Apósitos de alginato para la úlcera venosa de la pierna
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010182.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 19 agosto 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Susan O’Meara is guarantor of the review. She conceived, designed and co‐ordinated the original review; extracted the data, checked the quality of data extraction, undertook and checked quality assessment and analysed and interpreted data. She performed statistical analysis and checked quality of statistical analysis; completed the first draft of the review; made an intellectual contribution to, and approved final review prior to submission, and advised on the review. She performed previous work that was the foundation of the current review. She screened records for inclusion and edited the review for the first update.
Marrisa Martin St. James contributed the following to the original review: extracted data, undertook quality assessment and analysed data; contributed to writing or editing of the review; made an intellectual contribution to the review and wrote to study authors/experts/companies.
Una Adderley screened records for inclusion and edited the review for the first update.
Contributions of editorial base
Nicky Cullum: edited the protocol; advised on methodology, interpretation and content.
Joan Webster (Editor): approved the final review prior to submission.
Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited the protocol and review versions.
Ruth Foxlee: designed the search strategy, edited the search methods section and ran the searches.
Sources of support
Internal sources
-
Department of Health Sciences, University of York, UK.
External sources
-
NIHR Programme Grants for Applied Research, UK.
-
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant Reference Number RP‐PG‐0407‐10428). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgements
We are very grateful to the following peer referees who provided valuable feedback on the draft of the first version of this review: Una Adderley; Laura Bolton; Jac Dinnes; Ruth Foxlee; Elmer Villanueva; and Joan Webster. Thanks are also due to Belen Corbacho and Jeppe Schroll for help with translation, and to Elizabeth Royle who copy edited the review. Finally, we should like to express appreciation for the support we have received from the staff of the Cochrane Wounds Group ‐ to Ruth Foxlee and Rocio Rodriguez‐Lopez for advising on the search strategy and running the database searches, and to Sally Bell‐Syer for helpful advice and assistance with preparing the draft review.
CRG Funding Acknowledgement:
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Wounds Group.
Disclaimer:
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Aug 19 | Alginate dressings for venous leg ulcers | Review | Susan O'Meara, Marrissa Martyn‐St James, Una J Adderley | |
| 2013 Apr 30 | Alginate dressings for venous leg ulcers | Review | Susan O'Meara, Marrissa Martyn‐St James | |
| 2012 Nov 14 | Alginate dressings for venous leg ulcers | Protocol | Susan O'Meara, Marrissa Martyn‐St James | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Aged; Female; Humans; Male;
PICO

Flow diagram of the trial selection process.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison: 2 Hydrocolloid dressings compared with alginate dressings, outcome: 2.1 Proportion of ulcers healed at 6 and 12 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 1 Proportion of ulcers healed at 6 weeks.
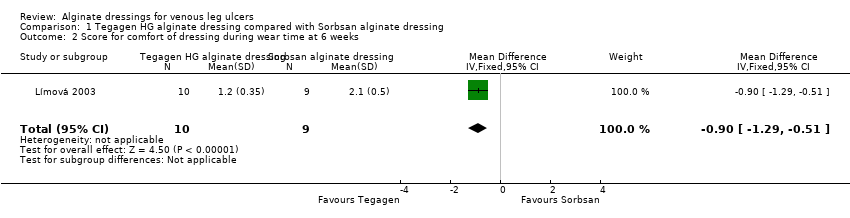
Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 2 Score for comfort of dressing during wear time at 6 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 3 Score for comfort during dressing removal at 6 weeks.

Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 4 Exudate absorption score at 6 weeks.
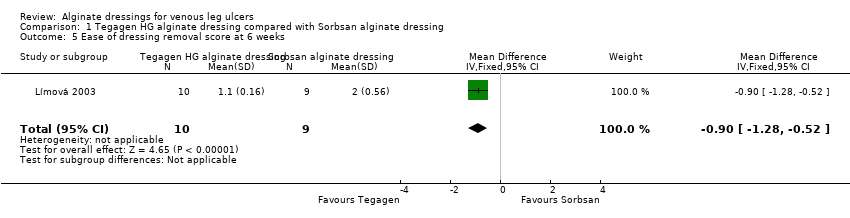
Comparison 1 Tegagen HG alginate dressing compared with Sorbsan alginate dressing, Outcome 5 Ease of dressing removal score at 6 weeks.

