静脈性下腿潰瘍に対するアルギネート創傷被覆剤
Appendices
Appendix 1. Glossary
The source for all definitions is The Free Dictionary 2015.
Aetiology: the underlying cause of diseases and disorders.
Autolytic: the destruction of tissues or cells of an organism by the action of substances, such as enzymes, that are produced within the organism.
Debride/debridement: the removal of foreign material and dead or damaged tissue from a wound.
Exudate: fluid, which leaks out of a wound.
Fibrinolytic therapy: the use of special drugs to break up blood clots.
Haemodynamic(s): the study of the forces involved in the circulation of blood.
Haemostatic: retarding or stopping bleeding.
Macerate/maceration: the softening and breaking down of skin resulting from prolonged exposure to moisture.
Slough: a layer or mass of dead tissue separated from surrounding living tissue, as in a wound, a sore, or an inflammation.
Vasoactive: causing constriction or dilation of blood vessels.
Appendix 2. British National Formulary categories of dressings
Basic wound contact dressings
Low‐adherence dressings
Low‐adherence dressings are usually cotton pads that are placed directly in contact with the wound. These dressings can be used as interface layers under secondary absorbent dressings and are suitable for clean, granulating, lightly exuding wounds without necrosis; they protect the wound bed from direct contact with secondary dressings. Variants include tulle dressings (manufactured from cotton or viscose fibres impregnated with soft white or yellow paraffin) and knitted viscose dressings (BNF 2015). Examples include Atrauman® (Hartmann) and Tricotex® (Smith and Nephew).
Absorbent dressings
Absorbent dressings have an absorbent cellulose or polymer wadding layer and are suitable for use on moderately to heavily exuding wounds. These may be applied directly to the wound and may be used as secondary absorbent layers in the management of heavily exuding wounds (BNF 2015). Examples include DryMax® Extra (Aspen Medical) and KerraMax® (Ark Therapeutics).
Advanced wound dressings
Hydrogel dressings
Hydrogel dressings consist of cross‐linked insoluable polymers (i.e. starch or carboxymethylcellulose) and up to 96% water. They are supplied in flat sheets, or as an amorphous hydrogel or as beads. These dressings are generally used to donate liquid to dry, sloughy wounds and facilitate autolytic debridement of necrotic tissue. Some also have the ability to absorb very small amounts of exudate. A secondary, non‐absorbent dressing is required with these dressings (BNF 2015). Examples include ActiFormCool® (Activa) and Intrasite Hydrosorb® (Hartmann).
Vapour‐permeable films and dressings
Vapour‐permeable dressings come in the form of a transparent film, usually with an adhesive base, which is applied to the wound. Vapour‐permeable films and membranes allow the passage of water vapour and oxygen but are impermeable to water and micro‐organisms. They are suitable for lightly‐exuding wounds, but not for infected, large, heavily‐exuding wounds. Most commonly, they are used as a secondary dressing over alginates or hydrogels (BNF 2015). Examples include OpSite® (Smith and Nephew) and Tegaderm® (3M).
Soft polymer dressings
Dressings with soft polymer, often a soft silicone polymer, in a non‐adherent or gently‐adherent layer are suitable for use on lightly‐to moderately‐exuding wounds. For moderately‐ to heavily‐exuding wounds, an absorbent secondary dressing can be added, or a soft polymer dressing with an absorbent pad can be used (BNF 2015). Examples include Mepilex® (Mölnlycke) and Urgotul® (Urgo).
Hydrocolloid dressings
Hydrocolloid dressings are occlusive dressings usually composed of a hydrocolloid matrix bonded onto a vapour‐permeable film or foam backing. When in contact with wound exudate these dressings form a gel to facilitate rehydration in lightly‐ to moderately‐exuding wounds (BNF 2015). Examples include DuoDERM® Extra Thin (ConvaTec) and Tegaderm® Hydrocolloid (3M).
Foam dressings
Foam dressings contain hydrophilic foam and are suitable for all types of exuding wounds. They vary in their ability to absorb exudates; some are suitable only for lightly to moderately exuding wounds, while others have a greater fluid‐handling capacity. These can be used in combination with other primary wound contact dressings, but are usually placed over ulcers prior to the application of compression bandages or hosiery, with the intention of promoting healing, and preventing the bandages from sticking to the wound. Saturated foam dressings can cause maceration of healthy skin if left in contact with the wound. If used under compression bandaging or compression garments, the fluid‐handling capacity of foam dressings may be reduced (BNF 2015). Examples include and Biatain® Non‐Adhesive (Coloplast) and Tielle® Plus (Systagenix).
