Services de télérééducation après les accidents vasculaires cérébraux
Résumé scientifique
Contexte
La télérééducation offre une alternative aux services de rééducation. Les technologies de l'information et de la communication sont utilisées pour faciliter la communication à distance entre professionnels de santé et patients. L'utilisation de la télérééeducation devient plus accessible avec l’accélération et la sophistication des technologies de communication Toutefois, on ne sait pas encore très bien dans quelle mesure ce modèle de prestation est efficace, par rapport à la rééducation dispensée en face à face ou lorsqu'il est ajouté aux soins habituels.
Objectifs
Déterminer si la télérééducation permet d'améliorer la capacité à accomplir les activités de la vie quotidienne chez les survivants d'un accident vasculaire cérébral, par rapport à (1) la rééducation en présentiel (lorsque le clinicien et le patient se trouvent au même endroit et que la rééducation est assurée en face à face) ; ou à (2) l'absence de rééducation ou de soins habituels.
Nos critères de jugement secondaires étaient de déterminer si l'utilisation de la télérééducation conduit à une plus grande indépendance dans les soins personnels et la vie domestique ainsi qu’à une amélioration de la mobilité, de l'équilibre, de la qualité de vie liée à la santé, de la dépression, des fonctions des membres supérieurs, des fonctions cognitives ou de la communication fonctionnelle, par rapport à la rééducation en présentiel et à l'absence de rééducation. En outre, nous avons recherché à rapporter la présence d'événements indésirables, le rapport coût‐efficacité, la faisabilité et les niveaux de satisfaction des utilisateurs associés aux interventions de télérééducation.
Stratégie de recherche documentaire
Nous avons consulté le Registre des Essais du Groupe Cochrane sur les accidents vasculaires cérébraux (juin 2019), le Registre Central Cochrane des Essais Contrôlés (la Bibliothèque Cochrane, numéro 6, 2019), MEDLINE (Ovid, 1946 à juin 2019), Embase (1974 à juin 2019), et huit autres bases de données. Nous avons consulté les registres d’essais et les listes de références.
Critères de sélection
Essais contrôlés randomisés (ECR) sur la télérééducation en cas d’accident vasculaire cérébral. Nous avons inclus des études comparant la télérééducation à la rééducation en présentiel ou à l'absence de rééducation. En outre, nous avons synthétisé et décrit les résultats des ECR qui comparent deux méthodes différentes de fourniture de services de télérééducation sans groupe alternatif. Nous avons inclus des programmes de rééducation qui combinent la télérééducation et la rééducation en présentiel, à condition que la plus grande partie de l'intervention se fasse par télérééducation.
Recueil et analyse des données
Deux auteurs de la revue ont, de manière indépendante, sélectionné les essais sur la base de critères d'inclusion prédéfinis, extrait les données et évalué les risques de biais. Un troisième auteur de la revue a réglé les désaccords éventuels. Les auteurs de la revue ont contacté les chercheurs pour leur demander les informations manquantes. Nous avons utilisé GRADE pour évaluer la qualité des données probantes et interpréter les résultats.
Résultats principaux
Nous avons inclus 22 essais dans la revue, soit un total de 1937 participants. La taille des études varie de 10 à 536 participants, et la qualité des informations était inadéquate, notamment concernant les procédures de génération de séquences aléatoires et d’assignation secrète Nous avons noté dans plusieurs études une présentation sélective des résultats et des données des résultats incomplètes. Les modes d’intervention et les comparaisons pouvant varier, il était souvent inapproprié de combiner les études. Les stratégies d’interventions comprenaient les programmes de soutien à la sortie de l'hôpital, l’entraînement des membres supérieurs, la rééducation des membres inférieurs et de la mobilité et la thérapie de la communication pour les personnes souffrant de troubles du langage post‐AVC Les études ont été menées soit à la sortie de l'hôpital, soit auprès de patients en phase subaiguë ou chronique après AVC.
Critère de jugement principal: nous avons trouvé des données probantes de qualité moyenne indiquant qu’il n’y avait pas de différence dans les activités de la vie quotidienne entre les patients ayant bénéficié d'une intervention de téléréadaptation après leur sortie de l'hôpital et ceux ayant reçu les soins habituels (sur la base de 2 études avec 661 participants (différence moyenne standardisée (DMS) ‐0,00, intervalle de confiance (IC) de 95% ‐0,15 à 0,15)). Nous avons trouvé des preuves de faible qualité indiquant qu’il n’y avait pas de différence d’effet sur les activités de la vie quotidienne entre les programmes de télérééducation et ceux de physiothérapie en présentiel (sur la base de 2 études avec 75 participants : DMS 0,03, 95 % IC ‐0,43 à 0,48). Critères de jugement secondaires : nous avons trouvé des données probantes indiquant qu’il n’y avait pas de différence entre la télérééducation et la rééducation en présentiel concernant l’équilibre (sur la base de 3 études avec 106 participants : DMS 0,08, 95%CI ‐0,30 à 0,46). Le regroupement de trois études (569 participants) a montré des données probantes de qualité moyenne indiquant qu’en matière de qualité de vie liée à la santé, il n’y a aucune différence entre les patients ayant bénéficié d'interventions de soutien après leur sortie de l'hôpital et ceux ayant reçu les soins habituels (DMS 0,03, IC à 95 % ‐0,14 à 0,20). De même, en regroupant les six études (1145 participants) nous avons trouvé des données probantes de qualité moyenne indiquant qu’il n’y avait pas de différence dans les symptômes de dépression entre les programmes de télé‐soutien après la sortie de l'hôpital et les soins habituels (DMS ‐0,04, 95 % IC ‐0,19 à 0,11). Nous n'avons trouvé aucune différence entre les groupes pour la rééducation des fonctions des membres supérieurs (sur 3 études avec 170 participants: différence moyenne (DM) 1,23, IC 95% ‐2,17 à 4,64, données probantes de faible qualité) entre un programme informatique de télérééducation pour les membres supérieurs et une thérapie en présentiel. Les données probantes sont insuffisantes pour tirer des conclusions sur les effets de la télérééducation sur la mobilité ou sur le niveau de satisfaction des participants à l'égard de l'intervention. Aucune étude n'a évalué le rapport coût‐efficacité de la télérééducation; cependant, cinq de nos études ont rapporté les critères de jugement de l'utilisation des services de santé ou les coûts des interventions fournies dans le cadre concerné. Deux études font état d'effets indésirables, bien qu'aucun événement indésirable grave lié aux essais n'ait été signalé.
Conclusions des auteurs
Bien qu'il y ait maintenant un nombre croissant d'essais contrôlés randomisés testant l'efficacité de la télérééducation, il est difficile de tirer des conclusions sur ses effets car, et les interventions et les comparateurs varient beaucoup d'une étude à l'autre. En outre, nous avons trouvé peu d’études suffisamment puissantes et plusieurs études incluses dans cette revue risquaient d'être biaisées. À ce stade, il n'existe que des données probantes de faible ou moyenne qualité permettant de déterminer si la télérééducation est un moyen de rééducation plus efficace ou bien tout aussi efficace que les autres approches. Il n'a pas été démontré que les programmes de télérééducation à court terme après la sortie de l'hôpital réduisent les symptômes de dépression, améliorent la qualité de vie ou renforcent l'indépendance dans les activités de la vie quotidienne, par rapport aux soins habituels. Les études comparant la télérééducation et la thérapie en présentiel n'ont pas non plus trouvé de résultats significativement différents entre les groupes, ce qui suggère que la télérééducation n'est pas de moindre qualité. Certaines études ont indiqué que la télérééducation était moins coûteuse, mais sans fournir d’informations sur le rapport coût‐efficacité. Seuls deux essais ont rapporté si des événements indésirables s'étaient produits ou non: ces essais n’ont signalé aucun événement indésirable grave en lien avec la télérééducation. Ce domaine est encore en émergence et d'autres études sont nécessaires pour tirer des conclusions plus définitives. Si cette revue a analysé l'efficacité de la télérééducation lors d'essais randomisés, par ailleurs les études qui utilisent des méthodes mixtes pour évaluer l'acceptabilité et la faisabilité des interventions de télésanté sont extrêmement précieuses pour en mesurer les résultats.
PICO
Résumé simplifié
Services de télérééducation après les accidents vasculaires cérébraux
Problématique de la revue
Cette revue a pour but de rassembler des données probantes en faveur de l'utilisation de la télérééducation après un accident vasculaire cérébral. Notre objectif consiste à comparer la télérééducation à la thérapie dispensée en face à face et à l'absence de thérapie (soins habituels).
Contexte
Les accidents vasculaires cérébraux (AVC) sont une cause fréquente de handicap chez l’adulte. Après un accident vasculaire cérébral, il est fréquent de rencontrer des difficultés dans les activités quotidiennes telles que marcher, se doucher, s'habiller et participer à des activités communautaires. De nombreuses personnes ont besoin d'une rééducation après un AVC ; celle‐ci est généralement assurée par des professionnels de santé dans un hôpital ou un dispensaire. Des études récentes ont examiné la possibilité d'utiliser des technologies telles que le téléphone ou l'internet pour aider les patients à communiquer avec les professionnels de santé sans avoir à quitter leur domicile. Cette approche, appelée télérééducation, pourrait être un moyen de rééducation plus pratique et moins coûteux. Elle pourrait être utilisée pour améliorer toute une série de critères de jugement, notamment le fonctionnement physique et l'humeur.
Caractéristiques des études
Nous avons recherché des études en juin 2019 et sélectionné 22 portant sur 1937 personnes ayant subi un AVC. Ces études ont utilisé un large éventail de traitements, y compris des programmes thérapeutiques conçus pour améliorer les fonctions des bras et la marche et des programmes de conseils et soutien aux patients à la sortie d’hospitalisation pour AVC.