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 6 and 12 weeks.
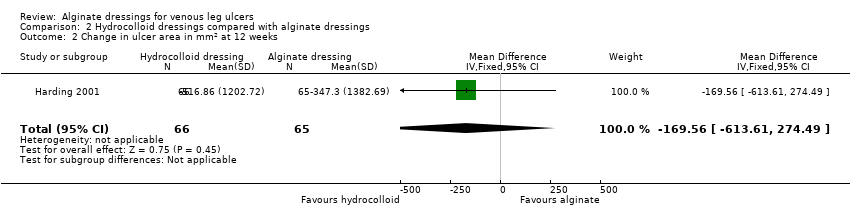
Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 2 Change in ulcer area in mm2 at 12 weeks.
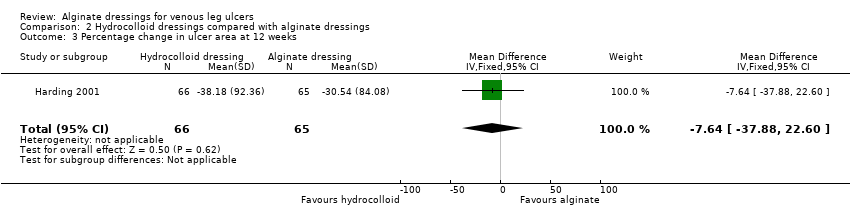
Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 3 Percentage change in ulcer area at 12 weeks.

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 4 Mean wear time (days).

Comparison 2 Hydrocolloid dressings compared with alginate dressings, Outcome 5 Proportion of participants experiencing adverse events.

Comparison 3 Plain non‐adherent dressings compared with alginate dressings, Outcome 1 Proportion of ulcers healed at 12 weeks.
| alginate dressing (Sorbsan®) compared to alternative alginate dressing (Tegagen ™ High Gelling) for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alternative alginate dressing (Tegagen ™High Gelling) | Alginate dressing (Sorbsan®) | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Proportion of participants with healed ulcers | Study population1 | RR 6.00 | 20 | ⊕⊝⊝⊝ | ||
| Low1 | ||||||
| 91 per 1000 | 546 per 1000 | |||||
| High1 | ||||||
| 204 per 1000 | 1000 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported (only reported mean percentage change in ulcer area, with no variance estimate, and no adjustment for baseline area). |
| Adverse effects | See comment | See comment | Not estimable | 0 | See comment | Limited information provided. |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 30 days have been taken from a meta‐analysis of RCTs evaluating different types of compression. The low risk estimate is based on a subset of participants with larger baseline ulcer area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). Estimate of baseline risk could not be estimated from study population because no participants healed in the Tegagen ™ HG group. | ||||||
| alginate dressing compared to hydrocolloid dressing for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hydrocolloid dressing | Alginate dressing | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | One RCT presented time to healing, but did not report a reliable estimate (not based on censored data). |
| Proportion of participants with healed ulcers at 6 weeks | Study population1 | RR 0.42 | 84 | ⊕⊝⊝⊝ | ||
| 233 per 1000 | 98 per 1000 | |||||
| Low1 | ||||||
| 91 per 1000 | 38 per 1000 | |||||
| High1 | ||||||
| 204 per 1000 | 86 per 1000 | |||||
| Proportion of participants with healed ulcers at 12 weeks | Study population | RR 1.02 | 131 | ⊕⊝⊝⊝ | ||
| 258 per 1000 | 263 per 1000 | |||||
| Low | ||||||
| 311 per 1000 | 317 per 1000 | |||||
| High | ||||||
| 696 per 1000 | 710 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Three RCTs reported change in wound area, but not with baseline adjustment. |
| Proportion of participants experiencing adverse effects at 6 weeks | 227 per 1000 | 334 per 1000 | RR 1.47 | 40 | ⊕⊝⊝⊝ | |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | One RCT assessed health‐related quality of life but did not use a validated tool, and only reported percentages of participants who had improved. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 30 days (and 90 days for the 12 week outcome) have been taken from a meta‐analysis of RCTs evaluating different types of compression. The low risk estimate is based on a subset of participants with larger baseline ulcer area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). | ||||||
| alginate dressing compared to plain non‐adherent dressing for venous leg ulceration | ||||||
| Patient or population: people with venous leg ulceration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Plain non‐adherent dressing | Alginate dressing | |||||
| Time to healing | See comment | See comment | Not estimable | 0 | See comment | Assessment of time to healing mentioned in RCT report, but estimates not provided. |
| Proportion of participants with healed ulcers | Study population1 | RR 1.08 | 60 | ⊕⊝⊝⊝ | ||
| 800 per 1000 | 864 per 1000 | |||||
| Low1 | ||||||
| 311 per 1000 | 336 per 1000 | |||||
| High1 | ||||||
| 696 per 1000 | 752 per 1000 | |||||