Alginate dressings
Alginate dressings are available as flat, freeze‐dried porous sheets, or as flexible fibre dressings (e.g. packing tape), designed for packing cavity wounds. The base constituents include calcium alginate or calcium sodium alginate, derived from brown seaweed. Alginate dressings are designed to form a soft gel once in contact with wound exudate. Purported benefits of alginate dressings include high absorbency of fluid from moderately to heavily exuding wounds and the ability to maintain a moist wound environment, thereby promoting autolytic debridement (the body's own process of breaking down dead tissue lying on top of the wound bed). Calcium ions present in the dressings help to control bleeding by aiding blood clotting; a potential disadvantage is that blood clots may cause the dressing to adhere to the wound surface. Alginate dressings are designed to function most effectively in a moist environment, and are not suitable for use with dry wounds, or those covered with hard, necrotic tissue; heavy bleeding is a contraindication to use (BNF 2015; Boateng 2008). In the UK, alginate dressings are common to many wound care formularies, where they are often recommended for the management of wounds with moderate to high amounts of exudate. Examples of alginate dressings currently available in the UK include Algosteril® (Smith and Nephew) and Tegaderm® Alginate (3M).
Capillary‐action dressings
Capillary‐action dressings consist of an absorbent core of hydrophilic fibres held between two low‐adherent wound‐contact layers to ensure no fibres are shed on to the wound surface. Wound exudate is taken up by the dressing and retained within the highly absorbent central layer. Capillary‐action dressings are suitable for use on all types of exuding wounds, but particularly on sloughy wounds where removal of fluid from the wound aids debridement (BNF 2015). Examples include Advadraw® (Advancis) and Vacutex® (Protex).
Odour‐absorbant dressings
Dressings containing activated charcoal are used to absorb odour from wounds. Many odour absorbent dressings are intended for use in combination with other dressings, and are often used in conjunction with a secondary dressing to improve absorbency (BNF 2015). Examples include CarboFLEX® (ConvaTec) and CliniSorb® Odour Control Dressings (CliniMed).
Antimicrobial dressings
Honey
Medical‐grade honey has antimicrobial and anti‐inflammatory properties and can be used for acute or chronic wounds. It is available in sheet‐dressing form or as a honey‐base topical application applied directly to the wound and covered with a primary low‐adherence wound dressing (BNF 2015). Examples include Medihoney® (Medihoney) and Mesitran® (Aspen Medical).
Iodine
Iodine has a long history of use as a skin and wound disinfectant. It is thought to have a wide spectrum of antimicrobial activity but is deactivated by wound exudate. There are currently two types of iodine‐based preparations used in wound management: povidone iodine and cadexomer iodine. Povidone iodine is available as various topical applications (solution, ointment and spray) and as an impregnated wound dressing. The impregnated dressing may be used as a wound contact layer for abrasions and superficial burns, and has also been used with chronic wounds (BNF 2015; Jeffcoate 2009). An example is Inadine® (Systagenix). Cadexomer iodine is available as different topical applications (paste, ointment and powder) and is used to absorb exudate and for debridement (BNF 2015). Examples include Iodoflex® (Smith and Nephew) and Iodosorb® (Smith and Nephew).
Silver
Antimicrobial dressings containing silver are available as: low‐adherent dressings, with knitted fabric of activated charcoal, soft polymer dressings, hydrocolloid dressings, foam dressings and alginate dressings. Silver ions exert an antimicrobial effect in the presence of wound exudate. Antimicrobial dressings containing silver should be used only when infection is suspected on the basis of clinical signs or symptoms (BNF 2015). Examples include Acticoat® (Smith and Nephew), Aquacel® Ag (ConvaTec) and Biatain® Ag (Coloplast).
Other antimicrobial dressings
A number of dressings that are impregnated with other antimicrobial agents, such as chlorhexidine and polyhexanide, are also available (BNF 2015). Examples include chlorhexidine gauze dressing, BP 1993 and Suprasorb® X + PHMB (Activa).
Specialised dressings
Protease‐modulating matrix dressings
Protease‐modulating matrix dressings alter the activity of proteolytic enzymes in chronic wounds (BNF 2015). Examples include Promogran® (Systagenix) and UrgoStart® (Urgo).