Principaux résultats
Comme ces études sont très différentes, il a été rarement pertinent de combiner les résultats pour déterminer l'effet global. Nous avons constaté que, pour les activités de la vie quotidienne, les patients ayant bénéficié d'une télérééducation, ceux ayant reçu une thérapie en face à face et ceux n’ayant reçu aucune thérapie (soins habituels) ont obtenu des résultats similaires. À l'heure actuelle, les recherches sont encore insuffisantes pour montrer si la télérééducation est un moyen de rééducation plus efficace. Certaines études indiquent qu’elle est moins coûteuse, mais on manque d’informations sur le rapport coût‐efficacité. Seuls deux essais ont rapporté si des événements indésirables s'étaient produits ou non: ces essais n’ont signalé aucun événement indésirable grave en lien avec la télérééducation. D'autres essais sont nécessaires.
Qualité des données probantes
Dans l’ensemble, la qualité des données probantes était faible ou moyenne. Pour chaque critère de jugement, la qualité des données probantes est limitée en raison du petit nombre de participants à l'étude et de la mauvaise communication des détails de l'étude.
Authors' conclusions
Summary of findings
| Telerehabilitation compared with in‐person rehabilitation for stroke | ||||
| Patient or population: people with stroke Settings: living in the community Intervention: telerehabilitation Comparison: in‐person rehabilitation | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of Participants | Quality of the evidence | Comments |
| Independence in ADL post‐intervention | No significant difference found on total ADL function score: MD 0.59 (‐5.50 to 6.68) (Analysis 1.1) | 75 (2 studies) | ⊕⊕⊝⊝ | |
| Self‐care and domestic life Post‐intervention | Outcome not assessed in included studies | |||
| Mobility post‐intervention | Outcome not assessed in included studies | |||
| Balance post‐intervention | No significant difference found on balance outcomes: MD 0.48 (‐1.36 to 2.32) (Analysis 1.2) | 106 participants (3 studies) | ⊕⊕⊝⊝ | |
| Self‐reported health‐related quality of life post‐intervention | Outcome not assessed in included studies (where the comparison was telerehabilitation versus in‐person rehabilitation) | |||
| Depression post‐intervention | Outcome not assessed in included studies (where the comparison was telerehabilitation versus in‐person rehabilitation) | |||
| Upper limb function post‐intervention | No significant difference found on total UL function score: MD 1.23 (‐2.17 to 4.64) (Analysis 1.3) | 170 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to risk of bias. bDowngraded one level due to imprecision related to small sample size. | ||||
| Telerehabilitation (post hospital discharge support) compared with usual care for stroke | ||||
| Patient or population: people with stroke Settings: living in the community Intervention: telerehabilitation Comparison: usual care | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of Participants | Quality of the evidence | Comments |
| Independence in ADL post‐intervention | No significant difference found on total ADL function score: SMD ‐0.00 (‐0.15 to 0.15) (Analysis 2.1) | 661 | ⊕⊕⊕⊝ | |
| Self‐care and domestic life post‐intervention | Outcome not assessed in included studies | |||
| Mobility post‐intervention | No significant difference found in gait speed: MD 0.01 (‐0.12 to 0.14) (Analysis 2.2) | 144 (1 study) | ⊕⊕⊝⊝ | |
| Balance post‐intervention | Outcome not assessed in included studies | |||
| Self‐reported health‐related quality of life post‐intervention | No significant difference found in self‐reported quality of life: SMD 0.03 (‐0.14 to 0.20) (Analysis 2.3) | 569 | ⊕⊕⊕⊝ | |
| Depression post‐intervention | No significant difference found in depressive symptoms SMD ‐0.04 (‐0.19 to 0.11) (Analysis 2.4) | 1145 | ⊕⊕⊕⊝ | |
| Upper limb function post‐intervention | No significant difference found in upper limb function: SMD 0.33 (‐0.21 to 0.87) (Analysis 2.5) | 54 (2 studies) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to risk of bias. bDowngraded one level due to imprecision related to small sample size. | ||||
Background
Description of the condition
Stroke is one of the most common causes of death and acquired disability worldwide (Thrift 2017). Survivors of stroke commonly experience a range of symptoms affecting motor function, speech, swallowing, vision, sensation and cognition, and recovery can be slow and incomplete (Crichton 2016; Langhorne 2011). These symptoms often lead to difficulty managing activities and limited participation in home and community activities. Approximately half of stroke survivors access some form of rehabilitation on discharge from acute services (National Institutes of Health 2014; Stroke Foundation 2017). Rehabilitation programmes are often lengthy and resource intensive. Therefore, determining the most effective and efficient ways to deliver stroke rehabilitation services is a matter of priority.
Description of the intervention
Telerehabilitation is the provision of rehabilitation services to patients at a remote location using information and communication technologies (Brennan 2009). Communication between the patient and the rehabilitation professional may occur through a variety of technologies such as the telephone, Internet‐based videoconferencing and sensors (such as pedometers). Virtual reality programmes may also be used as a medium for therapy; the patient completes therapy tasks within a computer‐generated virtual environment, and data are transmitted to the therapist (Rogante 2010). Telerehabilitation consultations may include assessment, diagnosis, goal‐setting, therapy, education, and monitoring (Russell 2009).
Stemming from the broader approach of telehealth, telerehabilitation has been described as an alternative method of delivering conventional rehabilitation services rather than a subspecialty (Winters 2002). There has been increasing interest in the use of telerehabilitation over time as technologies have become increasingly prevalent and more sophisticated (Brochard 2010; Galea 2019); however, translation into clinical practice has been slow and barriers experienced early in the development of the field persist (Standing 2018).
Many examples in the current literature demonstrate the scope of telerehabilitation. For example, home assessments to determine the need for modifications have been completed remotely by occupational therapists using a combination of still photography, telephone calls, and videoconferencing technology (Ninnis 2019). Physiotherapists have used telerehabilitation for treatment of musculoskeletal conditions and post‐surgical care (Richardson 2017; Van Egmond 2018), and speech pathologists have demonstrated the feasibility of providing aphasia rehabilitation using asynchronous telerehabilitation (Hill 2018).
How the intervention might work
Telerehabilitaton has been described simply as an alternative method of providing rehabilitation. Therefore, in theory, the mechanisms leading to recovery should mirror those associated with conventional rehabilitation programmes. It is now well established that organised, interdisciplinary stroke care reduces the likelihood of institutional care and long‐term disability and increases independence in activities of daily living (Kalra 2007; Pollock 2014). Improvements in function after completion of rehabilitation programmes have been attributed to a combination of physiological recovery, reorganisation within the brain (known as neuroplasticity), and compensation (Kwakkel 2004).
One of the key advantages of telerehabilitation is that it provides the opportunity for people who are isolated to access rehabilitation services. This feature is particularly beneficial in vast countries such as Canada and Australia, where many people live long distances away from specialised rehabilitation centres. People in rural and remote areas are unlikely to have access to rehabilitation teams with expertise in stroke, and they may not have access to rehabilitation clinicians at all. Eliminating the need for travel to rehabilitation centres may also benefit people with severely restricted mobility who have difficulty travelling or are unable to travel. Telerehabilitation is also likely to be beneficial in low‐resource settings where access to health professionals is poor but access to devices such as mobile phones is present.
Telerehabilitation services may also be used to complement and enhance the quality of current rehabilitation services. Stroke survivors have expressed concern regarding the lack of available long‐term support and ongoing unmet rehabilitation needs (Ullberg 2016). It is possible that the use of telerehabilitation may help to address these gaps by supporting patients as they resume life roles on discharge from inpatient facilities.
Furthermore, the use of telerehabilitation may result in cost savings in various ways. Reduced travel time (for clinicians who visit patients in their own home) may mean that clinicians are able to fit more consultations into a single day. In addition, it may be possible to discharge patients from inpatient rehabilitation facilities earlier and offer telerehabilitation as a way of continuing the rehabilitation programme. Furthermore, telerehabilitation may provide a mechanism for increasing the dose of therapy without an increase in face‐to‐face supervision.
Despite its apparent advantages, the challenges associated with telerehabilitation are well documented (Standing 2018; Theodoros 2008). One of the key issues facing clinicians is how to conduct assessments or provide interventions that are typically 'hands on', for example, assessment of muscle strength. The inability to conduct hands‐on assessment or treatment means that therapists need to modify current techniques, for example, by utilising family members or teaching the patient ways to perform the intervention independently (Russell 2009).
Furthermore, clinicians and patients may not possess the technical expertise to establish systems and to troubleshoot information and communication technologies. It has been recommended that service providers ensure that technical requirements are met (such as having adequate bandwidth), provide access to technical support and provide training to all users (clinicians and patients). Concerns have also been raised about the security of data transfer and how patient confidentiality can be maintained (American Telemedicine Association 2010).
Why it is important to do this review
Changes in the demographics of the population mean that the burden of stroke is projected to increase (Feigin 2017). New approaches that are demonstrated to be clinically sound and cost‐effective will be required. Increasing interest in telerehabilitation suggests that this area will continue to grow (Brochard 2010; Galea 2019), and there is great potential to implement effective telehealth interventions in low and middle‐income countries. Furthermore, clinical guidelines for stroke recommend telerehabilitation for people without access to centre‐based rehabilitation services (Blacquiere 2017). However, establishment of telerehabilitation services may be expensive because of the costs of equipment, training, and ongoing technical support. Therefore, it is important to determine whether telerehabilitation services once established may result in the desired outcomes.
Our first version of this review, published in 2013, included 10 RCTs which were heterogeneous in terms of the aims of their intervention (Laver 2013). Based on the lack of information available at that time, we were unable to reach conclusions about the effectiveness of telerehabilitation after stroke. Another recently published systematic review examined the effectiveness of telerehabilitation after stroke and, using different inclusion criteria, identified 11 RCTs (Chen 2015). The authors concluded that despite the relatively small field of evidence from which to draw information, telerehabilitation was non‐inferior to conventional rehabilitation approaches in improving activities of daily living and motor function (Chen 2015).
Given the growth of research in this area and the potential for telerehabilitation to improve access to, and quality of, rehabilitation services while reducing costs, an update of our previous review was warranted. Furthermore, health services are increasingly offering telerehabilitation services for their clients so evaluation of this approach is important.
