderately severe exacerbations Inclusion criteria: clinical diagnosis of COPD (GOLD criteria), current or ex‐smoker, aged 40 to 80 years, presenting as an outpatient with signs and symptoms of an exacerbation (change in dyspnoea, sputum volume and colour, and cough), able to produce sputum sample, 1 or 2 of: positive sputum Gram's stain, clinically relevant decrease in lung function or ≥ 2 exacerbations in the previous year Exclusion criteria: pneumonia, exacerbation or use of antibiotics or prednisolone 4 weeks prior to enrolment (except ≤ 5 mg prednisolone), other disease influencing lung function, maintenance antibiotics, hypersensitivity to amoxicillin‐clavulanic acid, serious medical or psychiatric co‐morbidity, uncontrolled diabetes mellitus, home oxygen therapy Baseline demographics: 35 patients included; mean age 67 years; 60% male; mean FEV1/FVC 40% Spirometrically confirmed COPD: yes Severity of exacerbation: moderate | |
| Interventions | Follow‐up: 28 days for primary outcome and 4 months for new exacerbations Treatment group: amoxicillin‐clavulanic acid 1.5 g/day for 7 days and oral prednisolone 30 mg for 7 days Control group: placebo for 7 days and oral prednisolone 30 mg for 7 days | |
| Outcomes | Resolution of exacerbation (patient reported symptom diary) Relapses of exacerbations within 28 days Chronic respiratory questionnaire Clinical COPD questionnaire | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgment of yes or no |
| Intention‐to‐treat‐analysis | Low risk | All patients were analysed in the groups to which they were randomised |
| Methods | RCT | |
| Participants | Participants: patients recruited from pulmonary departments received antibiotic or placebo on an outpatient basis in case of self‐reported worsening of respiratory symptoms Inclusion criteria: aged > 40 years, chronic bronchitis (defined as continuous cough and expectoration, present for at least 3 months of the year, in more than 2 consecutive years), FEV1 < 80% predicted Exclusion criteria: reversible obstruction, cancer, liver insufficiency, renal insufficiency, heart failure, pneumonia Baseline demographics: 335 patients included; mean age 63 years; 73% male; mean FEV1 1.37 L/s Spirometrically confirmed COPD: yes Severity of exacerbation: mild to moderate | |
| Interventions | Mean follow‐up: 5 days Treatment group: amoxicillin‐clavulanic acid 2 g/day (oral) for 5 days Control group: placebo for 5 days | |
| Outcomes | Treatment success/failure (patient‐reported symptoms and clinical signs) at 5 days (not analysed in this systematic review) Dyspnoea (not analysed in this systematic review because data were not in format that we could use) Adverse events | |
| Notes | According to an author of the study (personal communication with Dr. Blasi, March 2006) data after 14 days of follow‐up were available but not published and not made available for this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Intention‐to‐treat‐analysis | High risk | Only patients with complete follow‐up were analysed |
| Methods | Randomised double‐blinded placebo‐controlled trial | |
| Participants | Participants: patients admitted to hospital with exacerbation (increasing symptoms such as dyspnoea, sputum volume or cough) of COPD Inclusion criteria: clinical diagnosis of COPD at the time of hospital admission Exclusion criteria: antibiotic treatment during the previous 2 weeks, left ventricular failure, stroke, pneumonia, pneumothorax, non‐cutaneous cancer, coma, temperature > 38 °C, psychological disorders related to COPD Baseline demographics: 90 patients included; mean age 68 years, 84% male, mean FEV1 % predicted (SD) 29.98% (11.07) Spirometrically confirmed COPD: yes Severity of exacerbation: severe | |
| Interventions | Mean follow‐up: 7.2 days Treatment group: trimethoprim‐sulphamethoxazole 1.9 g/day or amoxicillin/clavulanic acid 1.9 g/day orally for 8 days Control group: placebo for 8 days | |
| Outcomes | Length of hospital stay Re‐exacerbations (in 3 months ‐ not analysed in this systematic review) | |
| Notes | All patients were treated with theophylline, inhaled bronchodilators and oxygen. If the numerical score was high or FEV1 < 40% they received, 6‐methylprednisolone 0.75 mg/kg/day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Arithmetic combination |
| Allocation concealment (selection bias) | Low risk | Through hospital pharmacy |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not enough information provided |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | Randomised double‐blinded placebo‐controlled trial | |
| Participants | Participants: 173 patients were recruited from the community with stable COPD. 116 developed exacerbations (increased dyspnoea, sputum volume or sputum purulence) and each time was randomly assigned to receive antibiotics or placebo Inclusion criteria: aged > 35 years; clinical diagnosis of COPD, not asthma; FEV1 and FVC < 70% predicted, TLC > 80% Exclusion criteria: if FEV1 increased to 80% of predicted post bronchodilator use; other disease serious enough to influence their quality of life or clinical course (e.g. cancer, left ventricular failure, stroke) or other disease likely to require antibiotics (e.g. recurrent sinusitis or UTI) Baseline demographics: 116 patients included; mean age 67 years, 80% male, mean FEV1 % predicted (SD): 33.9% (13.7) Spirometrically confirmed COPD: yes Severity of exacerbation: mild to moderate | |
| Interventions | Follow‐up: 21 days Treatment group: trimethoprim/sulphamethoxazole 1.9 g/day or amoxicillin 1 g/day or doxycycline 0.1 to 0.2 g/day orally for 10 days Control group: placebo for 10 days | |
| Outcomes | Treatment failure (patient‐reported symptoms) | |
| Notes | The analysis was based on number of patients with first exacerbations (only first exacerbation). Side effects were not analysed as they were expresses as % of all exacerbations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Neither patients nor medical staff knew which medication was active" |
| Blinding of outcome assessment (detection bias) | Low risk | "Medical staff" |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | RCT | |
