静脈性下腿潰瘍に対するアルギネート創傷被覆剤
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Design: open, multicenter RCT ‐ 3 centres Country: UK (2 centres) and France (1 centre) Setting: centres specialising in the treatment of leg ulceration Sample size calculation: not reported Ethical approval: not reported Informed consent: not reported | |
| Participants | 44 patients recruited from centres specialising in the treatment of leg ulceration. Inclusion criteria: males and females over 18 years of age presenting with an ulcer of any aetiology ≤ 7.5 cm in diameter, producing moderate to heavy amounts of exudate. Exclusion criteria: none specified
Numbers randomised: Group 1 (hydrocolloid dressing): 21 participants Group 2 (alginate dressing): 23 participants
Mean participant age: Group 1 (hydrocolloid dressing): 71 years (SD 10) Group 2 (alginate dressing): 65 years (SD 11)
Number male: Group 1 (hydrocolloid dressing): 10/21 (48%) Group 2 (alginate dressing): 13/23 (57%)
Number of participants with ulcers of venous, mixed or other aetiology: Group 1 (hydrocolloid dressing): 17/21 (81%), 3/21 (14%), 1/21 (5%) Group 2 (alginate dressing): 19/23 (83%), 3/23 (13%), 1/23 (4%)
ABPI: not reported
Unit of analysis: Participant
Number of participants who had limited mobility or were immobile: Group 1 (hydrocolloid dressing): 13/21 (62%) Group 2 (alginate dressing): 11/23 (48%)
Baseline ulcer area mm2 ‐ median (range): Group 1 (hydrocolloid dressing): 491 (64‐2081) Group 2 (alginate dressing): 611 (60‐1830)
Baseline ulcer duration months ‐ median (range): Group 1 (hydrocolloid dressing): 9 (1‐47) Group 2 (alginate dressing): 12 (1‐120)
Ulcer infection: not reported Participant ulcer history: not reported
Number of participants with heavily exuding ulcers: Group 1 (hydrocolloid dressing): 1/21 (5%) Group 2 (alginate dressing): 5/23 (22%)
Comments: a moderately‐exuding wound was defined as one requiring a dressing change every second day with a conventional dressing (tulle), or every third day with an absorbent dressing. A heavily‐exuding wound was defined as needing a dressing change daily, or more frequently, with a conventional dressing, or every second day with a more absorbent dressing
The trial authors reported that there was an imbalance as participants in Group 1 had ulcers which were smaller and of shorter duration at baseline | |
| Interventions | Group 1: hydrocolloid‐fibrous dressing (Aquacel, manufacturer not reported) Group 2: alginate dressing (Kaltostat, ConvaTec) The dressing was changed if there had been leakage, if infection was suspected, if a participant complained of pain, or once it had been in place for 7 days. A standardised secondary dressing and bandaging regimen applied over the primary dressing consisted of an occlusive hydrocolloid (DuoDerm Extra Thin) as the secondary dressing and, if indicated, orthopaedic padding and a Class 3C compression bandage (Tensopress) Description of compression therapy: Class 3C compression bandage (Tensopress) Length of treatment: 6 weeks, or until healing if sooner Follow‐up: 6 weeks | |
| Outcomes | Review‐relevant outcomes: Time to healing: not reported. Proportion of ulcers healed: reported, photography and planimetry used to measure the wound on enrolment, on days 14 and 28, and on completion of the trial period Change in ulcer size: reported. Healing rate: not reported. Quality of life: not reported. Costs: reported, direct costs (primary dressings, compression treatment and saline wound cleanser) and indirect costs (nurse time, calculated at GBP 2.03 per dressing change) measured. Costs based on 1995 Drug Tariff prices and manufacturer's costs for the hydrocolloid dressing.Total dressing cost was calculated per participant. Mean cost to achieve a healed ulcer was calculated by treatment group. Mean wear time was estimated from the first to last dressing change Pain: reported, pain on dressing removal assessed on a scale of 0‐5 (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = excruciating, 5 = unable to respond). Debridement: not reported. Haemostasis: not reported. Dressing performance ‐ exudate handling: secondary reference mentions 'noting leakage', but exudate levels not reported in the results Dressing performance – adherence/sticking: secondary reference mentions 'ease of removal', but not reported in the results. Adverse events: reported, safety monitored by documenting all adverse events
Other outcomes assessed by the trial: none reported. | |
| Notes | Sponsor: ConvaTec Ltd (trial number not reported) Number participants withdrawing and reasons: Group 1 (hydrocolloid dressing): 5/21 (24%) participant request, 1; adverse events, 4 Group 2 (alginate dressing): 7/23 (30%) all due to adverse events Adverse events that prompted withdrawal described as: bleeding; increased erythema; and deterioration of the ulcer (not reported by group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Twenty‐one patients were randomized to the Hydrocolloid dressing and 23 to the alginate.” (Armstrong 1996) Comment: sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Subjects were then randomized to the primary dressings under investigation by the use of sealed envelopes opened in a numerical order.” Comment: no statements about whether the envelopes were opaque |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “An open, comparative, randomized, multi‐centre trial design was adopted.” (Armstrong 1996) Comment: no statement regarding blinding of participants or study personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “An open, comparative, randomized, multi‐centre trial design was adopted.” (Armstrong 1996) Comment: no statement regarding blinded outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Data were analysed on an intention‐to‐treat basis.” |
| Selective reporting (reporting bias) | High risk | Comment: the reported outcome of proportion of participants achieving a 7‐day wear time was not mentioned in the methods section. Two outcomes in the secondary reference (exudate handling ability of the dressing and ease of removal) were not reported. Trial protocol not available (email communication with trial sponsor). |
| Methods | Design: open, multicenter RCT ‐ 4 centres Country: UK (4 centres) Setting: community Sample size calculation: a sample size of 90 evaluable participants (i.e. either completed the 12 week trial or healed) estimated as having 80% power to detect between‐group difference in mean wear time of 1 day at 5% significance, assuming a SD of 1.7 days Ethical approval: ethical approval was granted from the relevant local ethics committees Informed consent: participants were recruited once written informed consent was obtained | |
| Participants | Recruited 131 participants with moderately‐ to heavily‐exuding leg ulcers. Inclusion criteria: people with moderately‐ to heavily‐exuding leg ulcers of varying aetiology, provided they were suitable for treatment with either dressing. Wound aetiology assessed using patient history and Doppler. Exclusion criteria: people who had been in the trial previously and those with: a known history of poor compliance with treatments, wounds too large for the dressings, or dry eschar on the wound
Numbers randomised: Group 1 (hydrocolloid dressing): 66 participants Group 2 (alginate dressing): 65 participants
Mean participant age (range): Group 1 (hydrocolloid dressing): 75.53 years (35‐93) Group 2 (alginate dressing): 77.6 years (43‐97)
Number male: Group 1 (hydrocolloid dressing): 21/66 (32%) Group 2 (alginate dressing): 12/65 (18%)
Number participants with ulcers of venous, mixed, arterial or diabetic aetiology: Group 1 (hydrocolloid dressing): 54/66 (82%), 9/66 (13%), 2/66 (3%), 1/66 (2%) Group 2 (alginate dressing): 49/65 (75%), 6/65 (10%), 9/65 (13%), 1/66 (2%)
ABPI: not reported Unit of analysis: participant Participant mobility: not reported Baseline ulcer area: not reported Exclusion criteria included wounds that were too large for the dressings. Dressings used were 5 cm x 5 cm and 10 cm x 10 cm