Appendix 3. Search strategies for Ovid Medline, Ovid Embase and EBSCO CINAHL
Ovid Medline
1 exp Alginates/ (4449)
2 (alginate* or activheal or algisite or algosteril or curasorb or kalostat or melgisorb or seasorb or sorbalgon or sorbsan or suprasorb a or tegaderm or tegagel or urgosorb).tw. (6053)
3 or/1‐2 (6601)
4 exp Leg Ulcer/ (9728)
5 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw. (3571)
6 or/4‐5 (10421)
7 3 and 6 (68)
8 randomized controlled trial.pt. (238104)
9 controlled clinical trial.pt. (39335)
10 randomized.ab. (193802)
11 placebo.ab. (90703)
12 clinical trials as topic.sh. (79028)
13 randomly.ab. (133232)
14 trial.ti. (71766)
15 or/8‐14 (538944)
16 (animals not (humans and animals)).sh. (1600596)
17 15 not 16 (490866)
18 7 and 17 (21)
19 2012*.ed. (680299)
20 18 and 19 (3)
Ovid Embase
1 exp alginic acid/ (8396)
2 (alginate* or activheal or algisite or algosteril or curasorb or kalostat or melgisorb or seasorb or sorbalgon or sorbsan or suprasorb a or tegaderm or tegagel or urgosorb).tw. (9024)
3 or/1‐2 (11076)
4 exp leg ulcer/ (5951)
5 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw. (5370)
6 or/4‐5 (7825)
7 3 and 6 (121)
8 Randomized controlled trials/ (22788)
9 Single‐Blind Method/ (15232)
10 Double‐Blind Method/ (84730)
11 Crossover Procedure/ (31212)
12 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. (920231)
13 (doubl$ adj blind$).ti,ab. (88524)
14 (singl$ adj blind$).ti,ab. (9443)
15 or/8‐14 (952716)
16 animal/ (716985)
17 human/ (8455793)
18 16 not 17 (478469)
19 15 not 18 (920958)
20 7 and 19 (27)
21 2012*.em. (1182739)
22 20 and 21 (1)
EBSCO CINAHL
S7S3 AND S6
S6S4 OR S5
S5TI ( alginate* or activheal or algisite or algosteril or curasorb or kalostat or melgisorb or seasorb or sorbalgon or sorbsan or suprasorb a or tegaderm or tegagel or urgosorb ) OR AB ( alginate* or activheal or algisite or algosteril or curasorb or kalostat or melgisorb or seasorb or sorbalgon or sorbsan or suprasorb a or tegaderm or tegagel or urgosorb )
S4(MH "Alginates")
S3S1 OR S2
S2TI (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris) OR AB (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris)
S1(MH "Leg Ulcer+")
Appendix 4. Dressing manufacturers contacted regarding ongoing or recently completed trials of alginate dressings
3M
Activa
Aspen Medical
Coloplast
ConvaTec
Covidien
Hartmann
MedLogic
Mölnlycke
Smith & Nephew Healthcare
Urgo
Appendix 5. Data extraction form
| Trial identifier | First author and year (country where trial undertaken) |
| Methods | Design (number of study centres): e.g. pragmatic RCT (5 study centres). Sample size calculation: yes or no, if yes, summarise estimation details. Treatment setting. Ethical approval reported: yes or no. Informed consent reported: yes or no. |
| Participant characteristics at baseline | Number of patients randomised overall and recruitment setting: Inclusion criteria: Ulcer diagnosis method: e.g. Doppler Exclusion criteria:
Unit of randomisation/analysis:
Numbers randomised per treatment group: Group 1 (comparator dressing): xx participants/limbs/ulcers Group 2 (alginate dressing): xx participants/limbs/ulcers
Participant age in years – mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Number (%) male: Group 1 (comparator dressing): Group 2 (alginate dressing):
Number (%) of participants with co‐morbidities such as diabetes: Group 1 (comparator dressing): Group 2 (alginate dressing): Number (%) of participants with ulcer aetiology venous/mixed/arterial/other: Group 1 (comparator dressing): Group 2 (alginate dressing): ABPI: Group 1 (comparator dressing): Group 2 (alginate dressing):
Number (%) of participants according to different categories of mobility (e.g. fully mobile, walks with aid, confined to bed or wheelchair): Group 1 (comparator dressing): Group 2 (alginate dressing):
Health‐related quality of life score (instrument) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Number (%) of participants with previous history of leg ulceration: Group 1 (comparator dressing): Group 2 (alginate dressing):
Ulcer surface area (in, e.g. cm2) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Ulcer duration (in, e.g. months) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Number (%) of participants with ulcer infection: Group 1 (comparator dressing): Group 2 (alginate dressing):
Ulcer‐related pain score (instrument) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Percentage of sloughy or necrotic material on the wound bed ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Participant baseline exudate levels (instrument) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Comments: |
| Intervention characteristics | Group 1: description of comparator dressing, e.g. hydrocolloid dressing (proprietary name, manufacturer)
Group 2: description of alginate dressing (proprietary name, manufacturer)
Details of other care, common to all treatment groups (e.g. wound cleansing, debridement, compression, also planned frequency of dressing changes)
Length of treatment:
Length of follow‐up:
Comments: |
| Outcomes | Reporting of outcomes specified in the review – yes or no; if yes, give details of assessment and statistical methods: Time to healing: Proportion of ulcers healed: Change in ulcer size: Healing rate: Health‐related quality of life: Costs: e.g. including cost or cost‐effectiveness estimations as well as measurements of resource use such as number of dressing changes, dressing wear time and nurse time Pain: e.g. at dressing change, in between dressing changes or over the course of treatment Debridement: e.g. measured as percentage of sloughy or necrotic material remaining on the wound bed Haemostasis: management of bleeding Dressing performance – exudate handling: Dressing performance – adherence/sticking to wound bed: Adverse events – number per group together with descriptions: Other outcomes assessed by the trial: |
| Outcome data | Time to healing (in, e.g. weeks) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Number (%) of ulcers healed at (state time point): Group 1 (comparator dressing): Group 2 (alginate dressing):
Change in ulcer size (either as absolute change, e.g. in cm2 or as % change relative to baseline) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Healing rate (e.g. cm2 per week) ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Health‐related quality of life score ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Cost or other resource use estimation ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Ulcer‐related pain score ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Debridement, e.g. percentage of sloughy or necrotic material on the wound bed ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Haemostasis, as reported: Group 1 (comparator dressing): Group 2 (alginate dressing):
Dressing performance (exudate handling) exudate levels/score ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Dressing performance (adherence/sticking to wound bed), using score as reported ‐ mean (SD) [median (range)]: Group 1 (comparator dressing): Group 2 (alginate dressing):
Number (%) patients experiencing adverse events (with description of events): Group 1 (comparator dressing): Group 2 (alginate dressing):
Comments: |
| Notes | Name of trial sponsor: Trial registration number:
Number (%) participants withdrawing and reasons: Group 1 (comparator dressing): Group 2 (alginate dressing): |