| Participants | Participants: patients at general practitioner visit for new or aggravated respiratory symptoms Inclusion criteria: chronic bronchitis (persistent or recurrent cough with diffuse physical signs in the chest, in which X‐ray had excluded other disease) with exacerbation (worsening characterised by 1 or more of the following: increased cough, increased volume of sputum, increased purulence of sputum, increased breathlessness or fever) Exclusion criteria: none Baseline demographics: 58 patients included; mean age 59 years, 53% male, FEV1 not reported Spirometrically confirmed COPD: no Severity of exacerbation: mild to moderate | |
| Interventions | Mean follow‐up: 14 days Treatment group: oxytetracycline 1 g/day (oral) for 5 days Control group: placebo for 5 days | |
| Outcomes | Treatment success/failure (patient reported) | |
| Notes | Patients with severe exacerbations were not included because antibiotics were deemed indispensable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Identical bottles, key to numbers was kept by another person |
| Blinding of participants and personnel (performance bias) | Low risk | Identical bottles and capsules |
| Blinding of outcome assessment (detection bias) | Low risk | Practitioners were blinded |
| Intention‐to‐treat‐analysis | High risk | Patients with possible toxic effects from drugs were excluded |
| Methods | RCT | |
| Participants | Participants: hospitalised patients with acute exacerbations of COPD Inclusion criteria: aged > 45 years, diagnosis of COPD (GOLD criteria), acute exacerbation (Anthonisen 1 and 2) Exclusion criteria: inability to take oral medication, fever (> 38.5 °C), antibiotic treatment for > 24 hours, extensive treatment with corticosteroids (> 30 mg > 4 days), history of severe exacerbation requiring mechanical ventilation, lung malignancy, other infectious disease requiring antibiotic therapy, heart failure (NYHA III‐IV), apparent immunodeficiency, impaired renal function (creatinine clearance < 20 mL/min) Baseline demographics: 223 patients included; 265 exacerbations; mean age 72 years; 59.6% male; mean FEV1 (SD) doxycycline group 43.9% (17.2%), placebo group 46.9% (18.5%) Spirometrically confirmed COPD: yes Severity of exacerbation: moderate to severe | |
| Interventions | Mean follow‐up: 30 days Treatment group: 7‐day course of oral doxycycline, IV prednisolone taper Control group: 7‐day course of placebo, IV prednisolone taper | |
| Outcomes | Primary outcome: clinical response on day 30 (success/failure) Secondary outcome: clinical success day 10, dyspnoea score, adverse events, mortality | |
| Notes | Analysis based on the number of exacerbations and patients (mortality) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | "Allocation sequence was kept in a safe at the hospital pharmacy"; "study medication was delivered in pre‐numbered containers" |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described (only "double‐blind") |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat and per‐protocol (we used only intention to treat) |
| Methods | RCT | |
| Participants | Participants: patients were instructed to take antibiotic or placebo without a doctor visit as soon as new or aggravated respiratory symptoms were present Inclusion criteria: aged < 65 years, regular employment, productive winter cough for > 3 years, during which time they had at least 2 illnesses with purulent sputum, causing loss of time from work Exclusion criteria: other disabling disease Baseline demographics: 88 patients included; mean 54 age years; 84% male; FEV1 not stated Spirometrically confirmed COPD: no Severity of exacerbation: mild to moderate | |
| Interventions | Mean follow‐up: 17 days Treatment group: oxytetracycline 1 g/day orally for 5 to 7 days Control group: placebo for 5 to 7 days | |
| Outcomes | Treatment success/failure (need for further antibiotics) Time off work Side effects | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fisher and Yate's table of random numbers |
| Allocation concealment (selection bias) | Low risk | "Key list was held by the hospitals pharmacist" |
| Blinding of participants and personnel (performance bias) | Low risk | "Dummy tablets... neither doctors nor patients knowing which was which" |
| Blinding of outcome assessment (detection bias) | Low risk | "Dummy tablets... neither doctors nor patients knowing which was which" |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | RCT | |
| Participants | Participants: patients recruited from bronchitis and asthma clinics received antibiotic or placebo as an outpatient based on case of self‐reported worsening of respiratory symptoms Inclusion criteria: aged 20 to 65 years, winter cough and sputum for at least 3 years, with shortness of breath on effort without evidence of other cause; some degree of disability from the bronchitis (e.g. limitation of normal activity, loss of time at work) Exclusion criteria: none Baseline demographics: 62 patients included; mean age, % male and FEV1 not stated Spirometrically confirmed COPD: no Severity of exacerbation: mild to moderate | |
| Interventions | Mean follow‐up: 14 days Treatment group: oxytetracycline 1 g/day (oral) for 7 days Control group: placebo for 7 days | |
| Outcomes | Improvement of symptoms (not analysed in this systematic review) Days of illness (not analysed in this systematic review) | |
| Notes | Second trial of the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers |
| Allocation concealment (selection bias) | Low risk | "Similar to that used by Elmes 1957" "identical appearance" |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind" "identical appearance" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not enough information |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Participants: patients with general practitioner visits for new or aggravated symptoms Inclusion criteria: aged > 18 years with acute exacerbation (subjective worsening owing to change in sputum (increased volume, change of viscosity or colour) possibly accompanied by cough or dyspnoea, lasting for more than 3 days or chronic bronchitis (defined as continuous cough and expectoration, present for at least 3 months of the year, in more than 2 consecutive years) Exclusion criteria: pneumonia (on auscultation or X‐ray), temperature > 38.5 °C, heart rate > 100 beats/min, antibiotics within the previous 7 days, pregnancy, allergy to penicillin, uncompensated heart disease, treatment with oral corticosteroids or immunosuppressants Baseline demographics: 270 patients included; mean age 60 years, 43% male. FEV1 not stated Spirometrically confirmed COPD: no Severity of exacerbation: mild to moderate | |