Baseline ulcer duration: not reported Ulcer infection: not reported Participant ulcer history: not reported Participant baseline exudate levels: not reported
Comments: trial authors reported that participants were well matched in terms of baseline wound characteristics, but no data were reported | |
| Interventions | Group 1: hydrocolloid fibre dressing (Aquacel, ConvaTec) Group 2: alginate dressing (Sorbsan®, Maersk) Both dressings available in 5 cm x 5 cm and 10 cm x 10 cm sizes. Both groups received an absorbant pad as a secondary dressing (Release, Johnson & Johnson). Dressings could be left in place for up to 7 days (according to manufacturers' instructions) and were changed according to clinical need. If the wound became infected, dressings in Group 2 were changed daily (manufacturers' instructions). If the wound became infected during the trial, systemic antibiotics were prescribed and the participant remained in the trial. If topical antibiotic treatment was required, the participant was withdrawn from the trial
Description of compression therapy: where clinically indicated, compression provided using orthopaedic padding and a Class 3C elastic bandage (SurePress, ConvaTec)
Length of treatment: 12 weeks, or until healing Follow‐up: participants were followed up until they healed, or for a maximum of 12 weeks | |
| Outcomes | Review relevant outcomes reported:
Time to healing: reported, wound size assessed using acetate tracings. Wound assessments performed on a weekly basis. Analysed using log rank test to compare Kaplan‐Meier survival curves Proportion of ulcers healed: reported Change in ulcer size: reported Healing rate: not reported Quality of life: not reported Costs: reported, cost ‐effectiveness calculated by comparing clinical costs with costs in the International Committee on Wound Management and the Health economic UK Guidelines. All materials used at each dressing change were used to estimate mean costs. Included an additional GBP 15 (USD 21.53) at each dressing change to reflect cost of nursing staff. Costs calculated using May 2000 Drug Tariff prices (with the exception of the orthopaedic wool). Estimated a mean cost associated with a 1 cm2 reduction and a 10% reduction in ulcer area per treatment group, and the mean cost of a healed ulcer Pain: reported, pain on dressing removal was assessed by participant self‐reporting on a four‐point scale ('no pain' to 'severe pain'). Also, pain on dressing removal was assessed by the participant on a “yes/no” basis Debridement: not reported Haemostasis: not reported Dressing performance – exudate handling: reported, assessed by investigator using 4‐point scale ranging from “poor” to “excellent” on a patient‐by‐patient basis Dressing performance – adherence/sticking: reported, assessed by investigator using 4‐point scale ranging from “poor” to “excellent” on a patient‐by‐patient basis. Also, at each dressing change dressings were assessed for adhesion on a "yes/no" basis Adverse events: not reported
Other outcomes assessed by the trial: ease of application and residue observed on dressing change Review authors' comment: the trial authors stated that ease of dressing removal, exudate handling and level of pain on dressing removal were assessed by both the investigator and participant | |
| Notes | Sponsor: ConvaTec (trial number not reported) Participant withdrawal: trial authors did not report on participant withdrawal in the primary reference. Secondary reference reported a larger sample size described as “mainly community based”. Possibility that primary reference only reported on the community‐based participants (not stated)
Number participants withdrawing based on review authors' comparison between primary and secondary references: Group 1 (hydrocolloid dressing): 1/67 (1.5%) Group 2 (alginate dressing): 4/69 (6%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized to one of the following dressings:” Comment: sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization to study treatment using sealed envelopes was made at this point and the patients' wounds dressed accordingly, . . . ” Comment: no statement that envelopes were opaque and sequentially numbered |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “This paper reports the results of an open, prospective, randomized, controlled, multicenter evaluation . . .” Comment: no statement regarding blinding of participants or study personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “Both the investigator and patient measured the secondary outcomes” Comment: no statement regarding blinded outcome assessment of primary outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Data were processed on an 'intention to treat' basis so that all information relating to a subject who participated in the study was retained |
| Selective reporting (reporting bias) | High risk | Quote: ”Both the investigator and patient measured the secondary outcomes.” Comment: only one set of results was presented for the secondary outcomes and it was not reported whether these were assessed by the participants or investigators
Quote: “Overall ease of application and removal was assessed by the investigator using a four‐point scale ranging from ‘poor’ to ‘excellent’ on a patient‐by‐patient basis.” Comment: results were not presented for the full 4‐point scale for ease of application – only the proportion recorded as “excellent” was reported |
| Methods | Design: open RCT Country: USA (2 centres) Setting: outpatients Sample size calculation: not reported Ethical approval: not reported Informed consent: one of the inclusion criteria was that participants should be willing to sign informed consent before enrolment | |
| Participants | Recruited 20 people from wound clinics. Inclusion criteria: outpatients ≥ 21 years, able to understand the product application and assessment procedures, and willing to sign informed consent. Required to have a venous insufficiency ulcer of at least 1 month duration, with area 3 cm2 to 100 cm2 and moderate (3‐5 ml) to large amount (> 5 ml) of exudate. Initial assessment included recording ulcer history and determination of ulcer status (stable, improving or worsening). Exclusion criteria: ABPI < 0.8, uncontrolled diabetes, underlying vasculitis, on immunosuppressive therapy, ulcer showing signs of infection, presence of pre‐existing local skin disease or condition that could affect trial results, or an allergy to the trial materials
Numbers randomised: Group 1 (alginate dressing, Tegagen ™ HG): 11 participants Group 2 (alginate dressing, Sorbsan®): 9 participants
Baseline data were reported for participants completing the trial: Group 1 (Tegagen ™ HG): 10 participants Group 2 (Sorbsan®): 9 participants
Participant age ‐ mean (range): Group 1 (alginate dressing, Tegagen ™ HG): 75.4 years (51‐88) Group 2 (alginate dressing, Sorbsan®): 72.1 years (45‐93)
Number male: Group 1 (alginate dressing, Tegagen ™ HG): 3/10 (30%) Group 2 (alginate dressing, Sorbsan®): 0/9 (0%)
ABPI: ABPI < 0.8 amongst exclusion criteria. No data reported by group. Unit of analysis: participant Participant mobility: not reported
Baseline ulcer area cm2 ‐ mean (range): Group 1 (alginate dressing, Tegagen ™ HG): 6.9 (1.0‐16.8) Group 2 (alginate dressing, Sorbsan®): 8.5 (1.6‐21.7)
Baseline ulcer duration ‐ mean (range): Group 1 (alginate dressing, Tegagen ™ HG): 6.1 months (2‐14) Group 2 (alginate dressing, Sorbsan®): 9.1 months (1‐24)
Participant ulcer history: not reported
Number of ulcers categorised as worsening, stable, improving (exact nature of this variable unclear from RCT report): Group 1 (alginate dressing, Tegagen ™ HG): 4/10 (40%), 6/10 (60%), 0/10 (0%) Group 2 (alginate dressing, Sorbsan®): 7/9 (77.8%), 2/9 (22.2%), 0/9 (0%)
Ulcer infection: participants with ulcers showing signs of infection were excluded.