Objectives
To determine whether the use of telerehabilitation leads to improved ability to perform activities of daily living amongst stroke survivors when compared with (1) in‐person rehabilitation (when the clinician and the patient are at the same physical location and rehabilitation is provided face‐to‐face); or (2) no rehabilitation or usual care.
Secondary objectives were to determine whether use of telerehabilitation leads to greater independence in self‐care and domestic life and improved mobility, balance, health‐related quality of life, depression, upper limb function, cognitive function, or functional communication when compared with in‐person rehabilitation and no rehabilitation. Additionally, we aimed to report on the presence of adverse events, cost‐effectiveness, feasibility, and levels of user satisfaction associated with telerehabilitation interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs. We considered cross‐over trials as RCTs in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included studies if they compared telerehabilitation with in‐person rehabilitation or no rehabilitation, two different methods of delivering telerehabilitation services, different doses of telerehabilitation or telerehabilitation plus usual care compared with usual care alone.
Types of participants
All study participants had received a clinical diagnosis of stroke as defined by the World Health Organization ("a syndrome of rapidly developing symptoms and signs of focal, and at times global, loss of cerebral function lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin") (WHO 1989). We included people with all types of stroke, at all levels of severity, and at all stages post‐stroke (acute, subacute, or chronic). We also included participants with subarachnoid haemorrhage. We excluded studies with participants of mixed aetiology (e.g. stroke and traumatic brain injury) unless data were available for stroke survivors only. We set no age limits; however, we planned to acknowledge the inclusion of any participants who were younger than 18 years of age.
Types of interventions
We included Interventions if they matched the following definition of telerehabilitation: "the delivery of rehabilitation services via information and communication technologies" (Brennan 2009). Clinically, this term encompasses a range of rehabilitation services that include assessment, prevention, intervention, supervision, education, consultation, and counselling. Interventions must have lasted longer than one session. Interactive and communication technologies included the telephone, the Internet, virtual reality, and monitoring via sensors or wearable devices. We included rehabilitation programmes that used "store and forward" methods of communication, or real‐time interaction. Interventions were provided by one or more health disciplines (e.g. we planned to include studies involving only one health profession and studies which involved a multidisciplinary intervention). We included rehabilitation programmes that used a combination of telerehabilitation and in‐person rehabilitation to conduct assessment or intervention, provided that the greater proportion of intervention was provided via telerehabilitation. We did not include the use of telerehabilitation when the purpose was to provide education or support for healthcare professionals rather than patient care.
Types of outcome measures
Primary outcomes
The primary outcome of interest was independence in activities of daily living assessed post‐intervention. In the review, this encompassed the self‐care, mobility and domestic life activity and participation domains derived from the International Classification of Functioning, Disability and Health (WHO 2010). Included assessment tools were those such as the Functional Independence Measure, the Barthel Index, Lawton Instrumental Activities of Daily Living, Frenchay Activities Index, and the Nottingham Extended Activities of Daily Living Index.
Secondary outcomes
-
Self‐care and domestic life.
-
Mobility (e.g. Timed Up and Go test, walking speed, functional ambulation category).
-
Balance.
-
Participant satisfaction with the intervention.
-
Self‐reported health‐related quality of life.
-
Depression.
-
Upper limb function (e.g. Action Research Arm Test, Wolf Motor Function Test, Fugl‐Meyer Upper Extremity measure).
-
Cognitive function (global measures such as the Mini Mental State Examination, or specific measures such as tests of attention or executive functioning).
-
Functional communication.
-
Cost‐effectiveness (as measured by comparing the costs and outcomes of each intervention approach).
-
Adverse events.
We also aimed to provide information on the feasibility of telerehabilitation for use with people after stroke by reporting on participant eligibility criteria and recruitment methods used in the individual studies identified.
Search methods for identification of studies
See the 'Specialized register' information at the Cochrane Stroke Group's website. We searched for relevant trials in all languages and arranged translation of trial reports where necessary.
Electronic searches
The searches for studies in our previous reviews were conducted in November 2012. The searches for this update were completed in July 2017 and then updated in June 2018, and again in June 2019.
We searched the Cochrane Stroke Group Trials Register, which was searched by the Managing Editor in November 2012, and by the Information Specialist in July 2017, June 2018, and June 2019 using the intervention code, telemedicine. In addition, we searched the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6) in the Cochrane Library (searched 4 June 2019) (Appendix 1), MEDLINE (Ovid, 1946 to 3 June 2019) (Appendix 2), Embase Ovid (1974 to Week 22, 2019) (Appendix 3), AMED Ovid (Allied and Complementary Medicine; 1985 to May 2019) (Appendix 4), CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 4 June 2019) (Appendix 5), PsycINFO Ovid (from 1806 to 4 June 2019) (Appendix 6), PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy, www.psycbite.com/ to 20 September 2018) (Appendix 7), OTseeker (www.otseeker.com to 20 September 2018) (Appendix 8), Physiotherapy Evidence Database (www.pedro.org.au to 20 September 2018) (Appendix 9), REHABDATA (www.naric.com/research/rehab/ to 20 September 2018) (Appendix 10), and the Health Technology Assessment Database (HTA) (www.crd.york.ac.uk/crdweb/ to 20 September 2018) (Appendix 11). We developed the MEDLINE search strategy with the help of the Cochrane Information Specialist and used a combination of controlled vocabulary and text‐word terms. We adapted this strategy for use with the other databases. Search words for trial registers and for other Web‐based databases included telerehabilitation, telemedicine, telehealth, videoconferencing, and stroke.
We also:
-
searched the following ongoing trials registers: US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) to 4 June 2019 (Appendix 12);
-
used the Cited Reference Search within Science Citation Index (SCI) and Social Science Citation Index (SSCI) to track relevant references;
-
searched ProQuest Dissertations and Theses (to 4 June 2019) (Appendix 13);
-
searched the UK Telemedicine and E‐health Information Service (www.teis.port.ac.uk/) for the first version of this review. The website was discontinued and was not searched for this version of the review; and
-
searched the grey literature using Open Grey (www.opengrey.eu) and Google Scholar (www.scholar.google.com) on 4 June 2019.
Searching other resources
To identify further published, unpublished and ongoing trials, we:
-
scanned the reference lists of all identified studies and reviews; and
-
scanned the abstracts of non–English language studies if they were available in English.
Data collection and analysis
Selection of studies
Two review authors (KEL and ZA) independently reviewed titles and abstracts of the records identified through searches and excluded obviously irrelevant studies. We obtained the full text of the remaining studies, and two review authors (KEL and ZA) selected studies for inclusion based on the inclusion criteria of the review. When unsure regarding inclusion of a particular study, a third review author (MC, SG or CS) made the final decision. We contacted trial authors for further details when required and documented the reasons for exclusion.
Data extraction and management
Two review authors (KEL and ZA) independently extracted study data and recorded information on a predesigned data extraction form. We extracted the following study details.
-
Citation details: title, authors, source and year of publication.
-
Participant inclusion and exclusion criteria.
-
Participant details: age, gender, location of stroke, time since onset of stroke and level of disability.
-
Recruitment details: numbers of people screened, eligible, recruited and randomly assigned; withdrawals.
-
Methodological quality: the Cochrane Collaboration's tool for assessing risk of bias.
-
Intervention details: descriptions of procedures, personnel involved, duration, dose and comparison interventions.
-
Outcome measures: measures used, by whom, when they were administered and how they were administered (in‐person or via information and communication technologies).
We contacted trial authors to ask for missing information when required. We resolved differences by discussion or by consultation with a third review author when necessary.
Assessment of risk of bias in included studies
Two review authors (KEL and ZA) independently assessed the risk of bias of included studies using Cochrane's 'Risk of bias' tool (Higgins 2011). This tool allows assessment of the following possible sources of bias: random sequence generation; allocation concealment; blinding of outcome assessors; incomplete outcome data; selective reporting; and any other potential sources of bias. We did not report on whether studies were able to blind participants or personnel because of the difficulties involved in achieving this in rehabilitation trials. We compared each study against the tool and assessed it as 'low risk', 'high risk', or 'unclear risk' of bias, depending on whether it met the criteria for each aspect of the tool. A third review author resolved any disagreements.
Measures of treatment effect
Two review authors (KEL and ZA) independently assigned outcome measures to the domain assessed (activities of daily living, participant satisfaction, health‐related quality of life, depression, mobility, upper limb function, cognitive function, functional communication). If more than one outcome measure was used in the same domain from the same study, we included the measure most frequently used across included studies.
We intended to conduct separate analyses between short‐term (less than three months after intervention) and long‐term (three months or longer) outcomes.
We planned to calculate risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs), or standardised mean differences (SMDs) and 95% CIs for continuous outcomes, as appropriate.
Unit of analysis issues
The unit of randomisation in these trials was the individual participant. For three‐armed trials in which telerehabilitation was compared with in‐person or no rehabilitation, we intended to enter half the sample size for the telerehabilitation group. Thus, each alternative intervention would be included in a separate comparison, and the number of participants in the telerehabilitation group would be divided equally between comparisons; the telerehabilitation group mean and standard deviation would remain unchanged.
Dealing with missing data
We contacted trial authors to request missing data. We converted available data, when possible, using the procedures detailed in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to deal with missing data as recommended by the Cochrane Handbook for Systematic Reviews of Interventions. When dropouts were clearly identified, we used the denominator of participants contributing data at the relevant outcome assessment.
Assessment of heterogeneity
When appropriate, we pooled results to present an estimate of treatment effect using a random‐effects model. We assessed heterogeneity by performing visual inspection of the forest plot along with the I2 statistic (Higgins 2011) where we considered that up to 40% heterogeneity might not be important, but higher levels indicated moderate or substantial heterogeneity.
Assessment of reporting biases
We sought to assess the impact of publication bias by searching clinical trials registers for studies. In addition, we investigated whether selective reporting occurred by comparing study protocols and the methods sections of papers with the results sections. We intended to assess small sample bias by preparing a funnel plot if we had 10 or more studies included in any meta‐analysis.
Data synthesis
We conducted a meta‐analysis based on a random‐effects model with 95% CIs using RevMan 5.3 (RevMan 2014). We explored heterogeneity as detailed below.