| Mean change in wound size, with adjustment for baseline size | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Adverse effects | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| Health‐related quality of life | See comment | See comment | Not estimable | 0 | See comment | Outcome not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Note: lower risk of the outcome is less favourable (i.e. lower risk of healing) than higher risk. Estimates for baseline low and high risks of healing at 90 days have been taken from a meta‐analysis of compression RCTs. The low risk estimate is based on a subset of participants with larger baseline ulcer surface area (greater than 5 cm squared). The high risk estimate is based on a subset of participants with smaller baseline ulcer surface area (5 cm squared or smaller). Most participants received a simple, low‐adherent dressing plus four‐layer bandage (O'Meara 2007). | ||||||
| Number of ulcers healed at 6 weeks: Group 1 (hydrocolloid dressing): 6/21 (29%) Group 2 (alginate dressing): 2/23 (9%) P value for between‐group difference not reported. Change in ulcer size – median change in area: Group 1 (hydrocolloid dressing): ‐205 mm2 Group 2 (alginate dressing): ‐162 mm2 The trial authors reported that the between‐group difference was not statistically significant. P value not reported. Change in ulcer size – median percentage change in ulcer area: Group 1 (hydrocolloid dressing): ‐42% Group 2 (alginate dressing): ‐26% The trial authors reported that the between‐group difference was not statistically significant. P value not reported. Review authors' comment: there was a reporting discrepancy between primary and secondary references for this outcome. The respective values in the secondary reference were ‐30.5% and ‐28.1%. | |
| Time to healing days ‐ mean (SD) [median (range)] (analysis based on healed participants only): Group 1 (hydrocolloid dressing): 41.823 days (SD 21.302) [42 (14 to 87)]; n = 17 Group 2 (alginate dressing): 56.588 days (SD 21.569) [56 (14 to 85)]; n = 17 Reported P value for between‐group difference in means = 0.053 P = 0.05 (log rank test) for difference in Kaplan‐Meier survival curves (analysis based on all randomised patients) Number of ulcers healed at 12 weeks: Group 1 (hydrocolloid dressing): 17/66 (26%) Group 2 (alginate dressing): 17/65 (26%) P value for between‐group difference not reported Change in ulcer area mm2 ‐ mean (SD) [median (range)]: Group 1 (hydrocolloid dressing): ‐516.86 mm2 (SD 1202.72) [‐301.13 (‐2494.84 to 5285.82)]; n = 66 Group 2 (alginate dressing): ‐347.30 mm2 (SD 1382.69) [‐132.83 (‐5144.08 to 5946.24)]; n = 65 P value for between‐group difference in means P = 0.48, reported by trial authors. Percentage change in ulcer area – mean (SD) [median (range)]: Group 1 (hydrocolloid dressing): ‐38.18% (SD 92.36) [‐67.67 (374.84 to ‐100.00)]; n = 66 Group 2 (alginate dressing): ‐30.54% (SD 84.08) [‐43.33 (411.74 to ‐100.00)]; n = 65 P value for between‐group difference in means = 0.64, reported by trial authors | |
| Number of ulcers healed at 6 weeks: Group 1 (alginate dressing, Tegagen ™ HG): 0/11 (0%) Group 2 (alginate dressing, Sorbsan®): 2/9 (22%) P value for between‐group difference not reported. Mean percentage change in wound area at 6 weeks: Group 1 (alginate dressing, Tegagen ™ HG): ‐33.7%, n = 10 Group 2 (alginate dressing, Sorbsan®): ‐29.6%, n = 9 The trial authors reported a P value of 0.88 for between‐group difference | |
| Time to healing/number of ulcers healed at 12 weeks: Group 1 (plain non‐adherent dressing): 24/30 (80%) Group 2 (alginate dressing): 26/30 (87%) P value for between‐group difference not reported The trial authors reported that results were similar for cumulative proportions healed estimated using life table analysis (no data or P value for between‐group difference presented) | |
| Number of ulcers healed at 6 weeks: Group 1 (hydrocolloid dressing): 4/22 (18%) Group 2 (alginate dressing): 2/18 (11%) P value for between‐group difference not reported Mean percentage change in ulcer area: Group 1 (hydrocolloid dressing): ‐57.1 Group 2 (alginate dressing): ‐34.9 The trial authors reported that the between‐group difference was not statistically different. P value not reported |