Number of participants with ulcers with foul odour: Group 1 (alginate dressing, Tegagen ™ HG): 1/10 (10.0%) Group 2 (alginate dressing, Sorbsan®): 2/9 (22.2%)
Number participants with medium to large amounts of exudate: Group 1 (alginate dressing, Tegagen ™ HG): 10/10 (100%) Group 2 (alginate dressing, Sorbsan®): 9/9 (100%) Breakdown by medium and large levels of exudate not reported
Number of participants with purulent serosanguineous exudate: Group 1 (alginate dressing, Tegagen ™ HG): 3/10 (30%) Group 2 (alginate dressing, Sorbsan®): 6/9 (66.7%)
Number of participants with macerated peri‐wound skin: Group 1 (alginate dressing, Tegagen ™ HG): 5/10 (50%) Group 2 (alginate dressing, Sorbsan®): 4/9 (44.4%)
Number of participants with necrotic tissue: Group 1 (alginate dressing, Tegagen ™ HG): 10/10 (100%) Group 2 (alginate dressing, Sorbsan®): 9/9 (100%)
Number of participants with ulcers that required debridement: Group 1 (alginate dressing, Tegagen ™ HG): 7/10 (70%) Group 2 (alginate dressing, Sorbsan®): 3/9 (33.3%)
Comment: recruited some participants with ulcers smaller than those described as eligible for inclusion | |
| Interventions | Group 1: alginate dressing (Tegagen ™ HG (High Gelling),3M) Group 2: alginate dressing (Sorbsan®, Dow Hickman) Need for debridement (surgical debridement not requiring anaesthesia) of fibrin or necrotic tissue from wounds assessed at weekly dressing changes. Dressing changes completed weekly. The secondary dressing (Tegasorb hydrocolloid dressing, 3M) the same for both groups. Applied 3M Cavilon No Sting Barrier Film (3M) if peri‐wound skin was denuded or macerated
Description of compression therapy: Medicopaste Bandage (Graham‐Field) and 3M Coban Self‐Adhesive Wrap (3M)
Length of treatment: 6 weeks, or until ulcer no longer required the use of an alginate dressing Follow‐up: participants followed up for a maximum of 6 weeks, or until the venous leg ulcer no longer required the use of a calcium alginate dressing | |
| Outcomes | Review‐relevant outcomes reported:
Time to healing: not reported Proportion of ulcers healed: reported, wounds assessed weekly using a wound tracing and a photograph Change in ulcer size: reported Healing rate: not reported Quality of life: not reported Costs: reported, recorded the number of dressing changes over the course of the trial Pain: reported, comfort during dressing wear and at removal assessed by asking participants if they experienced itching, pain, or other problems. Dressings rated on a scale of 1 (very good) to 5 (very poor) Debridement: reported, recorded the percentage of visits where necrotic tissue was observed and debridement required. The amount of necrotic tissue was graded as 0 = none, 1 = ≤25%, 2 = 26%‐50%, 3 = 51%‐75%, 4 = 76%‐99%, 5 = 100% Haemostasis: not reported Dressing performance – exudate handling: reported, estimated by observation of the wound and dressing at dressing removal (dry wound; small amount, 1‐2 ml exudate; medium amount, 3‐5 ml exudate; large, > 5 ml exudate). Dressings rated on scale ranging from 1 (very good) to 5 (very poor) in terms of exudate absorption Dressing performance ‐ ease of removal: reported, rated during weekly assessment visits on a scale ranging from 1 (very good) to 5 (very poor). Dressing adherence to wound bed rated as 'yes' or 'no' Adverse events: reported, peri‐wound skin condition classified as normal, denuded, macerated, or flaky/dry. Reported whether peri‐wound skin required medication
Other outcomes assessed by the trial: condition of the wound edge; type and amount of necrotic tissue; amount of granular tissue and epithelialisation; dressing comfort; ease of application; conformability of dressing to the site; itching or other problems; and dressing residue on wound bed | |
| Notes | Sponsor: a 'company sponsor' is mentioned as having provided training to evaluators, but name not provided. Number of participants withdrawing and reasons: Group 1 (alginate dressing, Tegagen ™ HG): 1/11(9%), left after 1 week due to an unrelated adverse event Group 2 (alginate dressing, Sorbsan®): no details regarding participant withdrawal reported, assume 0/9 (0) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients provided informed consent and were randomized to one of the treatment groups according to the protocol randomization schedule.” Comment: sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation method not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “The purpose of this open, randomized, controlled clinical study was to compare the performance characteristics and clinical effect of two calcium alginate dressings in the management of venous leg ulcers:” Comment: no statement regarding blinding of participants or study personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “All wound assessments and dressing performance evaluations were undertaken by either the primary investigator or her designate (RN staff member).” Comment: no statement regarding blinding of the outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “One patient in the Alginate A group (Group 1) left the study after 1 week due to an unrelated adverse event and was not included in the analyses.” Comment: reason for drop‐out stated as unrelated to treatment (although nature of the adverse event not reported) |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes described in the methods section matched those reported in the results section Unable to obtain trial protocol |
| Methods | Design: RCT (pilot study) Country: UK Setting: community Sample size calculation: not reported, trial was a pilot study Ethical approval and informed consent: trial authors described methods used in the discussion section of the paper, and indicated that ethical approval and informed consent were both obtained | |
| Participants | Recruited 60 patients from the community Inclusion criteria: people with ulcers < 10 cm2. All participants had ABPI measurements Exclusion criteria: ABPI < 0.8
Numbers randomised: Group 1 (plain non‐adherent dressing): 30 participants Group 2 (alginate dressing): 30 participants
Median participant age (range): Group 1 (plain non‐adherent dressing): 70 years (38‐88) Group 2 (alginate dressing): 78 years (44‐88)
Number of males: Group 1 (plain non‐adherent dressing): 13/30 (43%) Group 2 (alginate dressing): 10/30 (33%)
ABPI: ABPI < 0.8 an exclusion criterion. No data reported by group Unit of analysis: participant Participant mobility: not reported
Baseline ulcer size ‐ median (range): Group 1 (Plain non‐adherent dressing): 6.4 (1.1‐9.9) Group 2 (alginate dressing): 3.6 (0.9‐9.8) Unit of measurement not stated, assume cm2
Baseline ulcer duration, unit of measurement not stated, assume months ‐ median (range): Group 1 (plain non‐adherent dressing): 3 (1‐20) Group 2 (alginate dressing): 2 (1‐192)
Ulcer infection: not reported Participant ulcer history: not reported Participant baseline exudate levels: not reported | |