Subgroup analysis and investigation of heterogeneity
If we identified a sufficient number of comparable studies (eight or more), we planned to perform subgroup analyses to determine whether the effect on the primary outcome varied according to time since onset of stroke, severity of stroke, frequency of the intervention (occasions of service per week), intensity of the intervention (total hours of intervention), intervention approach selected (e.g. speech therapy, upper limb retraining), mode of delivery (e.g. telephone versus videoconferencing, real‐time communication versus 'store and forward'), and whether the intervention was provided by a multidisciplinary team or by members of a single discipline.
Sensitivity analysis
We intended to perform sensitivity analyses for all outcomes based on the methodological quality of studies (allocation concealment, blinding of outcome assessor, intention‐to‐treat analysis) to assess the impact of risk of bias in the included studies. We planned to conduct sensitivity analysis regardless of the level of heterogeneity detected. We also planned to conduct a sensitivity analysis to identify differences noted when a fixed‐effect versus a random‐effects model was used.
GRADE and Summary of findings
We used GRADE to interpret findings (Guyatt 2008), and presented 'Summary of findings' tables. The tables provide outcome‐specific information concerning the overall quality of evidence, magnitude of effect, and the sum of available data. When using GRADE, we downgraded the evidence from 'high quality' by one level for serious (or by two levels for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias. We presented the following outcomes in our 'Summary of findings' tables: activities of daily living, self‐care and domestic life, mobility, balance, self‐reported health‐related quality of life, depression, and upper limb function.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
We identified 189 studies by searching the Cochrane Stroke Group trials register and clinical trial registries, and 25,234 references by searching electronic databases, totaling 25,423 references. We reviewed 416 articles in full text and contacted study authors to request more information when required, excluding articles that clearly did not meet the inclusion criteria. Details of 29 excluded studies are provided in the 'Characteristics of excluded studies' table. We listed studies in the excluded studies section of the review if they prompted conversations between the review authors regarding their eligibility. We identified 22 ongoing studies (Characteristics of ongoing studies), and six studies which are awaiting classification (Characteristics of studies awaiting classification). Search details are presented in the flow diagram (Figure 1).

Study flow diagram.
Included studies
We included 22 RCTs, with a total of 1937 participants, in the review.
Sample characteristics
Included studies were conducted in the USA (n = 8), Canada (n = 3), the Netherlands (n = 2), Italy (n = 2), Germany (n = 2), China (n = 2), Taiwan (n = 1), Spain (n = 1), and Slovenia (n = 1). All studies were published within the previous 15 years (between 2004 and 2019). Sample sizes ranged from 10 to 536; most studies (71%, n = 16) included fewer than 100 participants (Table 1; Table 2).
| Study | Screened | Randomised | Allocated to intervention group | Allocated to control group | Assessed at follow‐up |
| Not reported | 49 | 23 | 26 | 41 | |
| Not reported | 10 | 5 | 5 | Unclear | |
| 691 | 536 | 263 | 273 | 486 | |
| 167 | 25 | 13 | 12 | 20 | |
| 97 | 54 | 27 | 27 | 50 | |
| 52 | 52 | 27 | 25 | 44 | |
| 232 | 124 | 62 | 62 | 124 analysed | |
| 62 | 19 | 9 | 10 | 16 | |
| Not reported | 11 | Not reported | Not reported | 9 | |
| Not reported | 16 | Not reported | Not reported | Not reported | |
| 416 | 100 | 37 | 35 (in‐person), 28 (usual care) | 91 | |
| 94 | 24 | 12 | 12 | 23 | |
| 115 | 31 | 15 | 16 | 30 | |
| 294 | 190 | 96 | 94 | 157 | |
| Not reported | 53 | 20 | 22 | Unclear | |
| Not reported | 10 | 5 | 5 | 10 | |
| Not reported | 36 | 18 | 18 | 36 | |
| 286 | 186 | 92 | 94 | 139 | |
| 1045 | 265 | 130 | 135 | 230 | |
| 161 | 38 | 19 | 19 | 32 | |
| Unclear | 17 | Unclear | Unclear | Unclear | |
| 186 | 91 | 46 | 45 | 80 |
| Study | Intervention | Comparison | Time after stroke | Country of study |
| Family therapy phone calls to assist with transition home (up to 26 phone calls over 6 months) | Usual care | Not reported however occurred on discharge from hospital to home | USA | |
| Web‐based exercises (posture and upper limb) and therapist weekly consultations | Provision of written exercises without additional therapist contact | Subacute | Slovenia | |
| Case management via 3 telephone calls and a home visit up to 24 weeks after discharge from an acute hospital following stroke | Usual care | Not reported; however, intervention was provided on discharge from acute facility | Netherlands | |
| Upper limb therapy targeting finger and wrist movements provided via a computerised programme in which explicit feedback on performance was provided. Regular teleconferencing occurred between participant and therapist. | Upper limb therapy targeting finger and wrist movements provided via a computerised programme whereby explicit feedback on performance was not provided. Regular teleconferencing occurred between participant and therapist. | Chronic phase | USA | |
| Exercise programme and electrical stimulation which was supervised and monitored remotely via a telerehabilitation system | Same programme of exercises but provided face to face in an outpatient therapy service | Subacute phase | China | |
| A programme designed to improve the person's functional mobility administered via televisits, use of an in‐home messaging device, and 5 telephone calls over a 3‐month period | Usual care | Subacute phase | USA | |
| Telehrehabilitaiton program designed to improve upper limb function and involving on‐screen games | Similar therapy content and same dose of therapy but provided in the clinic | Subacute | USA | |
| Lower limb therapy targeting ankle movements provided via a computerised programme in which explicit feedback on performance was provided. Teleconferencing was used regularly, and performance data were emailed to the therapist. | Lower limb therapy targeting ankle movements provided via a computerised programme whereby explicit feedback on performance was not provided. Teleconferencing was used regularly, and performance data were emailed to the therapist. | Chronic phase | USA | |
| A total of 12 therapy sessions (occupational therapy and physiotherapy) were conducted via a desktop videophone. Interventions included education, retraining of self‐care, functional mobility and posture, home modifications and therapy to improve function in impaired limbs. | The same intervention programme was delivered face‐to‐face. | Not reported | USA | |
| Upper limb therapy using the Home Care Activity Device (computer‐based programme) for 1 month | Usual care and generic exercises were provided by a physician | Chronic phase | Netherlands | |
| Living Well with Stroke intervention designed to reduce depressive symptoms in people with stroke and depression delivered via telephone | Living Well with Stroke (in‐person) Usual care | Subacute | USA | |
| Physical exercises especially balance and lower limbs provided remotely via telerehabilitation system | Similar exercises provided face‐to‐face | Chronic | Taiwan | |
| Virtual reality system used in the home with the aim of improving balance. Remote monitoring and phone call checks | Virtual reality system used in the clinic with the aim of improving balance | Chronic | Spain | |
| Case management intervention provided via home visits and telephone calls for 6 weeks following discharge from acute care | Participants were instructed to make an appointment with their general practitioner. | Acute phase | Canada | |
| Aphasia rehabilitation provided remotely via telerehabilitation | Aphasia rehabilitation provided in‐person | Chronic phase | Canada | |
| Upper limb therapy that was delivered using a virtual reality programme at home and supplemented by videoconferencing | Upper limb therapy that was delivered using a virtual reality programme and conducted in the clinic setting | Chronic phase | Italy | |
| Upper limb therapy that was delivered using a virtual reality telerehabilitation programme and that took place in the home | A programme of conventional upper limb exercises | Chronic phase | Italy | |
| Regular phone calls to discuss family functioning and risk factors after discharge from hospital | Phone number to call health professional if queries | Acute phase | Canada | |
| Stroke support service upon discharge from acute hospital | Usual care | Post‐acute phase | Germany | |
| An intervention to support the caregivers of stroke survivors by enhancing knowledge, skills, and coping. Delivered via email, online chat sessions, and online resources | Participants had access to some of the online resources. | Not reported | USA | |
| Rehabilitation for people with aphasia after discharge from hospital | Conventional therapy | Chronic phase | Germany | |
| Goal‐setting telephone follow‐up relating to health behaviours | Usual stroke education | Subacute phase | China |
Most participants in the included studies were aged in their 50s, 60s, and 70s. Similar numbers of men and women were included, with the exception of two studies (Chumbler 2012; Smith 2012), for which only men were recruited. Eight studies recruited participants in the acute stages post‐stroke and provided rehabilitation upon discharge from hospital (Bishop 2014; Boter 2004; Chen 2017; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Wan 2016), whereas the rest of the studies involved participants in subacute and chronic stages.
Criteria for participant inclusion and exclusion varied amongst studies. Thirteen studies stated that they excluded participants with significant cognitive impairment (Bishop 2014; Chen 2017; Chumbler 2012; Cramer 2019; Deng 2012; Huijgen 2008; Lin 2014; Llorens 2015; Meltzer 2018; Piron 2008; Piron 2009; Rochette 2013; Wan 2016), although this condition was defined differently between studies; four studies stated that participants needed to have a caregiver available (Bishop 2014; Forducey 2012; Meltzer 2018; Smith 2012).
As seen in Table 1 (where screening rates were reported) 1611 out of 3666 screened were randomised, resulting in a participation rate of 44%. This rate varied widely between studies, ranging from 15% (Carey 2007), to 100% (Chumbler 2012).
Interventions
All interventions were delivered in the participant's own home with the exception of one study in which participants were residing in a long‐term care facility (Lin 2014). An additional study found that some participants either could not or preferred not to use the telerehabilitation equipment within their homes (Meltzer 2018). In these situations, participants in the telerehabilitation group used equipment at a local healthcare centre or at the study site; however, they used equipment in a separate room and avoided contact with the study team and interventionists.