| Mean wear time days: Group 1 (hydrocolloid dressing): 4.112 days Group 2 (alginate dressing): 3.051 days The trial authors reported a between‐group difference of 1.029 days (95% CI 0.385 to 1.672), and that the difference was statistically significant in favour of Group 1 but the P value was not reported. Reviewer authors' comment: the difference in means reported by the trial authors does not follow from the mean values for each group Number participants achieving a 7‐day wear time on at least one occasion: Group 1 (hydrocolloid dressing): 9/21 (43%) Group 2 (alginate dressing): 3/23 (13%) The trial authors reported that the between‐group difference (30%, 95% CI 5% to 55%) was statistically significant but P value not reported Cost to heal one ulcer: Group 1 (hydrocolloid dressing): GBP 237.66 (total direct and indirect costs = GBP 1425.97 for total of 6 wounds healed) Group 2 (alginate dressing): GBP 687.31 (total direct and indirect costs = GBP 1374.61 for total of 2 wounds healed) Cost per wound healed calculated by review authors. Taking into account the number of participants completely healed in each group, the trial authors reported that the cost to achieve a healed wound using the Group 1 dressing was approximately one‐third of the cost of Group 2. Number dressing changes with no pain, mild pain, moderate pain, severe pain, excruciating pain, unable to respond, missing data: Group 1 (hydrocolloid dressing): (total of 192 dressing changes) 144 (75%), 38 (20%), 6 (3%), 2 (1%), 0 (0%), 0 (0%), 2 (1%) Group 2 (alginate dressing): (total of 224 dressing changes) 186 (83%), 29 (13%), 8 (3.5%), 0 (0%), 0 (0)%, 0 (0%), 1 (0.5%) Number of adverse events during the trial: Group 1 (hydrocolloid dressing): 32 adverse events reported during the trial. 4 were related to the primary dressing and 28 to the secondary dressing, of which 8 were attributed to maceration. Group 2 (alginate dressing): 32 adverse events reported during the trial. 3 were related to the primary dressing and 29 to the secondary dressing, of which 9 were attributed to maceration. | |
| Total number of dressing changes: Group 1 (hydrocolloid dressing): 1093 Group 2 (alginate dressing): 1186 Mean wear time days (SD) [median (range)]: Group 1 (hydrocolloid dressing): 3.632 days (1.878) [3 (1 to 13)]; n = 66 Group 2 (alginate dressing): 3.271 days (1.944) [3 (1 to 9)]; n = 65 The trial authors reported that the between‐group difference in means was P value < 0.001 Mean number of dressing changes per healed ulcer: Group 1 (hydrocolloid dressing): 7.4 Group 2 (alginate dressing): 12.1 P value for between‐group difference not reported Mean cost to achieve ulcer healing (based on patients healed): Group 1 (hydrocolloid dressing): GBP 1184.09 (USD 1699.71) Group 2 (alginate dressing): GBP 1200.73 (USD 1723.59) Mean cost per 1cm2 reduction in ulcer size (all patients randomised): Group 1 (hydrocolloid dressing): GBP 59.22 (USD 85.01) Group 2 (alginate dressing): GBP 92.27 (USD 132.46) Mean cost per 10% reduction in ulcer area: Group 1 (hydrocolloid dressing): GBP 80.15 (USD 115.06) Group 2 (alginate dressing): GBP 104.92 (USD 150.62) Percentage of dressing changes associated with no pain: Group 1 (hydrocolloid dressing): 82% Group 2 (alginate dressing): 62% The trial authors reported that the between‐group difference was statistically significant ( P value < 0.001). Numbers of dressings not reported Dressing performance – percentage recording “excellent” for overall ability to contain exudate: Group 1 (hydrocolloid dressing): 44% Group 2 (alginate dressing): 20% Unclear whether denominator was the number of participants or the number of dressing changes. Reported P value for between‐group difference = 0.002 Dressing performance – percentage recording “excellent” for overall ease of dressing removal: Group 1 (hydrocolloid dressing): 51% Group 2 (alginate dressing): 24% Unclear whether denominator was the number of participants or the number of dressing changes. Reported P value for between‐group difference = 0.006 Percentage of dressing changes with some adhesion to the wound bed: Group 1 (hydrocolloid dressing): 38% Group 2 (alginate dressing): 74% The trial authors reported that the between‐group difference was statistically significant (P value < 0.001) Number of dressing changes not reported Review authors' comments: there were some minor discrepancies between numbers in main text and tables for cost information (data from main text were recorded here); discrepancies between primary and secondary references for outcomes of ease of removal and exudate handling (data from primary reference recorded here); unclear whether reported outcomes relating to pain at dressing change, exudate handling, ease of dressing removal and adhesion were rated by participants or investigators (or both). | |