| Interventions | Group 1: plain non‐adherent dressing (proprietary name not reported, Johnson and Johnson) Group 2: alginate dressing (Tegagel, 3M) Weekly cleaning and re‐dressing of ulcers unless excessive exudate or infection occurred, in which case dressing and bandage changes were more frequent
Description of compression therapy: All participants fitted with a graduated compression bandaging system to produce and sustain 40 mmHg at the ankle (exact device not specified)
Length of treatment: 12 weeks Follow‐up: 12 weeks | |
| Outcomes | Review‐relevant outcomes reported: Time to healing: reported, healing assessment method not described. Data analysis was by life table, with comparison between‐groups by the log rank method Proportion of ulcers healed: reported Change in ulcer size: not reported Healing rate: not reported Quality of life: not reported Costs: not reported Pain: not reported Debridement: not reported Haemostasis: not reported Dressing performance – exudate handling: not reported Dressing performance – adherence/sticking: not reported Adverse events: not reported Other outcomes assessed by the trial: none reported. | |
| Notes | Sponsor: 3M Health Care Ltd (trial number not reported) Number of participants withdrawing and reasons: trial authors did not report on participant withdrawal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Following assessment for arterial disease, patients were entered into the trial and randomised to either of the two dressing types.” Comment: sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation method not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no statement regarding blinding of participants or study personnel |
| Blinding of outcome assessment (detection bias) | High risk | Quote: “One of the difficulties of running trials of this nature is that the person assessing efficacy cannot be ‘blind’ to the treatment that is being offered to the patient.” Comment: trial authors described the methods used in the discussion, and indicated that outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Analysis based on 'intention to treat' is a standard method.” Comment: trial authors described the methods used in the discussion, and indicated that ITT was undertaken |
| Selective reporting (reporting bias) | High risk | Quote: “Data analysis was by life table for time to healing, with comparison between‐groups by the log rank method.” Comment: trial authors described the methods used to assess time to healing, but no results data presented |
| Methods | Design: open RCT Country: UK Setting: hospital dermatology department Sample size calculation: not reported Ethical approval: not reported Informed consent: recruited consenting adults | |
| Participants | Recruited 40 people from a hospital dermatology department Inclusion criteria: people with a venous leg ulcer > 2.5 cm in diameter Exclusion criteria: any condition that might affect wound healing (infection, immune deficiency, treatment with steroids, malignant disease), ulcer not clearly of venous origin, systemic treatment that might affect ulcer healing (fibrinolytic or anticoagulant therapy), or if other treatment was deemed better
Numbers randomised: Group 1 (hydrocolloid dressing): 22 participants Group 2 (alginate dressing): 18 participants
Baseline participant age, % male, and mobility: The trial authors reported that there were no statistically significant differences at baseline in participants in terms of sex, age or mobility. No data were reported
ABPI: not reported Unit of analysis: participant
Baseline ulcer area cm2 ‐ mean: Group 1 (hydrocolloid dressing): 22.17 Group 2 (alginate dressing): 12.74
Baseline ulcer size, ulcer duration, and participant ulcer history: not reported Baseline ulcer infection: presence of infection was an exclusion criterion
Mean ulcer pain score at baseline (during previous 2weeks, lower score better): Group 1 (hydrocolloid dressing): 4.74 Group 2 (alginate dressing): 4.86
Participant baseline exudate levels: not reported Comments: trial authors commented that the mean ulcer size at enrolment was considerably larger in Group 1 | |
| Interventions | Group 1: hydrocolloid dressing: (Improved Formulation Granuflex, ConvaTec) Group 2: alginate dressing (type and manufacturer not reported) Wounds cleaned with physiological saline and dressings applied according to manufacturers' instructions. Participants re‐attended dermatology clinic for ulcer dressing whenever necessary.
Description of compression therapy: Compression bandaging applied to each participant, but details of type not reported.
Length of treatment: 6 weeks Follow‐up: trial dressing continued for 6 weeks, or until the ulcer healed | |
| Outcomes | Review relevant outcomes reported:
Time to healing: not reported Proportion of ulcers healed: reported, an acetate tracing of the ulcer made on the first and last day of the trial, and the ulcer area calculated using an image analyser Change in ulcer size: reported Healing rate: not reported Quality of life: reported, assessed over the previous 2 weeks at weeks 0, 2, 4 and 6 on a 5‐point scale (deteriorated markedly, deteriorated somewhat, no change, improved somewhat or improved markedly). Name of scale not provided and no mention of using a validated instrument to assess health‐related quality of life Costs: reported, completed a costing sheet, listing all materials used, at each dressing change. Nursing time not included and price year/tariff not provided. Frequency of dressing changes assessed as the mean number of changes over 2 weeks Assessment of wear time mentioned, but no details of assessment provided Pain: reported, pain was assessed over the previous 2 weeks at weeks 2, 4, and 6 using a 10‐point visual analogue scale (0 = no pain to 10 = worse pain). Pain that disturbed sleep assessed (as: often, sometimes, rarely) and also pain at dressing change (10‐point VAS as before) Debridement: not reported Haemostasis: not reported. Dressing performance – exudate handling: reported, the ability of dressings to contain exudate rated on a 6‐point scale (excellent, very good, good, fair, poor or awful) Dressing performance – adherence/sticking: reported, ease of removal assessed on a 6‐point scale (excellent, very good, good, fair, poor, awful). Adverse events: reported, all adverse events recorded, including severe pain and suspected infection of the ulcer
Other outcomes assessed by the trial: convenience of dressing changes; participant comfort; ease of application | |
| Notes | Sponsor: ConvaTec Ltd (trial number not reported)
Number of participants withdrawing and reasons: Group 1 (hydrocolloid dressing): 6/22 (27%) ‐ pain, 1; ulcer infection, 1; possible allergy, 1; dressing leakage, 1; misdiagnosis, 1; participant default, 1 Group 2 (alginate dressing): 6/18 (33%) ‐ pain, 4; ulcer infection, 2 Note: pain, ulcer infection and possible allergy classified by trial authors as adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Eligible patients were allocated randomly to treatment with either the alginate or Granuflex.” Comment: sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation method not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: “It was an open, randomised, parallel group trial . . . " Comment: no statement regarding blinding of participants or study personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “It was an open, randomised, parallel group trial . . . ” Comment: no statement regarding blinded outcome assessment |