Appendix 6. 'Risk of bias' criteria
1. Was the allocation sequence randomly generated?
Low risk of bias: the investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias: the investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear risk of bias: insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias: participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias: participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. envelopes were unsealed or non‐opaque or not sequentially‐numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially‐numbered, opaque and sealed.
3. Blinding of participants and personnel ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias: no blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding or blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
High risk of bias: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit judgement of ‘low risk’ or ‘high risk’, or the study did not address this outcome.
4. Blinding of outcome assessment ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
High risk of bias: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Unclear risk of bias: insufficient information to permit judgement of ‘low risk’ or 'high risk’, or the study did not address this outcome.
5. Were incomplete outcome data adequately addressed?
Low risk of bias: no missing outcome data, or reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias), or missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; or (for dichotomous outcome data), the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; or (for continuous outcome data), plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; or missing data have been imputed using appropriate methods.
High risk of bias: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; or (for dichotomous outcome data), the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; or (for continuous outcome data), plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; or ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation, or potentially inappropriate application of simple imputation.
Unclear risk of bias: insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided), or the study did not address this outcome.
6. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias: either the trial protocol is available and all of the pre‐specified outcomes that are in the protocol have been reported in the pre‐specified manner or, if the protocol is not available, it is clear that the published report includes all outcomes in the results section that are described as being assessed in the methods section.
High risk of bias: if the trial protocol is available either, not all of the pre‐specified outcomes that are in the protocol are reported, or one or more outcomes are reported using measurements, analysis methods or subsets of the data that are not pre‐specified; or one or more reported outcomes were not pre‐specified (unless there is justification for their reporting, such as an unexpected adverse event). If the trial protocol is not available either, not all of the trial’s outcomes have been reported in the results section that are described in the methods section, or one or more of the outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not described in the methods section of the report; or one or more of the reported outcomes were not described in the methods section.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.

Flow diagram of the trial selection process.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison: 2 Hydrocolloid dressings compared with alginate dressings, outcome: 2.1 Proportion of ulcers healed at 6 and 12 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 1 Proportion of ulcers healed at 6 weeks.
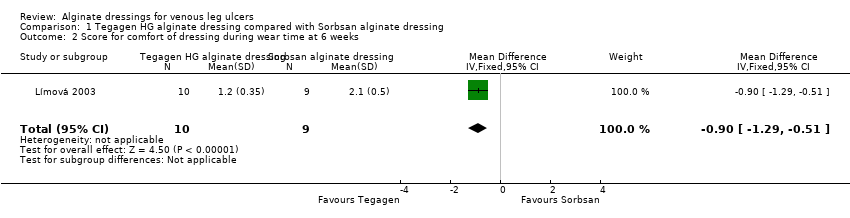
Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 2 Score for comfort of dressing during wear time at 6 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 3 Score for comfort during dressing removal at 6 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 4 Exudate absorption score at 6 weeks.
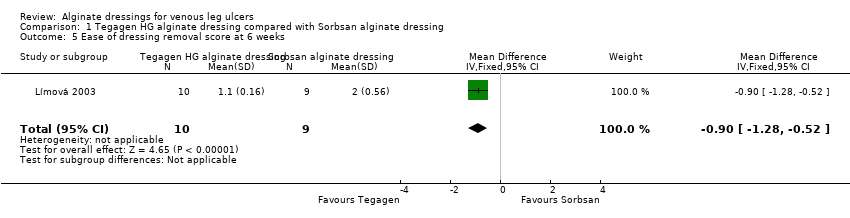
Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 5 Ease of dressing removal score at 6 weeks.

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 6 and 12 weeks.