| Interventions | Mean follow‐up: 8 days Treatment group: amoxicillin 1.5 g (oral) for 7 days Control group: placebo for 7 days | |
| Outcomes | Treatment failure (patient‐reported symptoms) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to treatment or placebo", with no more details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not enough information |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | Randomised double‐blind, placebo‐controlled trial | |
| Participants | Participants: recruited from 13 primary care centres Inclusion criteria: aged > 40 years, diagnosis of mild to moderate COPD (smoking history > 10 pack‐years, ratio of post‐bronchodilator FEV1:FVC of < 70%, post‐bronchodilator FEV1 > 50% of the predicted value), presence of an exacerbation (at least 1 of the following: increase of dyspnoea, increase in sputum volume, sputum purulence, or a combination) Exclusion criteria: antibiotic use in the previous 2 weeks, bronchial asthma, cystic fibrosis, bronchiectasis of origin other than COPD, active neoplasm, tracheotomy, need for hospital admission, immunosuppression, hypersensitivity to beta‐lactams, clavulanate or lactose, institutionalisation, unable to provide informed consent Baseline demographics: 310 patients included; mean age 68 years, 81% male, mean FEV1/FVC 62% Spirometrically confirmed COPD: yes Severity of exacerbation: mild to moderate | |
| Interventions | Mean follow‐up: 20 days Treatment group: amoxicillin/clavulanate 500/125 mg 3 times daily (oral) for 8 days Control group: placebo for 8 days | |
| Outcomes | Primary outcome: clinical cure/improvement or failure at the end of therapy visit (days 9 to 11, physician assessed) Re‐exacerbations (in 1 year ‐ not analysed in this systematic review) Adverse events | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding of participants and personnel (performance bias) | Low risk | Patients, investigators and data assessors were masked to treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Patients, investigators and data assessors were masked to treatment allocation |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | Randomised double‐blind, placebo‐controlled trial | |
| Participants | Participants: patients admitted to hospital with exacerbation of COPD Inclusion criteria: at the time of a hospital admission: increase in symptoms (cough, dyspnoea, and volume and purulence of sputum) Exclusion criteria: evidence of parenchymal consolidation on chest X‐ray or of other pulmonary or cardiac disease Baseline demographics: 19 patients included, mean age 67 years, % male, FEV1 not stated Spirometrically confirmed COPD: no Severity of exacerbation: severe | |
| Interventions | Mean follow‐up: 13 days Treatment group: cefaclor 1.5 g/day (oral) for 8 days Control group: placebo for 8 days | |
| Outcomes | Length of hospital stay | |
| Notes | Research letter to the editor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described ("double blind") |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described ("double blind") |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Participants: patients admitted to medical ICU with exacerbation of COPD and need for mechanical ventilation Inclusion criteria: aged > 40 years; COPD diagnosed on the basis of clinical history, physical examination, and chest radiograph; acute respiratory failure requiring mechanical ventilation within the first 24 h of admission Exclusion criteria: antimicrobial treatment in the previous 10 days, alveolar infiltrates on chest X‐rays, previously enrolled in the study. Known history of asthma or bronchiectasis, allergy to quinolone derivatives, pregnancy or breast feeding, terminally ill or immunocompromised, hepatic disease or severe renal impairment, gastrointestinal disease that could affect drug absorption, concomitant infection requiring systemic antibacterial therapy Baseline demographics: 93 patients included; mean age 66 years, 90% male, mean FEV1 0.77 L/s Spirometrically confirmed COPD: no Severity of exacerbation: severe | |
| Interventions | Mean follow‐up: 10 days All patients were monitored until their discharge from hospital Treatment group: ofloxacin 400 mg/day (oral) for 10 days Control group: placebo for 10 days | |
| Outcomes | Mortality | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to treatment or placebo using random numbers |
| Allocation concealment (selection bias) | Low risk | All drugs and placebo packages were prepared and numbered by the hospital pharmacy and were used consecutively. Assignments of patients were placed in closed envelopes with identification numbers that were stored in the ICU |
| Blinding of participants and personnel (performance bias) | Low risk | Identical appearance of the medication |
| Blinding of outcome assessment (detection bias) | Low risk | All study investigators and hospital staff were masked to the treatment status until data completion |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | Randomised, double‐blind, controlled trial | |
| Participants | Participants: patients admitted to hospital with exacerbation (not defined) of COPD Inclusion criteria: aged 45 to 75 years, chronic bronchitis (history of cough and expectoration on most days during at least 3 consecutive months in each of 2 or more successive years) Exclusion criteria: severe deformities of the spine or chest, localised or generalised specific lung disease, signs of cardiac insufficiency Baseline demographics: 19 patients included; mean age 62 years, 53% male Spirometrically confirmed COPD: no Severity of exacerbation: severe | |
| Interventions | Mean follow‐up: 10 days Treatment group: chloramphenicol 2 g/day for 10 day Control group: placebo for 10 day | |
| Outcomes | Mortality | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Patients: yes; personnel: no |
| Blinding of outcome assessment (detection bias) | High risk | Control group got a clinical examination on day 0 |