The primary aim of the intervention varied across the studies. Eight of the studies aimed to enhance care and well‐being after discharge from hospital through interventions which included goal‐setting, education about secondary prevention, family therapy, and case management (Bishop 2014; Boter 2004; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012; Wan 2016). Most of the remaining studies involved interventions which aimed to improve physical function (upper limb, lower limb), and mobility and balance (Bizovičar 2017; Carey 2007; Chen 2017; Chumbler 2012; Cramer 2019; Deng 2012; Forducey 2012; Huijgen 2008; Lin 2014; Llorens 2015; Piron 2008; Piron 2009). Specifically, six studies aimed to improve upper limb function through the use of customised computer‐based training programmes (Bizovičar 2017; Carey 2007; Cramer 2019; Huijgen 2008; Piron 2008; Piron 2009); four studies aimed to improve balance and mobility using customised telerehabilitation systems and communication between the participant and the therapist (Chumbler 2012; Deng 2012; Lin 2014; Llorens 2015); and one study involved exercises delivered remotely plus electrical stimulation with the aim of improving limb function, mobility, and balance (Chen 2017). One study used a combination of occupational therapy and physiotherapy to provide rehabilitation that often focused on remediation of impaired limbs (Forducey 2012). Two studies provided speech and language therapy for people with aphasia (Meltzer 2018; Vauth 2016).
Several different types of information and communication technologies were used to deliver telerehabilitation interventions. These included the telephone (Bishop 2014; Boter 2004; Kirkness 2017; Mayo 2008; Meltzer 2018; Rochette 2013; Saal 2015; Wan 2016), videoconferencing hardware and software (Bizovičar 2017; Carey 2007; Chen 2017; Cramer 2019; Deng 2012; Huijgen 2008; Lin 2014; Llorens 2015; Piron 2008; Piron 2009), and desktop videophones (Forducey 2012). Some studies used a combination of technologies: Chumbler 2012 used a combination of telephone calls, an in‐home messaging device and video recordings taken by a research assistant to be reviewed by the teletherapist; Smith 2012 used a combination of email, an online chat program and an online resource room (a virtual online library) established for caregivers of stroke survivors; and Chen 2017 utilised a telerehabilitation system which integrated electrical stimulation, measured physiological performance, and enabled data and medical records storage. The system used by Lin 2014 enabled videoconferencing plus monitoring of heart rate, oxygen saturation, and blood pressure.
Most interventions were conducted entirely by using information and communication technologies (Bishop 2014; Bizovičar 2017; Carey 2007; Chen 2017; Cramer 2019; Deng 2012; Forducey 2012; Huijgen 2008; Kirkness 2017; Lin 2014; Meltzer 2018; Piron 2008; Piron 2009; Rochette 2013; Saal 2015; Wan 2016). Four studies used a combination of telephone calls and home or clinic visits (Boter 2004; Llorens 2015; Mayo 2008; Saal 2015). The remaining study used 'store and forward' methods in which the research assistant video‐recorded the participant in his or her home and transmitted the information to the teletherapist for review (Chumbler 2012). The teletherapist was almost always described as being a health professional although details of their professional background was lacking in some studies.
Comparisons
Our preplanned comparisons were:
-
telerehabilitation compared with in‐person rehabilitation. We identified nine studies which tested this comparison (Chen 2017; Cramer 2019; Forducey 2012; Lin 2014; Llorens 2015; Meltzer 2018; Piron 2008; Piron 2009; Vauth 2016); and
-
telerehabilitation compared with no rehabilitation or usual care. Ten studies (Bizovičar 2017; Bishop 2014; Boter 2004; Chumbler 2012; Huijgen 2008; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012; Wan 2016) compared telerehabilitation with a level of care which would be considered as a usual level of care available to clients of the service.
We also included studies if they compared different forms of telerehabilitation. Two studies compared different models of telerehabilitation (Carey 2007; Deng 2012).
The remaining study was a three‐arm study which compared telerehabilitation with both in‐person intervention and no intervention (usual care) (Kirkness 2017).
A wide range of outcome measures were used to assess the effects of the range of intervention approaches. These included measures of physical function, independence in activities of daily living, quality of life, and participant satisfaction. All studies assessed outcome measures post‐intervention. Several studies included follow‐up at one month (Piron 2009; Smith 2012), three months (Carey 2007; Chumbler 2012; Llorens 2015), six months (Bishop 2014; Chen 2017; Mayo 2008; Wan 2016), or 12 months (Kirkness 2017; Rochette 2013; Saal 2015), after completion of the intervention.
Studies included in the meta‐analysis
We included data from 14 studies in the meta‐analysis. We could not use data from the remaining studies in our meta‐analysis. Reasons included: data were not presented in suitable format for pooling and not available from the author(s) (Bishop 2014; Forducey 2012; Huijgen 2008; Vauth 2016), studies compared two forms of telerehabilitation (Carey 2007; Deng 2012), or studies did not report on our primary or secondary outcomes (Meltzer 2018).
Excluded studies
We deemed 29 studies to be ineligible: five because of ineligible populations (e.g. traumatic brain injury or transient ischaemic attack), four because they were not randomised trials, and the remaining 20 because the intervention did not meet our definition of telerehabilitation (Characteristics of excluded studies).
Risk of bias in included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
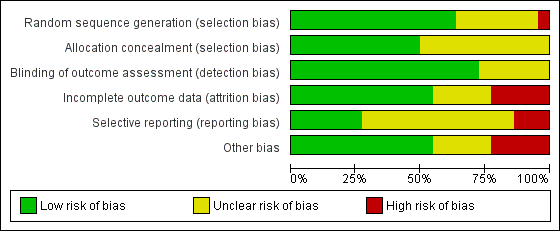
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation concealment was adequate in 11 studies (50%), therefore, considered at low risk of bias but there was an unclear risk of bias in the reports of the remaining studies.
Blinding
Partial blinding of participants and personnel was performed in one of the studies, in which participants were masked to the study objectives because of postponed informed consent procedures (Boter 2004). It was unclear (unclear risk of bias) whether the outcome assessor was blinded to intervention group allocation in six studies. The remaining studies clearly stated that the assessor was blinded to allocation and we therefore considered them to be at low risk of bias.
Incomplete outcome data
For 10 studies it was unclear as to whether there was risk of bias in relation to incomplete outcome data. We deemed that there was low risk of attrition bias in the remaining studies.
Selective reporting
We identified a number of studies as being free of selective reporting and considered at low risk of bias (Chen 2017; Cramer 2019; Piron 2009; Rochette 2013; Saal 2015; Wan 2016). In three studies, we identified a high risk of bias due to selective reporting (Chumbler 2012; Huijgen 2008; Smith 2012). It was unclear (unclear risk of bias) whether selective reporting occurred in the remaining studies.
Other potential sources of bias
We identified several studies as being at risk of bias because of small sample sizes where there were less than 50 participants or differences between groups at baseline, or both (Bishop 2014; Bizovičar 2017; Carey 2007; Chen 2017; Deng 2012; Forducey 2012; Huijgen 2008; Lin 2014; Llorens 2015; Piron 2008; Meltzer 2018; Vauth 2016). It was unclear whether other studies were at risk of other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2
Please refer also to the summary of findings Table for the main comparison; and summary of findings Table 2.
Telerehabilitation versus in‐person rehabilitation
Comparison 1.1: Telerehabilitation vs in‐person rehabilitation: activities of daily living
Three studies compared the effects of a physical therapy programme when delivered via telerehabilitation and when delivered in‐person (Chen 2017; Forducey 2012; Lin 2014). Two of these studies were pooled (Chen 2017; Lin 2014), whereas data from the third study was not reported in the paper in a way that could be used in the analysis and not available from the study authors (Forducey 2012). Pooling of the two studies with 75 participants showed no differences between groups post‐intervention (MD 0.59, 95% CI ‐5.5 to 6.68, I2 = 0%, low‐quality evidence) (Chen 2017; Lin 2014) (Analysis 1.1). We performed sensitivity analysis by removing Lin 2014 from the analysis due to unclear risk of bias for allocation concealment. This did not change the result (MD 1.6, 95% CI ‐5.32 to 8.52). The third study compared a telerehabilitation intervention delivered by physiotherapists and occupational therapists, in which the primary aim was restoration of physical function versus a more conventional rehabilitation approach delivered face‐to‐face (Forducey 2012). Both groups received the same dose of therapy. Participants receiving telerehabilitation communicated with the therapist via a desktop videophone connected to a standard home telephone line. The study authors reported that both telerehabilitation and control groups showed statistically significant improvement in activities of daily living. No significant differences in improvement were noted between groups.
Comparison 1.2: Telerehabilitation vs in‐person rehabilitation: balance
Three studies (with 106 participants) compared the effect of a physical therapy programme delivered using telerehabilitation with a physical therapy programme delivered in‐person and reported on balance outcomes (Chen 2017; Lin 2014; Llorens 2015). The effect of telerehabilitation compared to in‐person rehabilitation was found to be equivalent (MD 0.48, 95% CI ‐1.36 to 2.32, I2 = 0%, low‐quality evidence) (Analysis 1.2). Sensitivity analysis, involving removal of Lin 2014 from the analysis due to unclear risk of bias for allocation concealment, did not change the result (MD 0.53, 95% CI ‐1.33 to 2.38).
Telerehabilitation vs in‐person rehabilitation: participant satisfaction with the intervention
Two studies compared satisfaction between telerehabilitation and in‐person therapy (Cramer 2019; Piron 2008). We were unable to obtain the data required to pool these studies; however, both studies reported that participants in the intervention and control groups had high levels of satisfaction with the intervention, although Cramer 2019 reported that those in the telerehabilitation group reported slightly lower levels of satisfaction.
Telerehabilitation vs in‐person rehabilitation: health‐related quality of life
One study compared telehealth delivery of a programme of physiotherapy and occupational therapy with in‐person therapy (Forducey 2012). The investigators reported that although both groups reported improvement in health‐related quality of life, no differences between groups were evident. Data were not available in a form that could be presented in a forest plot.