| Total number of dressing changes over the course of the trial: Group 1 (alginate dressing, Tegagen ™ HG): 69 (65 scheduled and 4 unscheduled) Group 2 (alginate dressing, Sorbsan®): 61 (60 scheduled and 1 unscheduled) Mean (SD) comfort score during wear over number of visits: Group 1 (alginate dressing, Tegagen ™ HG): 1.2 (SD 0.35) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.1 (SD 0.50) over 51 visits; n = 9 The trial authors reported a P value of 0.0005 for the between‐group difference Mean (SD) comfort score during dressing removal over number of visits: Group 1 (alginate dressing, Tegagen ™ HG): 1.2 (SD 0.22) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 1.9 (SD 0.19) over 51 visits; n = 9 The trial authors reported a P value of 0.003 for the between‐group difference Percentage of visits where necrotic tissue was observed: Group 1 (alginate dressing, Tegagen ™ HG): 59.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 68.9% of 51 visits; n = 9 The trial authors reported a P value of 0.57 for the between‐group difference Percentage of visits where debridement was required: Group 1 (alginate dressing, Tegagen ™ HG): 18.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 40.7% of 51 visits; n = 9 The trial authors reported a P value of 0.18 for the between‐group difference Mean improvement in amount of necrotic tissue (lower score is better): Group 1 (alginate dressing, Tegagen ™ HG): 2.5 over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 1.5 over 51 visits; n = 9 The trial authors reported a P value of 0.38 for the between‐group difference Mean (SD) exudate absorption score over number of visits (lower score better): Group 1 (alginate dressing, Tegagen ™ HG): 1.3 (SD 0.45) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.1 (SD 0.48) over 51 visits; n = 9 The trial authors reported a P value of 0.002 for the between‐group difference Percentage of clinic visits with medium or large amount of exudate observed: Group 1 (alginate dressing, Tegagen ™ HG): 71.7% of 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 86.3% of 51 visits; n = 9 The trial authors reported a P value of 0.25 for the between‐group difference Mean (SD) ease of removal score over number of visits (lower is better): Group 1 (alginate dressing, Tegagen ™ HG): 1.1 (SD 0.16) over 55 visits; n = 10 Group 2 (alginate dressing, Sorbsan®): 2.0 (SD 0.56) over 51 visits; n = 9 The trial authors reported a P value of 0.002 for the between‐group difference Proportion of participants reporting dressing adherence to the wound bed: Group 1 (alginate dressing, Tegagen ™ HG): 0% Group 2 (alginate dressing, Sorbsan®): 27.8% The trial authors reported that the average percentage of dressing changes with adherence to the wound bed was significantly less in Group 1 (P value < 0.05). Percentage of clinic visits with observation of peri‐wound skin as macerated, denuded, requiring medication: Group 1 (alginate dressing, Tegagen ™ HG): 36.0%, 9.0%, 31.3% Group 2 (alginate dressing, Sorbsan®): 54.4%, 31.9%, 65.2% Reported P values for between‐group difference: macerated skin P value 0.30; denuded skin P value 0.04, medication required P value 0.07 | |
| None reported | |