| Intention‐to‐treat‐analysis | High risk | Drop‐outs were not analysed (only per‐protocol reported) |
| Methods | Randomised, double‐blinded, placebo‐controlled trial | |
| Participants | Participants: patients admitted to hospital with exacerbation of symptoms of chronic bronchitis Inclusion criteria: > 50 years old, history of chronic bronchitis > 5 years and a history during the past 6 weeks of an exacerbation, male, moderately‐to‐severely illness on admission (as judged by the receiving SHO), persistent purulent sputum and a PEFR < 200 L/min (unless too ill to do so) Exclusion criteria: allergy to penicillin, asthma, extensive bronchiectasis, active tuberculosis, lung cancer, sputum eosinophilia (> 10%) or blood urea > 60 mg/100 mL Baseline demographics: 30 patients, mean age 68 years, 100% males, FEV1 not reported Spirometrically confirmed COPD: No Severity of exacerbation: severe | |
| Interventions | Mean follow‐up: 14 days Treatment group: penicillin 6 million units/day for 14 days and streptomycin 1 g/day parenterally for 7 days Control group: placebo for 14 days | |
| Outcomes | Treatment failure (physician reported) | |
| Notes | Pilot trial of the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fisher and Yate's tables |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | "Placebo injection", "double blind" |
| Blinding of outcome assessment (detection bias) | Low risk | "blind assessors" |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
| Methods | Randomised double‐blinded controlled trial | |
| Participants | Participants: patients admitted to hospital with exacerbation of COPD Inclusion criteria: aged > 60 years old, history of chronic bronchitis > 5 years and a definite history during the previous 6 weeks of an exacerbation, male, failure of at least 1 previous treatment with antibiotics, moderately severely ill on admission (as judged by the receiving SHO), persistent purulent sputum and PEFR < 200 L/min Exclusion criteria: asthma, bronchiectasis, other pulmonary disease or sputum eosinophilia (> 10%) Baseline demographics: 259 patients included, mean age 71 years, 100% male, FEV1 not reported Spirometrically confirmed COPD: no Severity of exacerbation: severe | |
| Interventions | Mean follow‐up: 12 days Exacerbations were followed at the beginning and end of trial and 1 and 4 weeks later Treatment groups 1 and 2: tetracycline hydrochloride 2 g/day or chloramphenicol 2 g/day orally for 12 days Control group: placebo for 12 days | |
| Outcomes | Treatment failure (physician reported) day 12 Treatment failure (additional antibiotics) day 7 to 28 Adverse events | |
| Notes | Patients with very severe exacerbation were not included for ethical reasons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fisher & Yate's tables |
| Allocation concealment (selection bias) | Low risk | The total course of capsules for each patient was put into a sealed bottle by an independent pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | Identical capsules |
| Blinding of outcome assessment (detection bias) | Low risk | Assessments were made by independent trained observers |
| Intention‐to‐treat‐analysis | Low risk | No withdrawals |
| Methods | RCT | |
| Participants | Participants: patients with general practitioner visit for new or aggravated respiratory (increase in dyspnoea with or without sputum production) symptoms Inclusion criteria: aged > 18 years, positive diagnosis of asthma or COPD made by a pulmonary physician during the previous 10 years Exclusion criteria: daily use of oral corticosteroids or antimicrobial drugs, diabetes mellitus, alcoholism, history of pulmonary surgery or tuberculosis, severe bronchiectasis, a psychiatric history Baseline demographics: 61 patients included; mean age ˜ 52 years, % male and mean FEV1 not stated Spirometrically confirmed COPD: unclear Severity of exacerbation: mild to moderate | |
| Interventions | Mean follow‐up: 35 days Treatment group: amoxicillin 1.5 g or co‐trimoxazole 1.9 g/day orally for 7 days Control group: placebo for 7 days | |
| Outcomes | Treatment success/failure (patient reported symptoms) | |
| Notes | We included only the subgroup with COPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacist had the code of allocation |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Intention‐to‐treat‐analysis | Low risk | Analysed as intention to treat |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ICU: intensive care unit; IV: intravenous; NYHA: New York Heart Association; PEFR: peak expiratory flow rate; RCT: randomised controlled trial; SD: standard deviation; SHO: senior house officer; TLC: total lung capacity; UTI: urinary tract infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No comparison group | |
| No placebo group | |
| No placebo group | |
| No COPD exacerbations | |
| Participants did not have an exacerbation of COPD (stable patients) | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| Not an RCT | |
| No placebo group | |
| Not randomised and study had no placebo group | |
| No placebo group | |
| Not randomised. Matched pairs | |
| Protocol. Trial stopped due to recruitment problems | |
| No placebo group | |
| Use of long‐term prophylactic antibiotics | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| Not an RCT | |
| Prophylactic antibiotic use. Patients treated with azithromycin 500 mg/day for 3 days every 21 days during the winter months, and a control group without treatment | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| Not an RCT | |
| No clinical outcomes | |
| No placebo group | |
| No placebo group | |
| Not an RCT but a retrospective chart review | |
| No placebo group | |
| Study assessed outcomes of long‐term antibiotic use in stable patients (no exacerbation) | |
| No placebo group | |
| Study not in patients with COPD but in patients with acute bronchitis | |
| Study not in patients with COPD but in patients with acute bronchitis | |
| No placebo group. In addition the antibiotic treatment group also received fenspiride (from day 0 to day 30) and the control group received a placebo | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| Study compared participants with stable disease (no exacerbation) | |