| Incomplete outcome data (attrition bias) | High risk | Quote: “Statistical analysis was carried out on results from patients who completed the trial . . . " Quote: “Twelve patients were withdrawn before completion, six on the alginate and six on Granuflex . . . ” Comment: the analysis did not include all randomised participants |
| Selective reporting (reporting bias) | High risk | Comment: mean number of dressing changes assessed over 2‐week periods, but was not reported as such (e.g. "one or two alginate dressings"). Did not report all outcome categories for quality of life assessment, or data for outcomes of exudate handling and ease of dressing removal Trial protocol not available (email communication with trial sponsor) |
Abbreviations
> = greater than
< = less than
≤ = less than or equal to
ITT = intention‐to‐treat analysis
RCT = randomised controlled trial
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not an RCT (review article translated from German). | |
| Animal study. | |
| Alginate dressing not the only systematic difference across treatment arms (different compression systems used at different participating centres). | |
| Compared moist dressings (a mix of hydrocolloids, hydrogels, and alginates) with traditional dressings. Results not available separately for participants receiving alginate dressings. Information confirmed with author (RCT report published in Spanish). | |
| Did not report sufficient information to be judged as eligible for inclusion in this review. Unable to obtain the necessary information to make a judgement (no response to email to sent trial author). | |
| Not treatment of interest (topical agents not alginate dressings). | |
| Alginate dressing not the only systematic difference across treatment groups (alginate group only received secondary absorbant dressing). | |
| Did not report sufficient information to be judged as eligible for inclusion in this review. Unable to obtain the necessary information to make a judgement (no response to email to sent trial author). | |
| Not an RCT (participants allocated to groups on an alternating basis). | |
| Not treatment of interest (comparison of hydrocolloid dressings). | |
| Not an RCT (single‐arm trial of alginate dressings in pressure ulcers and non‐healing burns). | |
| Abstract with limited information. Full RCT report no longer available (email communication with trial author). | |
| Abstract with limited information. Full RCT report no longer available (email communication with trial sponsor). | |
| Not treatment of interest (protease‐modulating matrix dressing). | |
| Not treatment of interest (antifungal agent). | |
| Alginate dressing not the only systematic difference across treatment arms (alginate group only received secondary film dressing that was changed to a sterile swab dressing for 50% of the group half‐way through the trial). | |
| Alginate dressing not the only systematic difference across treatment arms (different secondary dressings used in each treatment group). | |
| Not treatment of interest (freeze‐dried alginate not dressings). | |
| Alginate dressing not the only systematic difference across treatment arms (different compression systems used in each treatment group). | |
| Not an RCT (participants allocated to groups on alternating basis). | |
| Not treatment of interest (hydrocolloid dressing). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 weeks Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.32, 111.04] |
| Analysis 1.1 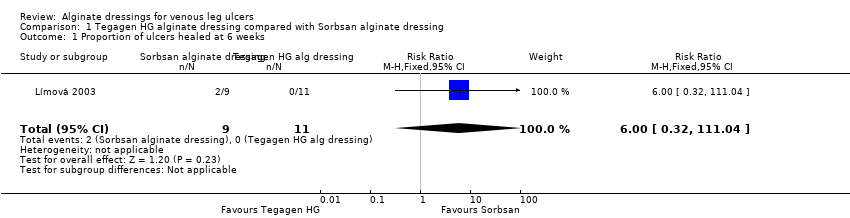 Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 1 Proportion of ulcers healed at 6 weeks. | ||||
| 2 Score for comfort of dressing during wear time at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.29, ‐0.51] |
| Analysis 1.2  Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 2 Score for comfort of dressing during wear time at 6 weeks. | ||||
| 3 Score for comfort during dressing removal at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐0.88, ‐0.52] |
| Analysis 1.3 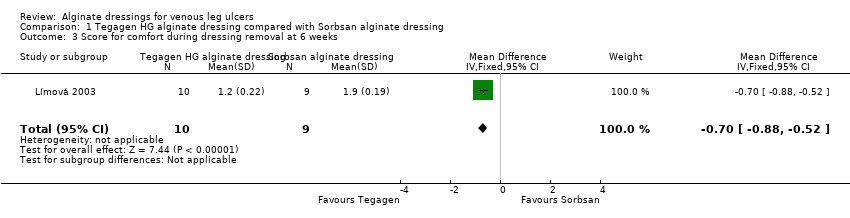 Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 3 Score for comfort during dressing removal at 6 weeks. | ||||
| 4 Exudate absorption score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.22, ‐0.38] |
| Analysis 1.4 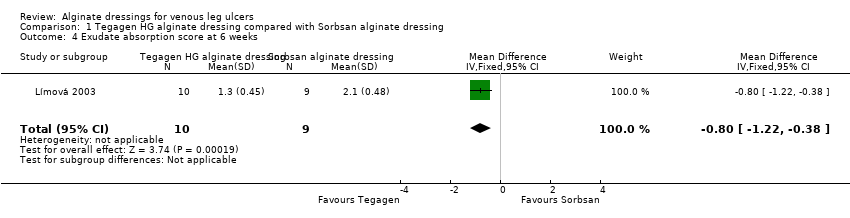 Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 4 Exudate absorption score at 6 weeks. | ||||
| 5 Ease of dressing removal score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.28, ‐0.52] |
| Analysis 1.5  Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 5 Ease of dressing removal score at 6 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 and 12 weeks Show forest plot | 3 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| Analysis 2.1  Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 6 and 12 weeks. | ||||
| 1.1 Healed at 6 weeks | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.21] |
| 1.2 Healed at 12 weeks | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.57, 1.81] |
| 2 Change in ulcer area in mm2 at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐169.56 [‐613.61, 274.49] |
| Analysis 2.2 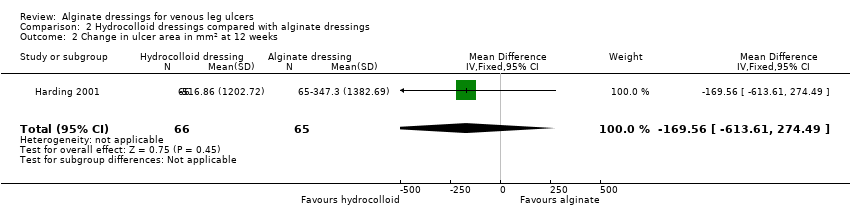 Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 2 Change in ulcer area in mm2 at 12 weeks. | ||||
| 3 Percentage change in ulcer area at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐7.64 [‐37.88, 22.60] |
| Analysis 2.3  Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 3 Percentage change in ulcer area at 12 weeks. | ||||
| 4 Mean wear time (days) Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.29, 1.02] |
| Analysis 2.4  Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 4 Mean wear time (days). | ||||
| 5 Proportion of participants experiencing adverse events Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.53, 4.03] |
| Analysis 2.5 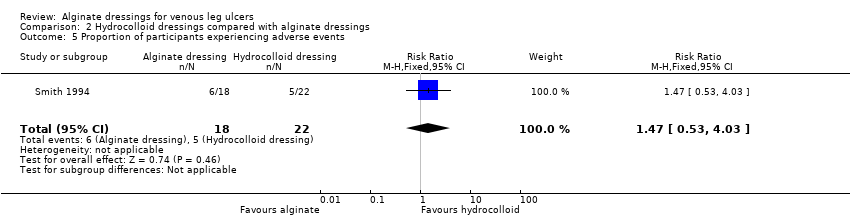 Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 5 Proportion of participants experiencing adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 12 weeks Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.36] |
| Analysis 3.1 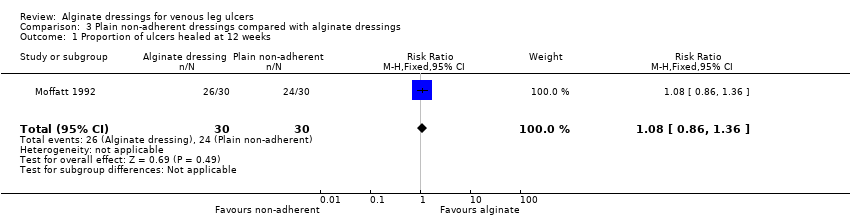 Comparison 3 Plain non‐adherent dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 12 weeks. | ||||

Flow diagram of the trial selection process.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison: 2 Hydrocolloid dressings compared with alginate dressings, outcome: 2.1 Proportion of ulcers healed at 6 and 12 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 1 Proportion of ulcers healed at 6 weeks.