Comparison 1.3: Telerehabilitation vs in‐person rehabilitation: upper limb function
We pooled three studies which consisted of a total of 170 participants and used a computer software program to retrain upper limb function (Cramer 2019; Piron 2008; Piron 2009). One of the studies compared the intervention versus the same intervention delivered in‐person (Piron 2008), another compared use of a virtual reality program provided via telerehabilitation versus conventional therapy delivered in‐person (Piron 2009), and the final study compared the same dose of therapy and similar content delivered via telerehabilitation or in the clinic (Cramer 2019). Participants in all studies were assessed with the Fugl‐Meyer Upper Extremity Scale post‐intervention. The impact of telerehabilitation on upper limb function was not different from the impact of the control intervention (MD 1.23, 95% CI ‐2.17 to 4.64, I2 = 42%, low‐quality evidence) (Analysis 1.3). Removal of Piron 2008 from the analysis (which was at risk of bias for allocation concealment) did not change the result (MD 1.47, 95% CI ‐2.99 to 5.93).
Comparison 1.4: Telerehabilitation vs in‐person rehabilitation: functional communication
Meltzer 2018 examined the difference between telerehabilitation and in‐person therapy for people with post‐stroke language disorders. The study authors reported that participants in both groups improved significantly on the Western Aphasia Battery aphasia quotient and Cognitive Linguistic Quick Test. There was no difference between groups (MD 1.10, 95% CI ‐2.52 to 4.72) (Analysis 1.4). An additional study looking at communication outcomes after stroke did not clearly present treatment outcomes and did not respond to our requests for more information (Vauth 2016).
Telerehabilitation versus usual care
Comparison 2.1: Telerehabilitation vs usual care: activities of daily living
Two studies, including 661 participants, delivered a post‐hospital discharge support programme, provided via a combination of telephone calls and home visits (Boter 2004; Mayo 2008). The control group received usual care, in which they did not receive any intervention within the research study; however, participants may or may not have received follow‐up from other sources. The estimated effect of telerehabilitation on activities of daily living, as measured by the Barthel Index, was SMD 0.00 (95% CI ‐0.15 to 0.15, I2 = 0%, moderate‐quality evidence) (Analysis 2.1). Removal of Mayo 2008 from the analysis, which was at risk of bias for allocation concealment, did not change the result (SMD 0.02, 95% CI ‐0.16 to 0.19). A third study found no difference between a post‐hospital discharge support programme and usual care on scores on the Frenchay Activities Index, which measures instrumental activities of daily living (Bishop 2014).
A further study compared a combination of technologies (video recordings, in‐home messaging, and phone calls) in an intervention designed to improve functional mobility versus usual care and reported no statistically significant differences between groups after the intervention was provided (Chumbler 2012).
Comparison 2.2: Telerehabilitation vs usual care: mobility
One study, which offered post‐hospital discharge support and case management (Mayo 2008), assessed mobility post‐intervention using the Timed Up and Go test and gait speed and reported no significant differences between groups post‐intervention or at follow‐up six months after stroke (MD 0.01, 95% CI ‐0.12 to 0.14, low‐quality evidence) (Analysis 2.3).
Telerehabilitation vs usual care: participant satisfaction with the intervention
Three studies reported outcomes related to participant satisfaction with the intervention using different scales (Boter 2004; Huijgen 2008; Lin 2014). We were unable to pool data due to the different nature of the measures used and inability to extract data suitable for pooling. One study compared post‐hospital discharge case management provided for up to six months with usual care and reported no significant differences in satisfaction with care between intervention and control groups (Boter 2004). A further study compared telerehabilitation physical therapy with in‐person physical therapy for people residing in long‐term care and found that participants in both groups reported moderately high levels of satisfaction with the intervention and there were no significant differences between groups (Lin 2014).
Comparison 2.3: Telerehabilitation vs usual care: self‐reported health‐related quality of life
Four studies reported outcomes for health‐related quality of life (Boter 2004; Mayo 2008; Rochette 2013; Saal 2015). We were able to pool three of these studies, with 569 participants, which compared post‐hospital discharge support with usual care (Mayo 2008; Rochette 2013; Saal 2015). Analysis showed similar outcomes between groups post‐intervention (SMD 0.03, 95% CI ‐0.14 to 0.20, I 2 = 5%, moderate‐quality evidence) (Analysis 2.3). We did not conduct sensitivity analysis as we considered all studies to be at risk of bias.
We were unable to pool results for the remaining study due to the way in which data were presented. Boter 2004, reported that participants in the intervention group who received a case management intervention had better scores in the domain of 'role limitations due to emotional health' on the Short Form (SF)‐36; however, no other significant differences were noted between groups.
Comparison 2.4: Telerehabilitation vs usual care: depression
Seven studies compared post‐hospital discharge support with usual care and examined the effect on depressive symptoms (Bishop 2014; Boter 2004; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012). We were able to pool data for six of the studies but data were not available in the necessary format for meta‐analysis from the remaining paper or study author (Bishop 2014). Analysis showed the post‐intervention effect of telerehabilitation was not greater than that of usual care when measured using tools quantifying depressive symptoms (SMD ‐0.04, 95% CI ‐0.19 to 0.11. I2 = 31%, moderate‐quality evidence) (Analysis 2.4). Removal of three studies, which we considered at risk of bias due to incomplete outcomes, did not change the result (SMD ‐0.19, 95% CI ‐0.51 to 0.13) (Boter 2004; Rochette 2013; Saal 2015). The remaining paper reported that there was no difference between groups when comparing scores from the Geriatric Depression Scale (short form) (Bishop 2014).
Comparison 2.5 Telerehabilitation vs usual care: upper limb function
Three studies compared telerehabilitation with usual care and assessed upper limb function (Bizovičar 2017; Chumbler 2012; Huijgen 2008). We were unable obtain data in a suitable format for one of the studies (Huijgen 2008). This particular study reported that there were no observed differences between groups on the Action Research Arm Test or the Nine‐Hole Peg Test after intervention (Huijgen 2008). We pooled the other two studies, with 54 participants; the result suggested similar outcomes in both groups (SMD 0.33, 95% CI ‐0.21 to 0.87, I2 = 0%, low‐quality evidence) (Bizovičar 2017; Chumbler 2012).
Costs and cost‐effectiveness of telerehabilitation
No studies included in our original review reported information about the cost‐effectiveness of telerehabilitation (Laver 2013). In this updated version of the review, four studies reported information about treatment costs or service utilisation, or both (Bishop 2014; Llorens 2015; Rochette 2013; Saal 2015). Bishop 2014 found that there was a significant reduction in visits to the doctor (for the stroke survivor and caregiver combined) in those receiving the intervention at the three‐month follow‐up assessment but this was not sustained at six months. Rochette 2013 found no significant differences between groups in unplanned use of health service, and Saal 2015 found no significant differences between groups in relation to medical care (over 3 months) or health service use (over 12 months). Llorens 2015 calculated the cost of the telerehabilitation intervention per participant to be $835.61 compared to $1490.23 for the in‐clinic programme, therefore, the difference in cost between the interventions was $654.72 per participant.
Adverse events
Two studies reported information about adverse events. One study reported that no adverse events occurred (Chen 2017). The other study reported that non‐serious adverse events considered related to the study occurred in six people in the telerehabilitation group (arm and shoulder pain) and five people in the control group (fatigue and arm and shoulder pain) (Cramer 2019).
Studies comparing two different telerehabilitation interventions
Two studies included in the review compared different forms of telerehabilitation (Carey 2007; Deng 2012). Although the main aim of the studies was different, with one study aiming to improve finger and wrist movement (Carey 2007), and the other study aiming to improve ankle movement (Deng 2012), these studies were similar with regard to the method of intervention and the comparison, and were conducted by the same research group. Both studies compared a computer program that provided feedback on movement and accuracy versus a program that provided less feedback. Teleconferencing was used in both studies to enable communication with the therapist. Carey 2007 found that both groups improved on measures of hand function after intervention, with no clear difference noted between the groups. Deng 2012 reported that, after intervention, both groups exhibited an increase in dorsiflexion during gait; this was significantly greater in the group that received more feedback.
Discussion
Summary of main results
We found 22 studies (with 1937 participants) that were eligible for inclusion in this review. Because of clinical heterogeneity between studies, there were few occasions where we were able to pool data.
Independence in activities of daily living
We pooled data from two trials with 661 participants that compared a post‐hospital discharge support programme with usual care. Data from these trials showed with moderate certainty that there was no evidence of a beneficial effect of telerehabilitation when compared with usual care. However, the moderate quality of evidence identified means that further research in this area is likely to change our confidence in the estimate of effect and may change the estimate . We pooled two trials (75 participants) that compared physical therapy provided using telerehabilitation with physical therapy provided in‐person and found only low certainty that there was no difference in outcome between groups. Two additional studies assessed independence in activities of daily living after telerehabilitation interventions (Chumbler 2012; Forducey 2012); one compared telerehabilitation versus face‐to‐face therapy, and the other compared telerehabilitation versus usual care, which may or may not have included any intervention. Both studies failed to find any significant differences in outcomes between the groups post‐intervention.
Secondary outcomes
We pooled three trials with 106 participants that compared telerehabilitation and in‐person rehabilitation and examined the effect on balance (Chen 2017; Lin 2014; Llorens 2015). There was low‐quality evidence of no difference between groups suggesting that neither approach was superior. Three trials that measured quality of life found, with moderate‐quality evidence, that those who received a post‐hospital discharge support programme did not have better outcomes than those that received usual care (Mayo 2008; Rochette 2013; Saal 2015). Similarly, pooling of six studies with 1145 participants found moderate‐quality evidence that those who received a post‐hospital discharge support program did not have lower levels of depression than those that had (Boter 2004; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012).
We pooled three trials with 170 participants that aimed to retrain upper limb function using a computer program administered via telerehabilitation (Cramer 2019; Piron 2008; Piron 2009). These studies were generally small; thus, evidence was insufficient to allow conclusions on whether the intervention was more effective than the comparison upper limb therapy programme.
It was inappropriate to conduct further analyses because of heterogeneity between studies. Limited information and insufficient evidence prevented conclusions regarding the effects of telerehabilitation on mobility, participant satisfaction, or functional communication. This update of the review identified studies that reported information about adverse events and costs; however, the information presented was limited and insufficient for us to reach conclusions about these outcomes.
We were unable to find any data related to our other secondary outcomes of self‐care and domestic life or cognitive function.