| This study terminated early (financial reasons) | |
| No clinical outcomes | |
| No placebo group | |
| Study looked at participants with stable disease (no exacerbation) | |
| Not an RCT but a retrospective cohort study | |
| Not an RCT | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| Not an RCT | |
| Not an RCT | |
| No placebo group | |
| No placebo group | |
| Not an RCT | |
| Placebo group began after 3 days of antibiotics in both groups | |
| No placebo group | |
| No placebo group | |
| No placebo group | |
| Study looked at participants with stable disease (no exacerbation) | |
| No placebo group | |
| Not an RCT but a narrative review | |
| No placebo group | |
| No placebo group | |
| Prophylactic antibiotic use | |
| No placebo group | |
| No placebo group | |
| No placebo group in trial. Moxifloxacin was compared to standard antibiotic therapy | |
| No placebo group | |
| Head‐to‐head trial of 2 different antibiotics regimens | |
| No placebo group | |
| No placebo group |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Utility of Antibiotic Treatment in Non‐purulent Exacerbations of Chronic Obstructive Pulmonary Disease: a Double Blinded, Randomized, Placebo‐controlled Trial of Security and Efficacy (AEPOC‐ATB) |
| Methods | RCT |
| Participants | Inclusion criteria: aged 40 to 90 years; COPD diagnosis according to GOLD guidelines; hospitalisation for any acute exacerbation of COPD; failure of outpatient treatment, increasing dyspnoea in the previous days; co‐morbidity that caused detriment of respiratory function |
| Interventions | Drug: moxifloxacin 400 mg administered once a day for 5 days Control: no intervention |
| Outcomes | Primary outcome measures: efficacy of treatment WITHOUT antibiotics in non‐purulent exacerbations of COPD (time frame: 6 months) |
| Starting date | July 2010 |
| Contact information | Nestor Soler, M.D., Ph.D. email:[email protected] |
| Notes | ‐ |
COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; ECG: electrocardiogram; IL: interleukin; RCT: randomised controlled trial; TNF‐α: tumour necrosis factor‐alpha.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure up to 4 weeks (no resolution or deterioration after trial medication of any duration or death when explicitly stated due to exacerbation or additional course of antibiotics) Show forest plot | 12 | 1636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.62, 0.81] |
| Analysis 1.1  Comparison 1 Antibiotics versus placebo, Outcome 1 Treatment failure up to 4 weeks (no resolution or deterioration after trial medication of any duration or death when explicitly stated due to exacerbation or additional course of antibiotics). | ||||
| 1.1 Outpatient | 7 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 1.2 Inpatient | 4 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.65, 0.91] |
| 1.3 ICU | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.08, 0.45] |
| 2 Treatment failure within 4 weeks ‐ current drugs only Show forest plot | 8 | 1175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.64, 0.91] |
| Analysis 1.2  Comparison 1 Antibiotics versus placebo, Outcome 2 Treatment failure within 4 weeks ‐ current drugs only. | ||||
| 2.1 Outpatient | 5 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 2.2 Inpatient | 3 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 3 Adverse events Show forest plot | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Antibiotics versus placebo, Outcome 3 Adverse events. | ||||
| 3.1 Diarrhoea | 3 | 698 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.62 [1.11, 6.17] |
| 3.2 Dyspepsia | 2 | 605 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.28, 1.73] |
| 3.3 Pain in mouth | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.73 [0.80, 74.98] |
| 3.4 Exanthema, itching | 3 | 698 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.83 [0.77, 19.11] |
| 3.5 Overall (adverse events not separated) | 5 | 1243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [1.03, 2.27] |
| 4 All‐cause mortality Show forest plot | 5 | 624 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.25, 1.16] |
| Analysis 1.4  Comparison 1 Antibiotics versus placebo, Outcome 4 All‐cause mortality. | ||||
| 4.1 Inpatients | 4 | 531 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.37, 2.79] |
| 4.2 ICU patients | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.06, 0.72] |
| 5 Duration of hospital stay (days) Show forest plot | 3 | 202 | Mean Difference (IV, Random, 95% CI) | ‐3.04 [‐8.83, 2.76] |
| Analysis 1.5  Comparison 1 Antibiotics versus placebo, Outcome 5 Duration of hospital stay (days). | ||||
| 6 Improvement in dyspnoea measured at the end of the study period Show forest plot | 2 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.96, 0.15] |
| Analysis 1.6  Comparison 1 Antibiotics versus placebo, Outcome 6 Improvement in dyspnoea measured at the end of the study period. | ||||
| 6.1 Outpatients | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.97, 0.97] |
| 6.2 Inpatients | 1 | 265 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.27, 0.07] |
| 7 Health‐related quality of life or functional status measures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Antibiotics versus placebo, Outcome 7 Health‐related quality of life or functional status measures. | ||||
| 8 Re‐exacerbations within ≥ 2 to 6 weeks since beginning of index exacerbation (rates) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Antibiotics versus placebo, Outcome 8 Re‐exacerbations within ≥ 2 to 6 weeks since beginning of index exacerbation (rates). | ||||
| 9 Days off work Show forest plot | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐5.18 [‐6.08, ‐4.28] |
| Analysis 1.9  Comparison 1 Antibiotics versus placebo, Outcome 9 Days off work. | ||||
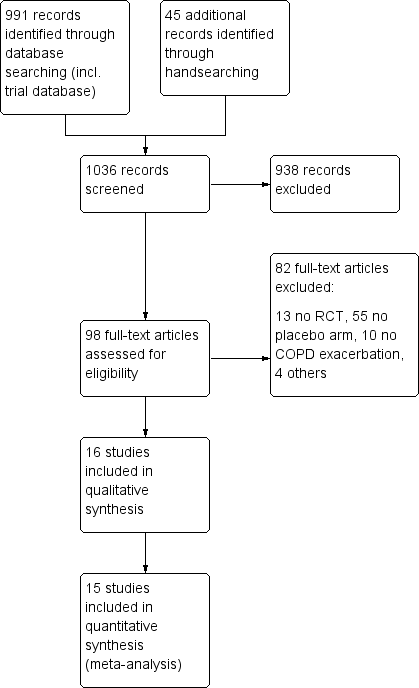
Study flow diagram.
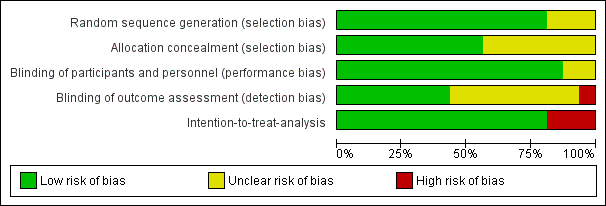
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
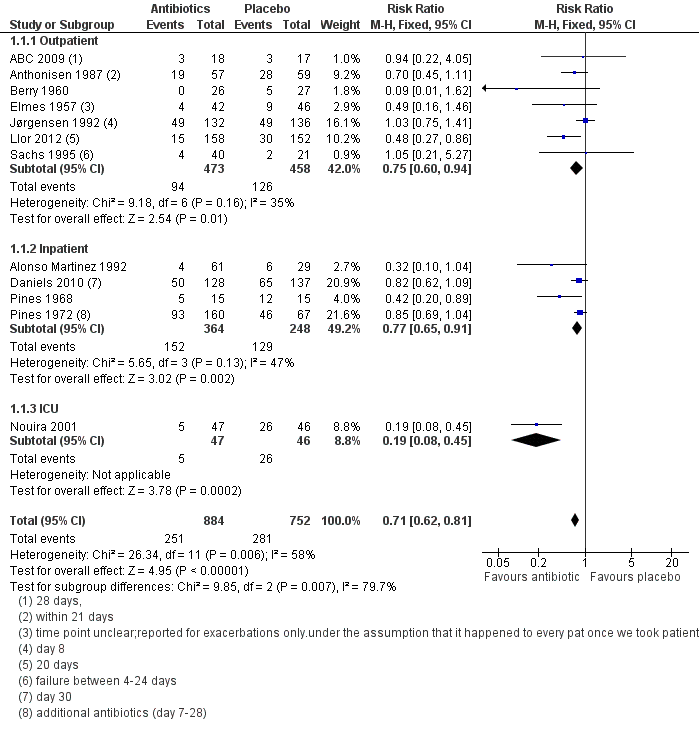
Forest plot of comparison: 1 Antibiotics versus placebo, outcome: 1.1 Treatment failure up to 4 weeks (no resolution or deterioration after trial medication of any duration or death when explicitly stated due to exacerbation or additional course of antibiotics).