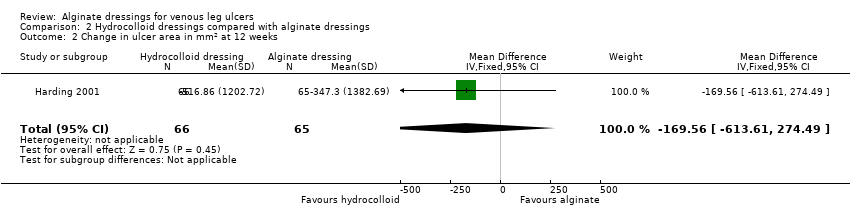
Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 2 Change in ulcer area in mm2 at 12 weeks.
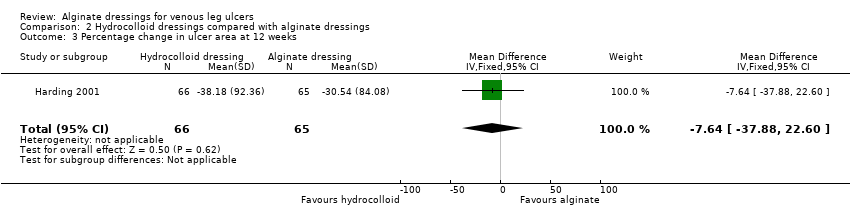
Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 3 Percentage change in ulcer area at 12 weeks.

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 4 Mean wear time (days).

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 5 Proportion of participants experiencing adverse events.

Comparison 3 Plain non‐adherent dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 12 weeks.
| alginate dressing (Sorbsan®) compared to alternative alginate dressing (Tegagen ™ High Gelling) for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alternative alginate dressing (Tegagen ™High Gelling) | Alginate dressing (Sorbsan®) | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Proportion of participants with healed ulcers | Study population1 | RR 6.00 | 20 | ⊕⊝⊝⊝ | ||
| Low1 | ||||||
| 91 per 1000 | 546 per 1000 | |||||
| High1 | ||||||
| 204 per 1000 | 1000 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported (only reported mean percentage change in ulcer area, with no variance estimate, and no adjustment for baseline area). |
| Adverse effects | See comment | See comment | Not estimable | 0 | See comment | Limited information provided. |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 30 days have been taken from a meta‐analysis of RCTs evaluating different types of compression. The low risk estimate is based on a subset of participants with larger baseline ulcer area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). Estimate of baseline risk could not be estimated from study population because no participants healed in the Tegagen ™ HG group. | ||||||
| alginate dressing compared to hydrocolloid dressing for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hydrocolloid dressing | Alginate dressing | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | One RCT presented time to healing, but did not report a reliable estimate (not based on censored data). |
| Proportion of participants with healed ulcers at 6 weeks | Study population1 | RR 0.42 | 84 | ⊕⊝⊝⊝ | ||
| 233 per 1000 | 98 per 1000 | |||||
| Low1 | ||||||
| 91 per 1000 | 38 per 1000 | |||||
| High1 | ||||||
| 204 per 1000 | 86 per 1000 | |||||
| Proportion of participants with healed ulcers at 12 weeks | Study population | RR 1.02 | 131 | ⊕⊝⊝⊝ | ||
| 258 per 1000 | 263 per 1000 | |||||
| Low | ||||||
| 311 per 1000 | 317 per 1000 | |||||
| High | ||||||
| 696 per 1000 | 710 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Three RCTs reported change in wound area, but not with baseline adjustment. |
| Proportion of participants experiencing adverse effects at 6 weeks | 227 per 1000 | 334 per 1000 | RR 1.47 | 40 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | One RCT assessed health‐related quality of life but did not use a validated tool, and only reported percentages of participants who had improved. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 30 days (and 90 days for the 12 week outcome) have been taken from a meta‐analysis of RCTs evaluating different types of compression. The low risk estimate is based on a subset of participants with larger baseline ulcer area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). | ||||||
| alginate dressing compared to plain non‐adherent dressing for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Plain non‐adherent dressing | Alginate dressing | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | Assessment of time to healing mentioned in RCT report, but estimates not provided. |
| Proportion of participants with healed ulcers | Study population1 | RR 1.08 | 60 | ⊕⊝⊝⊝ | ||
| 800 per 1000 | 864 per 1000 | |||||
| Low1 | ||||||
| 311 per 1000 | 336 per 1000 | |||||
| High1 | ||||||
| 696 per 1000 | 752 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Adverse effects | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 90 days have been taken from a meta‐analysis of compression RCTs. The low risk estimate is based on a subset of participants with larger baseline ulcer surface area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). | ||||||
| Number of ulcers healed at 6 weeks: Group 1 (hydrocolloid dressing): 6/21 (29%) Group 2 (alginate dressing): 2/23 (9%) P value for between‐group difference not reported. Change in ulcer size – median change in area: Group 1 (hydrocolloid dressing): ‐205 mm2 Group 2 (alginate dressing): ‐162 mm2 The trial authors reported that the between‐group difference was not statistically significant. P value not reported. Change in ulcer size – median percentage change in ulcer area: Group 1 (hydrocolloid dressing): ‐42% Group 2 (alginate dressing): ‐26% The trial authors reported that the between‐group difference was not statistically significant. P value not reported. Review authors' comment: there was a reporting discrepancy between primary and secondary references for this outcome. The respective values in the secondary reference were ‐30.5% and ‐28.1%. | |