Overall completeness and applicability of evidence
The previous version of this review identified 10 studies and this update of the review included 22 randomised trials demonstrating that the field is building, albeit slowly. Furthermore, we noted significant heterogeneity between the included studies with regard to the intervention used, the information and communication technologies involved, and the comparison intervention and outcomes assessed. Many studies involved small sample sizes. All studies were published over the past 15 years, demonstrating that this approach remains relatively new in rehabilitation. However, our review of the 22 trials provides information about the current state of telerehabilitation research. We also identified 22 ongoing studies, which suggests that research in this area is increasing. The quality of the evidence was low for most outcomes suggesting that further research is very likely to have an important impact on our confidence in the estimate of effect. Some comparisons and outcomes provided moderate‐quality evidence which still suggests that more research is required to provide more definitive information.
It is important to note that we excluded many trials because the intervention did not meet our predefined definition of telerehabilitation. There are now many published trials that involve a number of different methods of technology use and communication, and this approach is more likely to be offered by health services in clinical practice. For example, Van den Berg 2016 involved a caregiver‐mediated ehealth programme and video consultations to deliver a rehabilitation programme designed to improve physical function and independence. We excluded this study from our review as, although it involved telerehabilitation, there was a higher number of home visits performed by the therapist than video consultations. Guidelines from the World Health Organization suggest that digital health and non‐digital health interventions are more likely to be packaged together than delivered individually. The World Health Organization stated that digital health interventions are not a substitute for healthcare systems and that this form of service provision has significant limitations (WHO 2019).
Several studies evaluated interventions involving specialised software and hardware programs (Carey 2007; Deng 2012; Huijgen 2008; Lin 2014; Llorens 2015; Meltzer 2018; Piron 2008; Piron 2009). Although these studies provided important information regarding the effects of novel technologies, these intervention programs are not readily accessible to clinicians. As technology develops, purpose‐designed telerehabilitation systems are including more sophisticated features, such as vital sign monitoring and integration with medical records, and it is likely that these features will become more prevalent over time. In contrast, many studies that involve interventions such as counselling, goal‐setting, and case management use simpler methods of communication (such as teleconferencing or videoconferencing using readily available software). It is evident that different therapy approaches require use of different telerehabilitation systems. We found a recent emergence of studies conducted in low‐resources settings that utilise mobile phone technology to offer rehabilitation services to large numbers of people at low cost. However, these studies were either excluded due to their design or have not yet been completed (Kamwesiga 2018; Sureshkumar 2018). Future information about the efficacy of this approach has the potential to have great impact in these settings.
This updated review highlighted the growth in studies that aim to provide enhanced support for people after hospital discharge to reduce depressive symptoms following stroke. These studies offer a mix of psychosocial support, coaching, goal‐setting, education, and case management, and as such, are considered complex intervention trials. To determine the benefit or otherwise of delivery of such interventions using telerehabilitation, further studies that hold the intervention constant and change mode of delivery between groups are required.
Several of the studies in this review were primarily designed to evaluate the delivery of common rehabilitation interventions to stroke survivors via telerehabilitation (Chen 2017; Chumbler 2012; Cramer 2019; Forducey 2012; Lin 2014; Llorens 2015; Meltzer 2018; Vauth 2016). More research is required to investigate whether telerehabilitation can be used as an alternative or as a supplement to conventional therapy that is delivered face‐to‐face. Furthermore, although telerehabilitation is purported to reduce the cost of administering an intervention, none of the studies included in this review reported on cost‐effectiveness. Randomised trials are now beginning to describe the costs of telerehabilitation and compare these costs to the more expensive in‐person model of service delivery. Studies are also examining effect on health service utilisation following intervention, although there is currently insufficient evidence upon which to draw conclusions.
In addition, the studies included in this review provided little information regarding usability of information and communication technologies that are used to deliver telerehabilitation. Most studies used simple telephone or videoconferencing equipment, and few examples were provided of more complex technologies such as wearable sensors or remote monitoring or combinations of technology. Indeed, it is widely acknowledged that mixed‐methods studies are essential in this field in order to evaluate acceptability (for health professionals and healthcare recipients) as well as usability (Greenhalgh 2012).
Participants in these studies tended to be aged in their 50s, 60s, or 70s, whereas the average age of stroke is one to two decades older. It is commonly assumed that older people are less confident in using new technologies and may prefer to participate in face‐to‐face therapy. Some studies excluded patients with cognitive impairment, which may limit the transferability of this approach. None of the studies reported on participants' level of confidence or familiarity with technologies. Other research has suggested that therapists need to use a series of steps to support technology use within rehabilitation, such as positive first experiences and ongoing support (Hamilton 2018). More information is needed regarding the support required to administer telerehabilitation: whether a caregiver is required to assist, how much technology support is required, and whether the person needs to have a certain infrastructure in place (such as a high‐speed Internet connection). Studies rarely reported on these factors or how investigators dealt with issues of privacy and protection of data.
The use of technology to facilitate communication may lead to miscommunication. For example, the healthcare professional may make errors in assessment of the patient, or the patient may misunderstand advice or instructions provided by the healthcare professional. We were unable to identify any information in the included trials regarding harms associated with telerehabilitation, although one study reported that non‐serious adverse events had occurred due to pain and fatigue.
Quality of the evidence
Many studies involved small sample sizes; larger, more adequately‐powered studies are required to provide more conclusive evidence. The reporting of many studies was not consistent with the CONSORT guidelines (Schulz 2010), nor the extension which applies to equivalence trials (Piaggio 2012), and it was unclear in many cases whether studies were at risk of bias because of poor reporting and lack of clarification from study authors. In particular, in some cases, we were unable to determine whether the outcome assessor was blinded to the intervention, or whether allocation was concealed. Selective outcome reporting was apparent in several studies.
Potential biases in the review process
Our search strategy was comprehensive and included searches of clinical trial registers and the grey literature. However, with the sheer volume of citations reviewed, it is possible that we missed studies. Although we contacted the authors of included and ongoing studies, not all study authors responded. Therefore, the methodology of some studies was unclear, and we were unable to obtain some data for analyses.
We did not prespecify the outcome measurement tools that we would accept within this review. Where there were multiple measures used to measure the same outcome in a study, we used the measure which was most commonly used amongst included studies. This method of outcome selection could be open to selection bias. For future updates of the review, we will establish outcome measurements tools that we will include, as well as establish a hierarchy of measures.
Agreements and disagreements with other studies or reviews
This review identified a greater number of randomised trials than were described in previous reviews (Appleby 2019; Chen 2015; Tchero 2018). However, our conclusions are similar: despite the theoretical advantages of telerehabilitation, evidence is currently insufficient to allow conclusions on its effects.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
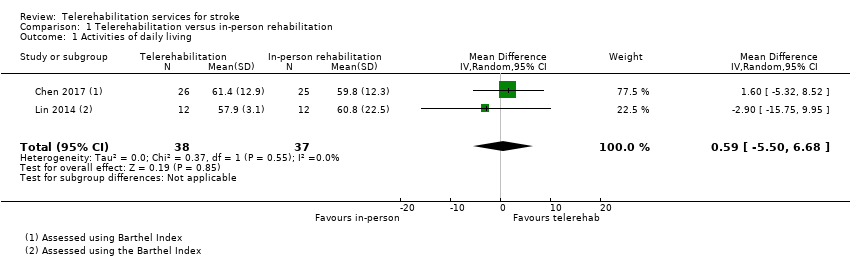
Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 1 Activities of daily living.
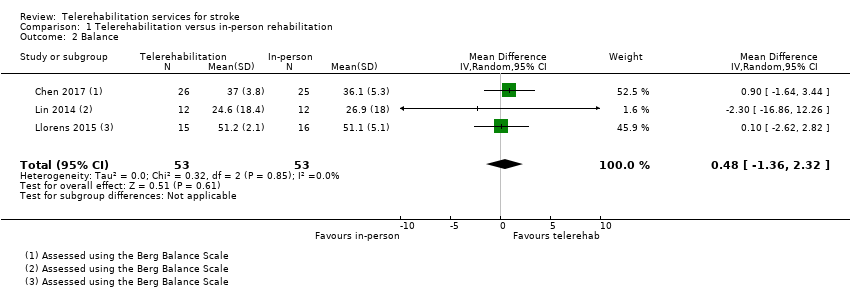
Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 2 Balance.
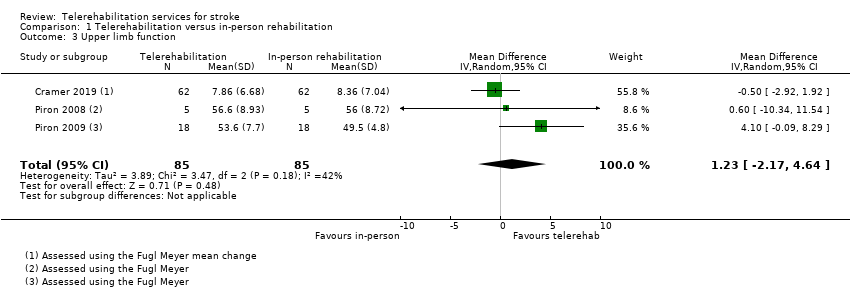
Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 3 Upper limb function.

Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 4 Functional communication.
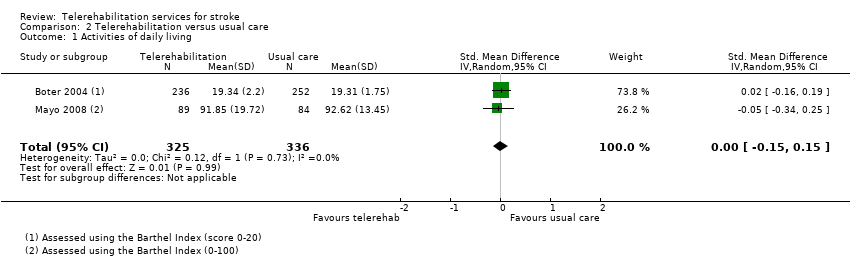
Comparison 2 Telerehabilitation versus usual care, Outcome 1 Activities of daily living.