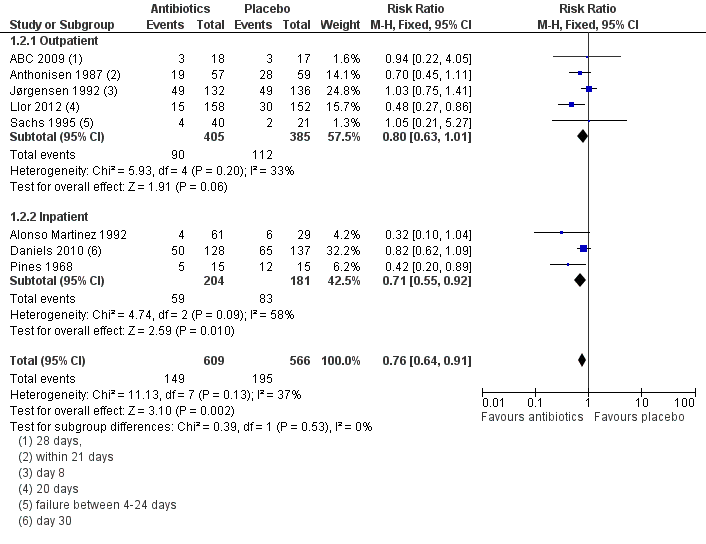
Forest plot of comparison: 1 Antibiotics versus placebo, outcome: 1.2 Treatment failure within 4 weeks ‐ current drugs only.

Forest plot of comparison: 1 Antibiotics versus placebo, outcome: 1.3 Adverse events.

Forest plot of comparison: 1 Antibiotics versus placebo, outcome: 1.4 All‐cause mortality.
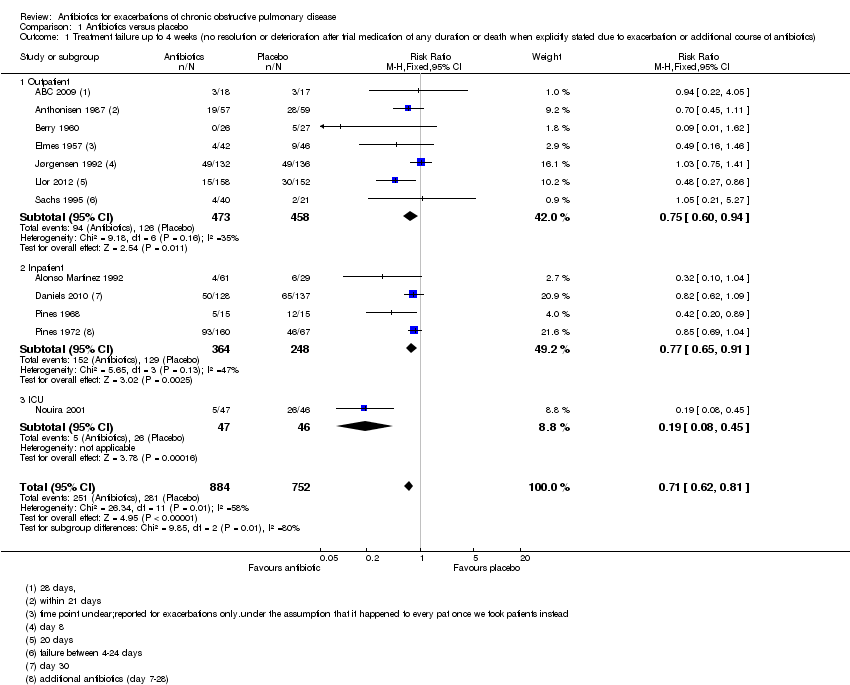
Comparison 1 Antibiotics versus placebo, Outcome 1 Treatment failure up to 4 weeks (no resolution or deterioration after trial medication of any duration or death when explicitly stated due to exacerbation or additional course of antibiotics).
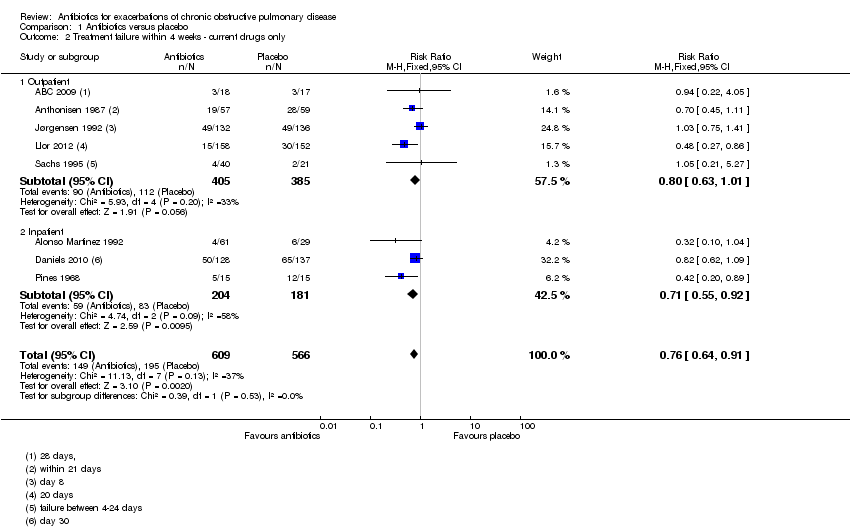
Comparison 1 Antibiotics versus placebo, Outcome 2 Treatment failure within 4 weeks ‐ current drugs only.

Comparison 1 Antibiotics versus placebo, Outcome 3 Adverse events.