| Time to healing days ‐ mean (SD) [median (range)] (analysis based on healed participants only): Group 1 (hydrocolloid dressing): 41.823 days (SD 21.302) [42 (14 to 87)]; n = 17 Group 2 (alginate dressing): 56.588 days (SD 21.569) [56 (14 to 85)]; n = 17 Reported P value for between‐group difference in means = 0.053 P = 0.05 (log rank test) for difference in Kaplan‐Meier survival curves (analysis based on all randomised patients) Number of ulcers healed at 12 weeks: Group 1 (hydrocolloid dressing): 17/66 (26%) Group 2 (alginate dressing): 17/65 (26%) P value for between‐group difference not reported Change in ulcer area mm2 ‐ mean (SD) [median (range)]: Group 1 (hydrocolloid dressing): ‐516.86 mm2 (SD 1202.72) [‐301.13 (‐2494.84 to 5285.82)]; n = 66 Group 2 (alginate dressing): ‐347.30 mm2 (SD 1382.69) [‐132.83 (‐5144.08 to 5946.24)]; n = 65 P value for between‐group difference in means P = 0.48, reported by trial authors. Percentage change in ulcer area – mean (SD) [median (range)]: Group 1 (hydrocolloid dressing): ‐38.18% (SD 92.36) [‐67.67 (374.84 to ‐100.00)]; n = 66 Group 2 (alginate dressing): ‐30.54% (SD 84.08) [‐43.33 (411.74 to ‐100.00)]; n = 65 P value for between‐group difference in means = 0.64, reported by trial authors | |
| Number of ulcers healed at 6 weeks: Group 1 (alginate dressing, Tegagen ™ HG): 0/11 (0%) Group 2 (alginate dressing, Sorbsan®): 2/9 (22%) P value for between‐group difference not reported. Mean percentage change in wound area at 6 weeks: Group 1 (alginate dressing, Tegagen ™ HG): ‐33.7%, n = 10 Group 2 (alginate dressing, Sorbsan®): ‐29.6%, n = 9 The trial authors reported a P value of 0.88 for between‐group difference | |
| Time to healing/number of ulcers healed at 12 weeks: Group 1 (plain non‐adherent dressing): 24/30 (80%) Group 2 (alginate dressing): 26/30 (87%) P value for between‐group difference not reported The trial authors reported that results were similar for cumulative proportions healed estimated using life table analysis (no data or P value for between‐group difference presented) | |
| Number of ulcers healed at 6 weeks: Group 1 (hydrocolloid dressing): 4/22 (18%) Group 2 (alginate dressing): 2/18 (11%) P value for between‐group difference not reported Mean percentage change in ulcer area: Group 1 (hydrocolloid dressing): ‐57.1 Group 2 (alginate dressing): ‐34.9 The trial authors reported that the between‐group difference was not statistically different. P value not reported |
| Mean wear time days: Group 1 (hydrocolloid dressing): 4.112 days Group 2 (alginate dressing): 3.051 days The trial authors reported a between‐group difference of 1.029 days (95% CI 0.385 to 1.672), and that the difference was statistically significant in favour of Group 1 but the P value was not reported. Reviewer authors' comment: the difference in means reported by the trial authors does not follow from the mean values for each group Number participants achieving a 7‐day wear time on at least one occasion: Group 1 (hydrocolloid dressing): 9/21 (43%) Group 2 (alginate dressing): 3/23 (13%) The trial authors reported that the between‐group difference (30%, 95% CI 5% to 55%) was statistically significant but P value not reported Cost to heal one ulcer: Group 1 (hydrocolloid dressing): GBP 237.66 (total direct and indirect costs = GBP 1425.97 for total of 6 wounds healed) Group 2 (alginate dressing): GBP 687.31 (total direct and indirect costs = GBP 1374.61 for total of 2 wounds healed) Cost per wound healed calculated by review authors. Taking into account the number of participants completely healed in each group, the trial authors reported that the cost to achieve a healed wound using the Group 1 dressing was approximately one‐third of the cost of Group 2. Number dressing changes with no pain, mild pain, moderate pain, severe pain, excruciating pain, unable to respond, missing data: Group 1 (hydrocolloid dressing): (total of 192 dressing changes) 144 (75%), 38 (20%), 6 (3%), 2 (1%), 0 (0%), 0 (0%), 2 (1%) Group 2 (alginate dressing): (total of 224 dressing changes) 186 (83%), 29 (13%), 8 (3.5%), 0 (0%), 0 (0)%, 0 (0%), 1 (0.5%) Number of adverse events during the trial: Group 1 (hydrocolloid dressing): 32 adverse events reported during the trial. 4 were related to the primary dressing and 28 to the secondary dressing, of which 8 were attributed to maceration. Group 2 (alginate dressing): 32 adverse events reported during the trial. 3 were related to the primary dressing and 29 to the secondary dressing, of which 9 were attributed to maceration. | |