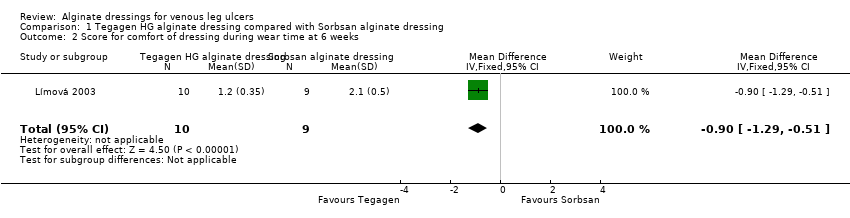
Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 2 Score for comfort of dressing during wear time at 6 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 3 Score for comfort during dressing removal at 6 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 4 Exudate absorption score at 6 weeks.
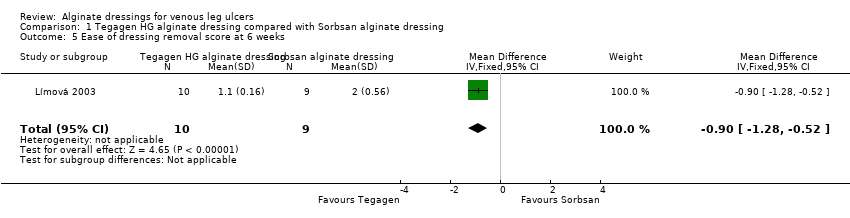
Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 5 Ease of dressing removal score at 6 weeks.

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 6 and 12 weeks.
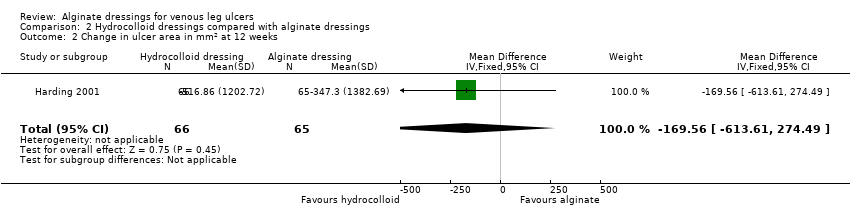
Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 2 Change in ulcer area in mm2 at 12 weeks.
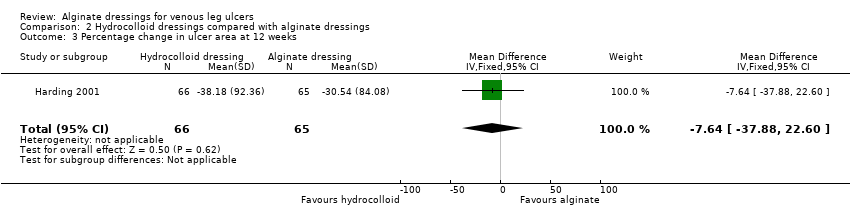
Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 3 Percentage change in ulcer area at 12 weeks.

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 4 Mean wear time (days).

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 5 Proportion of participants experiencing adverse events.

Comparison 3 Plain non‐adherent dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 12 weeks.
| alginate dressing (Sorbsan®) compared to alternative alginate dressing (Tegagen ™ High Gelling) for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alternative alginate dressing (Tegagen ™High Gelling) | Alginate dressing (Sorbsan®) | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Proportion of participants with healed ulcers | Study population1 | RR 6.00 | 20 | ⊕⊝⊝⊝ | ||
| Low1 | ||||||
| 91 per 1000 | 546 per 1000 | |||||
| High1 | ||||||
| 204 per 1000 | 1000 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported (only reported mean percentage change in ulcer area, with no variance estimate, and no adjustment for baseline area). |
| Adverse effects | See comment | See comment | Not estimable | 0 | See comment | Limited information provided. |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 30 days have been taken from a meta‐analysis of RCTs evaluating different types of compression. The low risk estimate is based on a subset of participants with larger baseline ulcer area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). Estimate of baseline risk could not be estimated from study population because no participants healed in the Tegagen ™ HG group. | ||||||
| alginate dressing compared to hydrocolloid dressing for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hydrocolloid dressing | Alginate dressing | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | One RCT presented time to healing, but did not report a reliable estimate (not based on censored data). |
| Proportion of participants with healed ulcers at 6 weeks | Study population1 | RR 0.42 | 84 | ⊕⊝⊝⊝ | ||
| 233 per 1000 | 98 per 1000 | |||||
| Low1 | ||||||
| 91 per 1000 | 38 per 1000 | |||||
| High1 | ||||||
| 204 per 1000 | 86 per 1000 | |||||
| Proportion of participants with healed ulcers at 12 weeks | Study population | RR 1.02 | 131 | ⊕⊝⊝⊝ | ||
| 258 per 1000 | 263 per 1000 | |||||
| Low | ||||||
| 311 per 1000 | 317 per 1000 | |||||
| High | ||||||
| 696 per 1000 | 710 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Three RCTs reported change in wound area, but not with baseline adjustment. |
| Proportion of participants experiencing adverse effects at 6 weeks | 227 per 1000 | 334 per 1000 | RR 1.47 | 40 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | One RCT assessed health‐related quality of life but did not use a validated tool, and only reported percentages of participants who had improved. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 30 days (and 90 days for the 12 week outcome) have been taken from a meta‐analysis of RCTs evaluating different types of compression. The low risk estimate is based on a subset of participants with larger baseline ulcer area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). | ||||||
| alginate dressing compared to plain non‐adherent dressing for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Plain non‐adherent dressing | Alginate dressing | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | Assessment of time to healing mentioned in RCT report, but estimates not provided. |
| Proportion of participants with healed ulcers | Study population1 | RR 1.08 | 60 | ⊕⊝⊝⊝ | ||
| 800 per 1000 | 864 per 1000 | |||||
| Low1 | ||||||
| 311 per 1000 | 336 per 1000 | |||||
| High1 | ||||||
| 696 per 1000 | 752 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Adverse effects | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 90 days have been taken from a meta‐analysis of compression RCTs. The low risk estimate is based on a subset of participants with larger baseline ulcer surface area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). | ||||||
| Number of ulcers healed at 6 weeks: Group 1 (hydrocolloid dressing): 6/21 (29%) Group 2 (alginate dressing): 2/23 (9%) P value for between‐group difference not reported. Change in ulcer size – median change in area: Group 1 (hydrocolloid dressing): ‐205 mm2 Group 2 (alginate dressing): ‐162 mm2 The trial authors reported that the between‐group difference was not statistically significant. P value not reported. Change in ulcer size – median percentage change in ulcer area: Group 1 (hydrocolloid dressing): ‐42% Group 2 (alginate dressing): ‐26% The trial authors reported that the between‐group difference was not statistically significant. P value not reported. Review authors' comment: there was a reporting discrepancy between primary and secondary references for this outcome. The respective values in the secondary reference were ‐30.5% and ‐28.1%. | |
| Time to healing days ‐ mean (SD) [median (range)] (analysis based on healed participants only): Group 1 (hydrocolloid dressing): 41.823 days (SD 21.302) [42 (14 to 87)]; n = 17 Group 2 (alginate dressing): 56.588 days (SD 21.569) [56 (14 to 85)]; n = 17 Reported P value for between‐group difference in means = 0.053 P = 0.05 (log rank test) for difference in Kaplan‐Meier survival curves (analysis based on all randomised patients) Number of ulcers healed at 12 weeks: Group 1 (hydrocolloid dressing): 17/66 (26%) Group 2 (alginate dressing): 17/65 (26%) P value for between‐group difference not reported Change in ulcer area mm2 ‐ mean (SD) [median (range)]: Group 1 (hydrocolloid dressing): ‐516.86 mm2 (SD 1202.72) [‐301.13 (‐2494.84 to 5285.82)]; n = 66 Group 2 (alginate dressing): ‐347.30 mm2 (SD 1382.69) [‐132.83 (‐5144.08 to 5946.24)]; n = 65 P value for between‐group difference in means P = 0.48, reported by trial authors. Percentage change in ulcer area – mean (SD) [median (range)]: Group 1 (hydrocolloid dressing): ‐38.18% (SD 92.36) [‐67.67 (374.84 to ‐100.00)]; n = 66 Group 2 (alginate dressing): ‐30.54% (SD 84.08) [‐43.33 (411.74 to ‐100.00)]; n = 65 P value for between‐group difference in means = 0.64, reported by trial authors | |