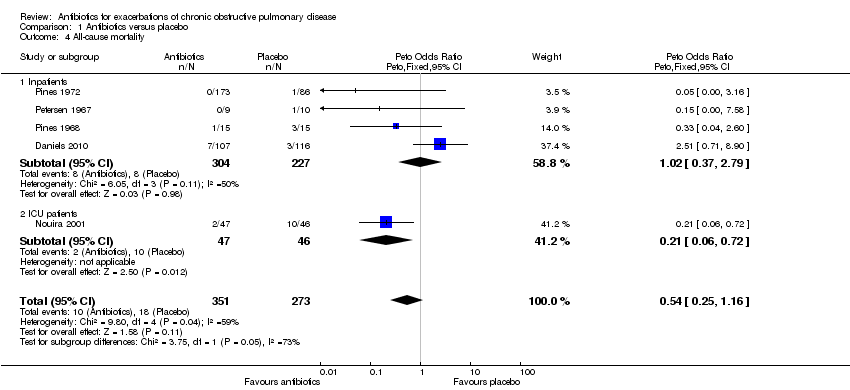
Comparison 1 Antibiotics versus placebo, Outcome 4 All‐cause mortality.

Comparison 1 Antibiotics versus placebo, Outcome 5 Duration of hospital stay (days).
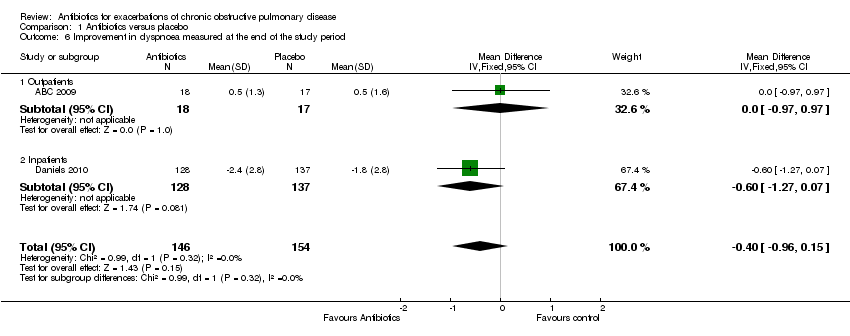
Comparison 1 Antibiotics versus placebo, Outcome 6 Improvement in dyspnoea measured at the end of the study period.
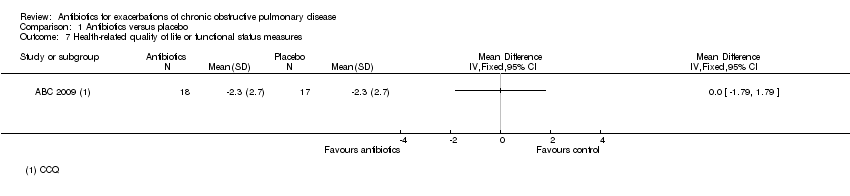
Comparison 1 Antibiotics versus placebo, Outcome 7 Health‐related quality of life or functional status measures.
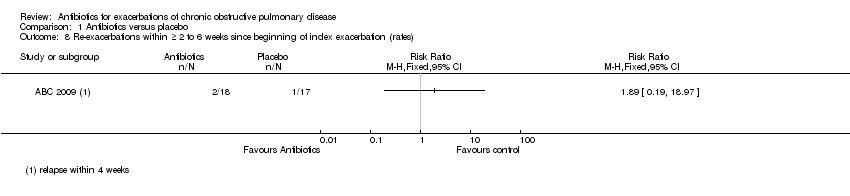
Comparison 1 Antibiotics versus placebo, Outcome 8 Re‐exacerbations within ≥ 2 to 6 weeks since beginning of index exacerbation (rates).
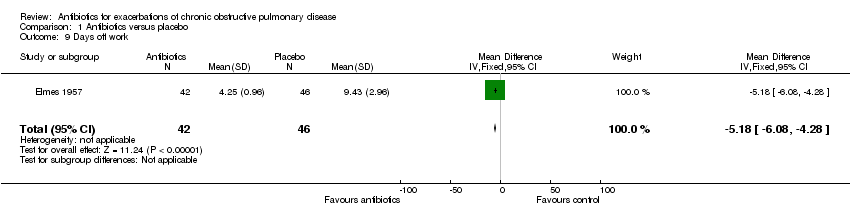
Comparison 1 Antibiotics versus placebo, Outcome 9 Days off work.