| Proportion of participants “improved remarkably” in quality of life at week 6: Group 1 (hydrocolloid dressing): 42.9% Group 2 (alginate dressing): 40.0% The trial authors did not report the number of participants completing the quality of life assessment. P value for between‐group difference not reported. There was a reporting discrepancy between “improved remarkably” and the pre‐defined categories for this outcome (deteriorated markedly, deteriorated somewhat, no change, improved somewhat or improved markedly) Number of dressings used per week: Group 1 (hydrocolloid dressing): 1‐3 Group 2 (alginate dressing): 1‐2 Wear time: The trial authors reported that both dressings were equivalent in terms of wear time, but no data by group or P value for between‐group difference were reported. Mean total approximate cost of materials: Group 1 (hydrocolloid dressing): GBP 431.73 Group 2 (alginate dressing): GBP 364.08 P value for between‐group difference not reported Mean ulcer pain score over past 2 weeks at 6 weeks: Group 1 (hydrocolloid dressing): 1.46 Group 2 (alginate dressing): 2.15 The trial authors reported that the between‐group difference was not statistically significant. P value not reported Change from baseline in mean ulcer pain score over past 2 weeks at 6 weeks: Group 1 (hydrocolloid dressing): ‐3.28 Group 2 (alginate dressing): ‐2.71 Estimated by review authors Mean pain score at dressing change at week 6: Group 1 (hydrocolloid dressing): 1.73 Group 2 (alginate dressing): 2.16 P value for between‐group difference not reported Change from baseline in mean pain score at dressing change at 6 weeks: Not reported and reviewer unable to estimate as data at week 6 only reported (no baseline) Proportion of participants reporting no sleep disturbance due to pain at week 2 and week 6: Group 1 (hydrocolloid dressing): 31.25%, 78.6% Group 2 (alginate dressing): 8.8%, 40.0% The trial authors did not report whether the proportions were for all participants enrolled or only those completing the trial. Reported P value for between‐group difference = 0.0721, but unclear to which time point this refers or if it is for the test across time points Dressing performance (exudate handling): The trial authors reported that the Group 2 dressing was “slightly superior” in terms of ability to contain exudate. However, no data by group or P value for between‐group difference were reported Proportion of participants reporting ‘excellent’ for ease of dressing removal: Group 1 (hydrocolloid dressing): 56.3% Group 2 (alginate dressing): 8.3% The trial authors did not report raw numbers and the denominator was not clear. Reported P value for between‐group difference was < 0.001. The trial authors reported that the Group 2 dressing often needed to be soaked off the ulcer. Number participants experiencing adverse events: Group 1 (hydrocolloid dressing): 5/22 (23%) (withdrawals: pain, 1; ulcer infection, 1; possible allergy, 1; not thought to warrant withdrawal ‐ wound infection, 1; pain and erythema at the final visit, 1) Group 2 (alginate dressing): 6/18 (33%) (withdrawals: pain, 4; ulcer infection, 2) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 weeks Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.32, 111.04] |
| 2 Score for comfort of dressing during wear time at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.29, ‐0.51] |
| 3 Score for comfort during dressing removal at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐0.88, ‐0.52] |
| 4 Exudate absorption score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.22, ‐0.38] |
| 5 Ease of dressing removal score at 6 weeks Show forest plot | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.28, ‐0.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 6 and 12 weeks Show forest plot | 3 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.48, 1.31] |
| 1.1 Healed at 6 weeks | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.21] |
| 1.2 Healed at 12 weeks | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.57, 1.81] |
| 2 Change in ulcer area in mm2 at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐169.56 [‐613.61, 274.49] |
| 3 Percentage change in ulcer area at 12 weeks Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐7.64 [‐37.88, 22.60] |
| 4 Mean wear time (days) Show forest plot | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.29, 1.02] |
| 5 Proportion of participants experiencing adverse events Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.53, 4.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of ulcers healed at 12 weeks Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.36] |