| Total number of dressing changes: Group 1 (hydrocolloid dressing): 1093 Group 2 (alginate dressing): 1186 Mean wear time days (SD) [median (range)]: Group 1 (hydrocolloid dressing): 3.632 days (1.878) [3 (1 to 13)]; n = 66 Group 2 (alginate dressing): 3.271 days (1.944) [3 (1 to 9)]; n = 65 The trial authors reported that the between‐group difference in means was P value < 0.001 Mean number of dressing changes per healed ulcer: Group 1 (hydrocolloid dressing): 7.4 Group 2 (alginate dressing): 12.1 P value for between‐group difference not reported Mean cost to achieve ulcer healing (based on patients healed): Group 1 (hydrocolloid dressing): GBP 1184.09 (USD 1699.71) Group 2 (alginate dressing): GBP 1200.73 (USD 1723.59) Mean cost per 1cm2 reduction in ulcer size (all patients randomised): Group 1 (hydrocolloid dressing): GBP 59.22 (USD 85.01) Group 2 (alginate dressing): GBP 92.27 (USD 132.46) Mean cost per 10% reduction in ulcer area: Group 1 (hydrocolloid dressing): GBP 80.15 (USD 115.06) Group 2 (alginate dressing): GBP 104.92 (USD 150.62) Percentage of dressing changes associated with no pain: Group 1 (hydrocolloid dressing): 82% Group 2 (alginate dressing): 62% The trial authors reported that the between‐group difference was statistically significant ( P value < 0.001). Numbers of dressings not reported Dressing performance – percentage recording “excellent” for overall ability to contain exudate: Group 1 (hydrocolloid dressing): 44% Group 2 (alginate dressing): 20% Unclear whether denominator was the number of participants or the number of dressing changes. Reported P value for between‐group difference = 0.002 Dressing performance – percentage recording “excellent” for overall ease of dressing removal: Group 1 (hydrocolloid dressing): 51% Group 2 (alginate dressing): 24% Unclear whether denominator was the number of participants or the number of dressing changes. Reported P value for between‐group difference = 0.006 Percentage of dressing changes with some adhesion to the wound bed: Group 1 (hydrocolloid dressing): 38% Group 2 (alginate dressing): 74% The trial authors reported that the between‐group difference was statistically significant (P value < 0.001) Number of dressing changes not reported Review authors' comments: there were some minor discrepancies between numbers in main text and tables for cost information (data from main text were recorded here); discrepancies between primary and secondary references for outcomes of ease of removal and exudate handling (data from primary reference recorded here); unclear whether reported outcomes relating to pain at dressing change, exudate handling, ease of dressing removal and adhesion were rated by participants or investigators (or both). | |
| Total number of dressing changes over the course of the trial: Group 1 (alginate dressing, Tegagen ™ HG): 69 (65 scheduled and 4 unscheduled) Group 2 (alginate dressing, Sorbsan®): 61 (60 scheduled and 1 unscheduled) Mean (SD) comfort score during wear over number of visits: Group 1 (alginate dressing, Tegagen ™ HG): 1.2 (SD 0.35) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.1 (SD 0.50) over 51 visits; n = 9 The trial authors reported a P value of 0.0005 for the between‐group difference Mean (SD) comfort score during dressing removal over number of visits: Group 1 (alginate dressing, Tegagen ™ HG): 1.2 (SD 0.22) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 1.9 (SD 0.19) over 51 visits; n = 9 The trial authors reported a P value of 0.003 for the between‐group difference Percentage of visits where necrotic tissue was observed: Group 1 (alginate dressing, Tegagen ™ HG): 59.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 68.9% of 51 visits; n = 9 The trial authors reported a P value of 0.57 for the between‐group difference Percentage of visits where debridement was required: Group 1 (alginate dressing, Tegagen ™ HG): 18.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 40.7% of 51 visits; n = 9 The trial authors reported a P value of 0.18 for the between‐group difference Mean improvement in amount of necrotic tissue (lower score is better): Group 1 (alginate dressing, Tegagen ™ HG): 2.5 over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 1.5 over 51 visits; n = 9 The trial authors reported a P value of 0.38 for the between‐group difference Mean (SD) exudate absorption score over number of visits (lower score better): Group 1 (alginate dressing, Tegagen ™ HG): 1.3 (SD 0.45) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.1 (SD 0.48) over 51 visits; n = 9 The trial authors reported a P value of 0.002 for the between‐group difference Percentage of clinic visits with medium or large amount of exudate observed: Group 1 (alginate dressing, Tegagen ™ HG): 71.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 86.3% of 51 visits; n = 9 The trial authors reported a P value of 0.25 for the between‐group difference Mean (SD) ease of removal score over number of visits (lower is better): Group 1 (alginate dressing, Tegagen ™ HG): 1.1 (SD 0.16) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.0 (SD 0.56) over 51 visits; n = 9 The trial authors reported a P value of 0.002 for the between‐group difference Proportion of participants reporting dressing adherence to the wound bed: Group 1 (alginate dressing, Tegagen ™ HG): 0% Group 2 (alginate dressing, Sorbsan®): 27.8% The trial authors reported that the average percentage of dressing changes with adherence to the wound bed was significantly less in Group 1 (P value < 0.05). Percentage of clinic visits with observation of peri‐wound skin as macerated, denuded, requiring medication: Group 1 (alginate dressing, Tegagen ™ HG): 36.0%, 9.0%, 31.3% Group 2 (alginate dressing, Sorbsan®): 54.4%, 31.9%, 65.2% Reported P values for between‐group difference: macerated skin P value 0.30; denuded skin P value 0.04, medication required P value 0.07 | |