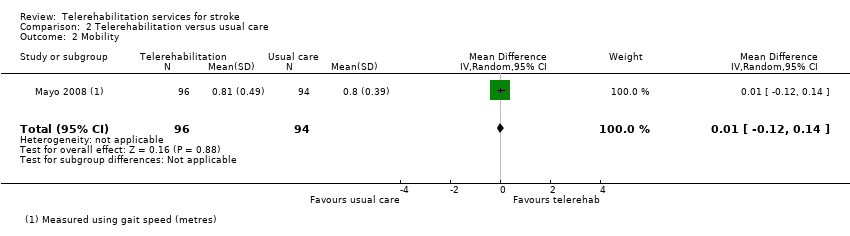
Comparison 2 Telerehabilitation versus usual care, Outcome 2 Mobility.

Comparison 2 Telerehabilitation versus usual care, Outcome 3 Health‐related quality of life.

Comparison 2 Telerehabilitation versus usual care, Outcome 4 Depression.

Comparison 2 Telerehabilitation versus usual care, Outcome 5 Upper limb function.
| Telerehabilitation compared with in‐person rehabilitation for stroke | ||||
| Patient or population: people with stroke Settings: living in the community Intervention: telerehabilitation Comparison: in‐person rehabilitation | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of Participants | Quality of the evidence | Comments |
| Independence in ADL post‐intervention | No significant difference found on total ADL function score: MD 0.59 (‐5.50 to 6.68) (Analysis 1.1) | 75 (2 studies) | ⊕⊕⊝⊝ | |
| Self‐care and domestic life Post‐intervention | Outcome not assessed in included studies | |||
| Mobility post‐intervention | Outcome not assessed in included studies | |||
| Balance post‐intervention | No significant difference found on balance outcomes: MD 0.48 (‐1.36 to 2.32) (Analysis 1.2) | 106 participants (3 studies) | ⊕⊕⊝⊝ | |
| Self‐reported health‐related quality of life post‐intervention | Outcome not assessed in included studies (where the comparison was telerehabilitation versus in‐person rehabilitation) | |||
| Depression post‐intervention | Outcome not assessed in included studies (where the comparison was telerehabilitation versus in‐person rehabilitation) | |||
| Upper limb function post‐intervention | No significant difference found on total UL function score: MD 1.23 (‐2.17 to 4.64) (Analysis 1.3) | 170 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to risk of bias. bDowngraded one level due to imprecision related to small sample size. | ||||
| Telerehabilitation (post hospital discharge support) compared with usual care for stroke | ||||
| Patient or population: people with stroke Settings: living in the community Intervention: telerehabilitation Comparison: usual care | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of Participants | Quality of the evidence | Comments |
| Independence in ADL post‐intervention | No significant difference found on total ADL function score: SMD ‐0.00 (‐0.15 to 0.15) (Analysis 2.1) | 661 | ⊕⊕⊕⊝ | |
| Self‐care and domestic life post‐intervention | Outcome not assessed in included studies | |||
| Mobility post‐intervention | No significant difference found in gait speed: MD 0.01 (‐0.12 to 0.14) (Analysis 2.2) | 144 (1 study) | ⊕⊕⊝⊝ | |
| Balance post‐intervention | Outcome not assessed in included studies | |||
| Self‐reported health‐related quality of life post‐intervention | No significant difference found in self‐reported quality of life: SMD 0.03 (‐0.14 to 0.20) (Analysis 2.3) | 569 | ⊕⊕⊕⊝ | |
| Depression post‐intervention | No significant difference found in depressive symptoms SMD ‐0.04 (‐0.19 to 0.11) (Analysis 2.4) | 1145 | ⊕⊕⊕⊝ | |
| Upper limb function post‐intervention | No significant difference found in upper limb function: SMD 0.33 (‐0.21 to 0.87) (Analysis 2.5) | 54 (2 studies) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to risk of bias. bDowngraded one level due to imprecision related to small sample size. | ||||
| Study | Screened | Randomised | Allocated to intervention group | Allocated to control group | Assessed at follow‐up |
| Not reported | 49 | 23 | 26 | 41 | |
| Not reported | 10 | 5 | 5 | Unclear | |
| 691 | 536 | 263 | 273 | 486 | |
| 167 | 25 | 13 | 12 | 20 | |
| 97 | 54 | 27 | 27 | 50 | |
| 52 | 52 | 27 | 25 | 44 | |
| 232 | 124 | 62 | 62 | 124 analysed | |
| 62 | 19 | 9 | 10 | 16 | |
| Not reported | 11 | Not reported | Not reported | 9 | |
| Not reported | 16 | Not reported | Not reported | Not reported | |
| 416 | 100 | 37 | 35 (in‐person), 28 (usual care) | 91 | |
| 94 | 24 | 12 | 12 | 23 | |
| 115 | 31 | 15 | 16 | 30 | |
| 294 | 190 | 96 | 94 | 157 | |
| Not reported | 53 | 20 | 22 | Unclear | |
| Not reported | 10 | 5 | 5 | 10 | |
| Not reported | 36 | 18 | 18 | 36 | |
| 286 | 186 | 92 | 94 | 139 | |
| 1045 | 265 | 130 | 135 | 230 | |
| 161 | 38 | 19 | 19 | 32 | |
| Unclear | 17 | Unclear | Unclear | Unclear | |
| 186 | 91 | 46 | 45 | 80 |
| Study | Intervention | Comparison | Time after stroke | Country of study |
| Family therapy phone calls to assist with transition home (up to 26 phone calls over 6 months) | Usual care | Not reported however occurred on discharge from hospital to home | USA | |
| Web‐based exercises (posture and upper limb) and therapist weekly consultations | Provision of written exercises without additional therapist contact | Subacute | Slovenia | |
| Case management via 3 telephone calls and a home visit up to 24 weeks after discharge from an acute hospital following stroke | Usual care | Not reported; however, intervention was provided on discharge from acute facility | Netherlands | |
| Upper limb therapy targeting finger and wrist movements provided via a computerised programme in which explicit feedback on performance was provided. Regular teleconferencing occurred between participant and therapist. | Upper limb therapy targeting finger and wrist movements provided via a computerised programme whereby explicit feedback on performance was not provided. Regular teleconferencing occurred between participant and therapist. | Chronic phase | USA | |
| Exercise programme and electrical stimulation which was supervised and monitored remotely via a telerehabilitation system | Same programme of exercises but provided face to face in an outpatient therapy service | Subacute phase | China | |
| A programme designed to improve the person's functional mobility administered via televisits, use of an in‐home messaging device, and 5 telephone calls over a 3‐month period | Usual care | Subacute phase | USA | |
| Telehrehabilitaiton program designed to improve upper limb function and involving on‐screen games | Similar therapy content and same dose of therapy but provided in the clinic | Subacute | USA | |
| Lower limb therapy targeting ankle movements provided via a computerised programme in which explicit feedback on performance was provided. Teleconferencing was used regularly, and performance data were emailed to the therapist. | Lower limb therapy targeting ankle movements provided via a computerised programme whereby explicit feedback on performance was not provided. Teleconferencing was used regularly, and performance data were emailed to the therapist. | Chronic phase | USA | |
| A total of 12 therapy sessions (occupational therapy and physiotherapy) were conducted via a desktop videophone. Interventions included education, retraining of self‐care, functional mobility and posture, home modifications and therapy to improve function in impaired limbs. | The same intervention programme was delivered face‐to‐face. | Not reported | USA | |
| Upper limb therapy using the Home Care Activity Device (computer‐based programme) for 1 month | Usual care and generic exercises were provided by a physician | Chronic phase | Netherlands | |
| Living Well with Stroke intervention designed to reduce depressive symptoms in people with stroke and depression delivered via telephone | Living Well with Stroke (in‐person) Usual care | Subacute | USA | |
| Physical exercises especially balance and lower limbs provided remotely via telerehabilitation system | Similar exercises provided face‐to‐face | Chronic | Taiwan | |
| Virtual reality system used in the home with the aim of improving balance. Remote monitoring and phone call checks | Virtual reality system used in the clinic with the aim of improving balance | Chronic | Spain | |
| Case management intervention provided via home visits and telephone calls for 6 weeks following discharge from acute care | Participants were instructed to make an appointment with their general practitioner. | Acute phase | Canada | |
| Aphasia rehabilitation provided remotely via telerehabilitation | Aphasia rehabilitation provided in‐person | Chronic phase | Canada | |
| Upper limb therapy that was delivered using a virtual reality programme at home and supplemented by videoconferencing | Upper limb therapy that was delivered using a virtual reality programme and conducted in the clinic setting | Chronic phase | Italy | |
| Upper limb therapy that was delivered using a virtual reality telerehabilitation programme and that took place in the home | A programme of conventional upper limb exercises | Chronic phase | Italy | |
| Regular phone calls to discuss family functioning and risk factors after discharge from hospital | Phone number to call health professional if queries | Acute phase | Canada | |
| Stroke support service upon discharge from acute hospital | Usual care | Post‐acute phase | Germany | |
| An intervention to support the caregivers of stroke survivors by enhancing knowledge, skills, and coping. Delivered via email, online chat sessions, and online resources | Participants had access to some of the online resources. | Not reported | USA | |
| Rehabilitation for people with aphasia after discharge from hospital | Conventional therapy | Chronic phase | Germany | |
| Goal‐setting telephone follow‐up relating to health behaviours | Usual stroke education | Subacute phase | China |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities of daily living Show forest plot | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐5.50, 6.68] |
| 2 Balance Show forest plot | 3 | 106 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.36, 2.32] |
| 3 Upper limb function Show forest plot | 3 | 170 | Mean Difference (IV, Random, 95% CI) | 1.23 [‐2.17, 4.64] |
| 4 Functional communication Show forest plot | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐2.52, 4.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities of daily living Show forest plot | 2 | 661 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.15, 0.15] |
| 2 Mobility Show forest plot | 1 | 190 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 3 Health‐related quality of life Show forest plot | 3 | 569 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.14, 0.20] |
| 4 Depression Show forest plot | 6 | 1145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 5 Upper limb function Show forest plot | 2 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.21, 0.87] |