| Number of ulcers healed at 6 weeks: Group 1 (alginate dressing, Tegagen ™ HG): 0/11 (0%) Group 2 (alginate dressing, Sorbsan®): 2/9 (22%) P value for between‐group difference not reported. Mean percentage change in wound area at 6 weeks: Group 1 (alginate dressing, Tegagen ™ HG): ‐33.7%, n = 10 Group 2 (alginate dressing, Sorbsan®): ‐29.6%, n = 9 The trial authors reported a P value of 0.88 for between‐group difference | |
| Time to healing/number of ulcers healed at 12 weeks: Group 1 (plain non‐adherent dressing): 24/30 (80%) Group 2 (alginate dressing): 26/30 (87%) P value for between‐group difference not reported The trial authors reported that results were similar for cumulative proportions healed estimated using life table analysis (no data or P value for between‐group difference presented) | |
| Number of ulcers healed at 6 weeks: Group 1 (hydrocolloid dressing): 4/22 (18%) Group 2 (alginate dressing): 2/18 (11%) P value for between‐group difference not reported Mean percentage change in ulcer area: Group 1 (hydrocolloid dressing): ‐57.1 Group 2 (alginate dressing): ‐34.9 The trial authors reported that the between‐group difference was not statistically different. P value not reported |
| Mean wear time days: Group 1 (hydrocolloid dressing): 4.112 days Group 2 (alginate dressing): 3.051 days The trial authors reported a between‐group difference of 1.029 days (95% CI 0.385 to 1.672), and that the difference was statistically significant in favour of Group 1 but the P value was not reported. Reviewer authors' comment: the difference in means reported by the trial authors does not follow from the mean values for each group Number participants achieving a 7‐day wear time on at least one occasion: Group 1 (hydrocolloid dressing): 9/21 (43%) Group 2 (alginate dressing): 3/23 (13%) The trial authors reported that the between‐group difference (30%, 95% CI 5% to 55%) was statistically significant but P value not reported Cost to heal one ulcer: Group 1 (hydrocolloid dressing): GBP 237.66 (total direct and indirect costs = GBP 1425.97 for total of 6 wounds healed) Group 2 (alginate dressing): GBP 687.31 (total direct and indirect costs = GBP 1374.61 for total of 2 wounds healed) Cost per wound healed calculated by review authors. Taking into account the number of participants completely healed in each group, the trial authors reported that the cost to achieve a healed wound using the Group 1 dressing was approximately one‐third of the cost of Group 2. Number dressing changes with no pain, mild pain, moderate pain, severe pain, excruciating pain, unable to respond, missing data: Group 1 (hydrocolloid dressing): (total of 192 dressing changes) 144 (75%), 38 (20%), 6 (3%), 2 (1%), 0 (0%), 0 (0%), 2 (1%) Group 2 (alginate dressing): (total of 224 dressing changes) 186 (83%), 29 (13%), 8 (3.5%), 0 (0%), 0 (0)%, 0 (0%), 1 (0.5%) Number of adverse events during the trial: Group 1 (hydrocolloid dressing): 32 adverse events reported during the trial. 4 were related to the primary dressing and 28 to the secondary dressing, of which 8 were attributed to maceration. Group 2 (alginate dressing): 32 adverse events reported during the trial. 3 were related to the primary dressing and 29 to the secondary dressing, of which 9 were attributed to maceration. | |
| Total number of dressing changes: Group 1 (hydrocolloid dressing): 1093 Group 2 (alginate dressing): 1186 Mean wear time days (SD) [median (range)]: Group 1 (hydrocolloid dressing): 3.632 days (1.878) [3 (1 to 13)]; n = 66 Group 2 (alginate dressing): 3.271 days (1.944) [3 (1 to 9)]; n = 65 The trial authors reported that the between‐group difference in means was P value < 0.001 Mean number of dressing changes per healed ulcer: Group 1 (hydrocolloid dressing): 7.4 Group 2 (alginate dressing): 12.1 P value for between‐group difference not reported Mean cost to achieve ulcer healing (based on patients healed): Group 1 (hydrocolloid dressing): GBP 1184.09 (USD 1699.71) Group 2 (alginate dressing): GBP 1200.73 (USD 1723.59) Mean cost per 1cm2 reduction in ulcer size (all patients randomised): Group 1 (hydrocolloid dressing): GBP 59.22 (USD 85.01) Group 2 (alginate dressing): GBP 92.27 (USD 132.46) Mean cost per 10% reduction in ulcer area: Group 1 (hydrocolloid dressing): GBP 80.15 (USD 115.06) Group 2 (alginate dressing): GBP 104.92 (USD 150.62) Percentage of dressing changes associated with no pain: Group 1 (hydrocolloid dressing): 82% Group 2 (alginate dressing): 62% The trial authors reported that the between‐group difference was statistically significant ( P value < 0.001). Numbers of dressings not reported Dressing performance – percentage recording “excellent” for overall ability to contain exudate: Group 1 (hydrocolloid dressing): 44% Group 2 (alginate dressing): 20% Unclear whether denominator was the number of participants or the number of dressing changes. Reported P value for between‐group difference = 0.002 Dressing performance – percentage recording “excellent” for overall ease of dressing removal: Group 1 (hydrocolloid dressing): 51% Group 2 (alginate dressing): 24% Unclear whether denominator was the number of participants or the number of dressing changes. Reported P value for between‐group difference = 0.006 Percentage of dressing changes with some adhesion to the wound bed: Group 1 (hydrocolloid dressing): 38% Group 2 (alginate dressing): 74% The trial authors reported that the between‐group difference was statistically significant (P value < 0.001) Number of dressing changes not reported Review authors' comments: there were some minor discrepancies between numbers in main text and tables for cost information (data from main text were recorded here); discrepancies between primary and secondary references for outcomes of ease of removal and exudate handling (data from primary reference recorded here); unclear whether reported outcomes relating to pain at dressing change, exudate handling, ease of dressing removal and adhesion were rated by participants or investigators (or both). | |