| Antibiotics for exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: patients with exacerbations of chronic obstructive pulmonary disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antibiotics versus placebo | |||||
| Treatment failure up to 4 weeks1 Subgroup: outpatient | 275 per 1000 | 206 per 1000 | RR 0.75 | 931 | ⊕⊕⊝⊝ | Antibiotics: ‐ amoxicillin‐clavulanic acid ‐ trimethoprim/sulphamethoxazole ‐ oxytetracycline ‐ amoxicillin ‐ amoxicillin and co‐trimoxazol |
| Treatment failure up to 4 weeks1 Subgroup: inpatient | 520 per 1000 | 401 per 1000 | RR 0.77 | 612 | ⊕⊕⊕⊕ | Antibiotics: ‐ amoxicillin‐clavulanic acid and trimethoprim/sulphamethoxazole ‐ doxycycline ‐ tetracycline hydrochloride or chloramphenicol ‐ penicillin and streptomycin |
| Treatment failure up to 4 weeks1 Subgoup: ICU | 565 per 1000 | 107 per 1000 | RR 0.19 | 93 | ⊕⊕⊕⊕ | Antibiotics: ‐ ofloxacine |
| All‐cause mortality Subgroup: inpatients | 35 per 1000 | 36 per 1000 | OR 1.02 | 531 | ⊕⊕⊝⊝ | Antibiotics: ‐ tetracycline hydrochloride or chloramphenicol ‐ penicillin and streptomycin ‐ chloramphenicol ‐ doxycycline |
| All‐cause mortality Subgroup: ICU | 217 per 1000 | 55 per 1000 | OR 0.21 | 93 | ⊕⊕⊕⊕ | Antibiotics: ‐ ofloxacine |
| Adverse events ‐ diarrhoea | 18 per 1000 | 45 per 1000 | OR 2.62 | 698 | ⊕⊕⊕⊕ | Antibiotics: ‐ amoxicillin‐clavulanic acid ‐ amoxicillin ‐ ofloxacine |
| Adverse events ‐ overall (adverse events not separated) | 74 per 1000 | 109 per 1000 | OR 1.53 | 1243 | ⊕⊕⊕⊝ | Antibiotics: ‐ amoxicillin‐clavulanic acid ‐ doxycycline ‐ amoxicillin ‐ ofloxacine |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 no resolution or deterioration after trial medication of any duration or death when explicitly stated due to exacerbation or additional course of antibiotics. 2 (‐ 1 inconsistency). Discrepancy between the statistical significance of the meta‐analysis that includes all trials (RR 0.75; 95% CI 0.60 to 0.94) vs. the meta‐analysis that is restricted to currently used drugs (amoxicillin‐clavulanic acid, trimethoprim/sulphamethoxazole, doxycycline, penicillin; RR 0.80; 95% CI 0.63 to 1.01). 3 (‐1 limitations). For one trial (Blasi) not all results are available. 4 (no downgrading). The heterogeneity was caused by two small trials that contribute only 14% to the pooled estimate. Two larger trials have the most weight in the meta‐analysis and show almost identical point estimates, therefore we did not downgrade for inconsistency. 5 (‐1 inconsistency). Substantial heterogeneity across trials with the largest trial showing an increase in mortality and the three small trials suggesting a decrease in mortality. 6 (‐ 1 imprecision). Wide 95% CI that precludes any conclusion about the effects of antibiotics on mortality in inpatients. 7 (no downgrading). The CI is relatively wide; however, because the upper limit of the 95% CI (0.72) as the most conservative effect estimate shows an at least moderate effect of antibiotics on mortality we did not downgrade. 8 (‐ 1 imprecision). Lower limit of 95% CI is very close to 1.0 and it is uncertain if lower limit would be below 1.0 with additional trials. | ||||||
| Study | Antibiotic | Dose | Duration | Currently available and used? | Co‐interventions | Control |
| Amoxicillin‐clavulanic acid (oral) | 1.5 g/day | 7 days | Yes | Oral prednisolone 30 mg for 7 days | Placebo for 7 days and oral prednisolone 30 mg for 7 days | |
| Amoxicillin‐clavulanic acid (oral) | 2 g/day | 5 days | Yes |
| Placebo | |
| Trimethoprim‐sulphamethoxazole or amoxicillin/clavulanic acid
| 1.9 g/day | 8 days | Yes |
|
| |
| Trimethoprim/sulphamethoxazole (oral) | 1.9 g/day | 10 days | Yes |
| Placebo | |
| Amoxicillin (oral) | 1 g/day | |||||
| Doxycycline(oral) | 0.1 to 0.2/day | |||||
| Oxytetracycline (oral) | 1 g/day | 5 days | No |
| Placebo | |
| Doxycycline (oral) |
| 7 days | Yes | IV prednisolone taper | Placebo plus IV prednisolone taper | |
| Oxytetracycline (oral) | 1 g/day | 5 to 7 days | No |
| Placebo | |
| Oxytetracycline (oral) | 1 g/day | 7 days | No |
| placebo | |
| Amoxicillin (oral) | 1.5 g/day | 7 days | Yes |
| placebo | |
| Amoxicillin/clavulanate (oral) | 1.5 g/day | 8 days | Yes | Placebo | ||
| Cefaclor (oral) | 1.5 g/day | 8 days | Yes |
| placebo | |
| Ofloxacin (oral) | 400 mg/day | 10 days | Yes |
| placebo | |
| Chloramphenicol (oral) | 2 g/day | 10 days | No |
| placebo | |
| Penicillin (parenterally) | 1 g/day | 14 days | Yes |
| placebo | |
| Tetracycline hydrochloride (oral) or Chloramphenicol | 2 g/day | 12 days | No |
| placebo | |
| Amoxicillin 1.5 g/day 1.9 g/day (oral) | 1.5 g/day | 7 days | yes |
| placebo | |
| or co‐trimoxazole | 1.9 g/day | |||||
| IV: intravenous. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure up to 4 weeks (no resolution or deterioration after trial medication of any duration or death when explicitly stated due to exacerbation or additional course of antibiotics) Show forest plot | 12 | 1636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.62, 0.81] |
| 1.1 Outpatient | 7 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 1.2 Inpatient | 4 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.65, 0.91] |
| 1.3 ICU | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.08, 0.45] |
| 2 Treatment failure within 4 weeks ‐ current drugs only Show forest plot | 8 | 1175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.64, 0.91] |
| 2.1 Outpatient | 5 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 2.2 Inpatient | 3 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 3 Adverse events Show forest plot | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Diarrhoea | 3 | 698 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.62 [1.11, 6.17] |
| 3.2 Dyspepsia | 2 | 605 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.28, 1.73] |
| 3.3 Pain in mouth | 1 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.73 [0.80, 74.98] |
| 3.4 Exanthema, itching | 3 | 698 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.83 [0.77, 19.11] |
| 3.5 Overall (adverse events not separated) | 5 | 1243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [1.03, 2.27] |
| 4 All‐cause mortality Show forest plot | 5 | 624 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.25, 1.16] |
| 4.1 Inpatients | 4 | 531 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.37, 2.79] |
| 4.2 ICU patients | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.06, 0.72] |
| 5 Duration of hospital stay (days) Show forest plot | 3 | 202 | Mean Difference (IV, Random, 95% CI) | ‐3.04 [‐8.83, 2.76] |
| 6 Improvement in dyspnoea measured at the end of the study period Show forest plot | 2 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.96, 0.15] |
| 6.1 Outpatients | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.97, 0.97] |
| 6.2 Inpatients | 1 | 265 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.27, 0.07] |
| 7 Health‐related quality of life or functional status measures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Re‐exacerbations within ≥ 2 to 6 weeks since beginning of index exacerbation (rates) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Days off work Show forest plot | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐5.18 [‐6.08, ‐4.28] |