| None reported | |
| Proportion of participants “improved remarkably” in quality of life at week 6: Group 1 (hydrocolloid dressing): 42.9% Group 2 (alginate dressing): 40.0% The trial authors did not report the number of participants completing the quality of life assessment. P value for between‐group difference not reported. There was a reporting discrepancy between “improved remarkably” and the pre‐defined categories for this outcome (deteriorated markedly, deteriorated somewhat, no change, improved somewhat or improved markedly) Number of dressings used per week: Group 1 (hydrocolloid dressing): 1‐3 Group 2 (alginate dressing): 1‐2 Wear time: The trial authors reported that both dressings were equivalent in terms of wear time, but no data by group or P value for between‐group difference were reported. Mean total approximate cost of materials: Group 1 (hydrocolloid dressing): GBP 431.73 Group 2 (alginate dressing): GBP 364.08 P value for between‐group difference not reported Mean ulcer pain score over past 2 weeks at 6 weeks: Group 1 (hydrocolloid dressing): 1.46 Group 2 (alginate dressing): 2.15 The trial authors reported that the between‐group difference was not statistically significant. P value not reported Change from baseline in mean ulcer pain score over past 2 weeks at 6 weeks: Group 1 (hydrocolloid dressing): ‐3.28 Group 2 (alginate dressing): ‐2.71 Estimated by review authors Mean pain score at dressing change at week 6: Group 1 (hydrocolloid dressing): 1.73 Group 2 (alginate dressing): 2.16 P value for between‐group difference not reported Change from baseline in mean pain score at dressing change at 6 weeks: Not reported and reviewer unable to estimate as data at week 6 only reported (no baseline) Proportion of participants reporting no sleep disturbance due to pain at week 2 and week 6: Group 1 (hydrocolloid dressing): 31.25%, 78.6% Group 2 (alginate dressing): 8.8%, 40.0% The trial authors did not report whether the proportions were for all participants enrolled or only those completing the trial. Reported P value for between‐group difference = 0.0721, but unclear to which time point this refers or if it is for the test across time points Dressing performance (exudate handling): The trial authors reported that the Group 2 dressing was “slightly superior” in terms of ability to contain exudate. However, no data by group or P value for between‐group difference were reported Proportion of participants reporting ‘excellent’ for ease of dressing removal: Group 1 (hydrocolloid dressing): 56.3% Group 2 (alginate dressing): 8.3% The trial authors did not report raw numbers and the denominator was not clear. Reported P value for between‐group difference was < 0.001. The trial authors reported that the Group 2 dressing often needed to be soaked off the ulcer. Number participants experiencing adverse events: Group 1 (hydrocolloid dressing): 5/22 (23%) (withdrawals: pain, 1; ulcer infection, 1; possible allergy, 1; not thought to warrant withdrawal ‐ wound infection, 1; pain and erythema at the final visit, 1) Group 2 (alginate dressing): 6/18 (33%) (withdrawals: pain, 4; ulcer infection, 2) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 weeks Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.32, 111.04] |
| 2 Score for comfort of dressing during wear time at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.29, ‐0.51] |
| 3 Score for comfort during dressing removal at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐0.88, ‐0.52] |
| 4 Exudate absorption score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.22, ‐0.38] |
| 5 Ease of dressing removal score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.28, ‐0.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 and 12 weeks Show forest plot | 3 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 1.1 Healed at 6 weeks | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.21] |
| 1.2 Healed at 12 weeks | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.57, 1.81] |
| 2 Change in ulcer area in mm2 at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐169.56 [‐613.61, 274.49] |
| 3 Percentage change in ulcer area at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐7.64 [‐37.88, 22.60] |
| 4 Mean wear time (days) Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.29, 1.02] |
| 5 Proportion of participants experiencing adverse events Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.53, 4.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 12 weeks Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.36] |