| Total number of dressing changes over the course of the trial: Group 1 (alginate dressing, Tegagen ™ HG): 69 (65 scheduled and 4 unscheduled) Group 2 (alginate dressing, Sorbsan®): 61 (60 scheduled and 1 unscheduled) Mean (SD) comfort score during wear over number of visits: Group 1 (alginate dressing, Tegagen ™ HG): 1.2 (SD 0.35) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.1 (SD 0.50) over 51 visits; n = 9 The trial authors reported a P value of 0.0005 for the between‐group difference Mean (SD) comfort score during dressing removal over number of visits: Group 1 (alginate dressing, Tegagen ™ HG): 1.2 (SD 0.22) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 1.9 (SD 0.19) over 51 visits; n = 9 The trial authors reported a P value of 0.003 for the between‐group difference Percentage of visits where necrotic tissue was observed: Group 1 (alginate dressing, Tegagen ™ HG): 59.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 68.9% of 51 visits; n = 9 The trial authors reported a P value of 0.57 for the between‐group difference Percentage of visits where debridement was required: Group 1 (alginate dressing, Tegagen ™ HG): 18.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 40.7% of 51 visits; n = 9 The trial authors reported a P value of 0.18 for the between‐group difference Mean improvement in amount of necrotic tissue (lower score is better): Group 1 (alginate dressing, Tegagen ™ HG): 2.5 over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 1.5 over 51 visits; n = 9 The trial authors reported a P value of 0.38 for the between‐group difference Mean (SD) exudate absorption score over number of visits (lower score better): Group 1 (alginate dressing, Tegagen ™ HG): 1.3 (SD 0.45) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.1 (SD 0.48) over 51 visits; n = 9 The trial authors reported a P value of 0.002 for the between‐group difference Percentage of clinic visits with medium or large amount of exudate observed: Group 1 (alginate dressing, Tegagen ™ HG): 71.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 86.3% of 51 visits; n = 9 The trial authors reported a P value of 0.25 for the between‐group difference Mean (SD) ease of removal score over number of visits (lower is better): Group 1 (alginate dressing, Tegagen ™ HG): 1.1 (SD 0.16) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.0 (SD 0.56) over 51 visits; n = 9 The trial authors reported a P value of 0.002 for the between‐group difference Proportion of participants reporting dressing adherence to the wound bed: Group 1 (alginate dressing, Tegagen ™ HG): 0% Group 2 (alginate dressing, Sorbsan®): 27.8% The trial authors reported that the average percentage of dressing changes with adherence to the wound bed was significantly less in Group 1 (P value < 0.05). Percentage of clinic visits with observation of peri‐wound skin as macerated, denuded, requiring medication: Group 1 (alginate dressing, Tegagen ™ HG): 36.0%, 9.0%, 31.3% Group 2 (alginate dressing, Sorbsan®): 54.4%, 31.9%, 65.2% Reported P values for between‐group difference: macerated skin P value 0.30; denuded skin P value 0.04, medication required P value 0.07 | |
| None reported | |
| Proportion of participants “improved remarkably” in quality of life at week 6: Group 1 (hydrocolloid dressing): 42.9% Group 2 (alginate dressing): 40.0% The trial authors did not report the number of participants completing the quality of life assessment. P value for between‐group difference not reported. There was a reporting discrepancy between “improved remarkably” and the pre‐defined categories for this outcome (deteriorated markedly, deteriorated somewhat, no change, improved somewhat or improved markedly) Number of dressings used per week: Group 1 (hydrocolloid dressing): 1‐3 Group 2 (alginate dressing): 1‐2 Wear time: The trial authors reported that both dressings were equivalent in terms of wear time, but no data by group or P value for between‐group difference were reported. Mean total approximate cost of materials: Group 1 (hydrocolloid dressing): GBP 431.73 Group 2 (alginate dressing): GBP 364.08 P value for between‐group difference not reported Mean ulcer pain score over past 2 weeks at 6 weeks: Group 1 (hydrocolloid dressing): 1.46 Group 2 (alginate dressing): 2.15 The trial authors reported that the between‐group difference was not statistically significant. P value not reported Change from baseline in mean ulcer pain score over past 2 weeks at 6 weeks: Group 1 (hydrocolloid dressing): ‐3.28 Group 2 (alginate dressing): ‐2.71 Estimated by review authors Mean pain score at dressing change at week 6: Group 1 (hydrocolloid dressing): 1.73 Group 2 (alginate dressing): 2.16 P value for between‐group difference not reported Change from baseline in mean pain score at dressing change at 6 weeks: Not reported and reviewer unable to estimate as data at week 6 only reported (no baseline) Proportion of participants reporting no sleep disturbance due to pain at week 2 and week 6: Group 1 (hydrocolloid dressing): 31.25%, 78.6% Group 2 (alginate dressing): 8.8%, 40.0% The trial authors did not report whether the proportions were for all participants enrolled or only those completing the trial. Reported P value for between‐group difference = 0.0721, but unclear to which time point this refers or if it is for the test across time points Dressing performance (exudate handling): The trial authors reported that the Group 2 dressing was “slightly superior” in terms of ability to contain exudate. However, no data by group or P value for between‐group difference were reported Proportion of participants reporting ‘excellent’ for ease of dressing removal: Group 1 (hydrocolloid dressing): 56.3% Group 2 (alginate dressing): 8.3% The trial authors did not report raw numbers and the denominator was not clear. Reported P value for between‐group difference was < 0.001. The trial authors reported that the Group 2 dressing often needed to be soaked off the ulcer. Number participants experiencing adverse events: Group 1 (hydrocolloid dressing): 5/22 (23%) (withdrawals: pain, 1; ulcer infection, 1; possible allergy, 1; not thought to warrant withdrawal ‐ wound infection, 1; pain and erythema at the final visit, 1) Group 2 (alginate dressing): 6/18 (33%) (withdrawals: pain, 4; ulcer infection, 2) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 weeks Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.32, 111.04] |
| 2 Score for comfort of dressing during wear time at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.29, ‐0.51] |
| 3 Score for comfort during dressing removal at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐0.88, ‐0.52] |
| 4 Exudate absorption score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.22, ‐0.38] |
| 5 Ease of dressing removal score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.28, ‐0.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 and 12 weeks Show forest plot | 3 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 1.1 Healed at 6 weeks | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.21] |
| 1.2 Healed at 12 weeks | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.57, 1.81] |
| 2 Change in ulcer area in mm2 at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐169.56 [‐613.61, 274.49] |
| 3 Percentage change in ulcer area at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐7.64 [‐37.88, 22.60] |
| 4 Mean wear time (days) Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.29, 1.02] |
| 5 Proportion of participants experiencing adverse events Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.53, 4.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 12 weeks Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.36] |

