Antisepsia de la piel para la reducción de las infecciones relacionadas con el catéter venoso central
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010140.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 julio 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Nai Ming Lai conceived the review question, coordinated and developed the review, performed the CENTRAL search, screened and selected the studies, entered the data, performed the analyses, developed the 'Summary of findings' tables, drafted the results, discussion, conclusions and abstract, edited the review, made an intellectual contribution to the draft review writing, approved the final version prior to submission and is guarantor for the review.
Nai An Lai made an intellectual contribution to the review writing and approved the final version prior to submission.
Elizabeth O’Riordan made an intellectual contribution to the review writing and approved the final version prior to submission.
Nathorn Chaiyakunapruk assisted in searching, provided some full‐text articles, made an intellectual contribution to the review writing and approved the final version prior to submission.
Kenneth Tan participated in study selection, data entry and cross‐checking, made an intellectual contribution to the review writing and approved the final version prior to submission.
Jacqueline Taylor participated in study selection, data entry and cross‐checking, edited the review draft and approved the final version prior to submission.
Contributions of editorial base:
Nicky Cullum (Editor): edited the protocol and the review; advised on methodology, interpretation and review content; approved the final review prior to submission.
Sally Bell‐Syer and Gill Rizzello (Managing Editors): coordinated the editorial process. Advised on methodology, interpretation and content. Edited the review.
Ruth Foxlee designed the search strategy, Amanda Briant and Reetu Child ran the searches and edited the search methods section.
Denise Mitchell: assisted in searching and provided full‐text articles
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Nai Ming Lai: none known.
Nai An Lai: none known.
Elizabeth O’Riordan: none known.
Nathorn Chaiyakunapruk: none known.
Jacqueline Taylor: none known.
Kenneth Tan: none known.
Acknowledgements
We are grateful for the contribution of peer reviewers Joan Webster, Susan O’Meara, Judith Tanner, Ankur Barua, Clifford Richardson, Mark Rodgers, Jane Nadel, Marian Brady, Gill Worthy, Victoria Steelman and Dayanithee Chetty for kindly spending time to comment on our draft protocol and review and suggesting improvements. We thank Meggan Harris for copyediting the review.
We also acknowledge the contribution by Dr Rachel Wel Lynn Ooi in assisting Nai Ming Lai in screening through the search results from CENTRAL to identify potentially relevant articles.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Jul 13 | Skin antisepsis for reducing central venous catheter‐related infections | Review | Nai Ming Lai, Nai An Lai, Elizabeth O'Riordan, Nathorn Chaiyakunapruk, Jacqueline E Taylor, Kenneth Tan | |
| 2012 Oct 17 | Skin antisepsis during catheter insertion for reducing central venous catheter related infections | Protocol | Nai An Lai, Nai Ming Lai, Elizabeth O'Riordan, Nathorn Chaiyakunapruk, Jacqueline E Taylor, Kenneth Tan | |
Differences between protocol and review
1. We have amended the title of the review by omitting the phrase "during catheter insertion". This was considered appropriate as all of our included studies examined skin antisepsis throughout the in‐dwelling period of the catheters with or without including the period of insertion, and keeping the phrase "during catheter insertion" would be misleading. We have revised the text of our review from Background through to the Methods where appropriate to reflect the change.
2. Under 'Why it is important to do this review', we changed the original statements "However, in some studies within the meta‐analysis, a combination of antiseptics were used; for example, chlorhexidine gluconate was sometimes evaluated in combination with alcohol. There remain some uncertainties regarding the best agent or combination of agents to be used for skin antisepsis" to the following: "However, the meta‐analysis only evaluated chlorhexidine gluconate and povidone‐iodine as skin antiseptics, and some studies within it assessed a combination of arterial catheters as well as central and peripheral venous catheters. Some uncertainties remain regarding the best agent, or combination of agents, for use as skin antisepsis for CVCs alone . . .". This was because in this review, the studies included also used a combination of agents, and there were no studies that assessed chlorhexidine gluconate separately, so the original statements did not justify the need for this review. Instead, the new statements more clearly reflect the differences between this review and the earlier review mentioned.
3. Under 'Types of studies', we added the following statements to further define the scope of our selection of studies: "We excluded cross‐over studies due to the possible contaminating effect of one intervention over another. We also excluded studies assessing CVCs for haemodialysis, as this is covered by another Cochrane review (McCann 2010)."
4. Under 'Selection of studies', we omitted the reference to unpublished studies because we did not find any unpublished study in our search of the trials registries.
5. Under Electronic searches, we updated the CENTRAL and MEDLINE search strategies in line with the updated indexing terms in each database.
6. Under 'Data extraction and management', we have re‐written paragraph 2 to the following to reflect what was actually done in the review.
"We found a discrepancy between the number of catheter and the number of patients in most studies, and this was due to multiple catheters being enrolled in some patients. However, we were unable to limit our analysis to one catheter per participant as none of the individual studies provided the adjusted results based on one catheter per participant."
7. We have added the section 'Unit of analysis issues' to describe how we would handle cluster‐RCTs.
8. Under 'Dealing with missing data', we revised our statement to include the absolute dropout rate in our consideration in assessing the risk of attrition bias, as a number of included studies had very high absolute dropout rates. Our revised statements are shown below:
"To assess whether the dropout rate was significant, we inspected the absolute dropout rate and the dropout rate in relation to the event rates for the intervention and the comparison groups. If the absolute dropout rate was 20% or more, we judged the study to be at high risk for incomplete outcome data. If the dropout rate was lower than 20%, we used a 'worst‐case‐scenario' method . . ."
9. Under 'Assessment of heterogeneity', we revised the statement to reflect what was actually done in the review, as follows:
"We found significant statistical heterogeneity in one analysis (Analysis 4.4) and provided a plausible explanation the possible reason for heterogeneity in the form of risk of attrition bias in some included studies. We decided to still provided the pooled estimate for this analysis and separated the studies based on the risk of attrition bias in our pre‐specified sensitivity analysis."
10. Under 'Sensitivity analysis', we re‐wrote the section as follows to reflect the information that we gathered in the review and removed any mention of intention‐to‐treat analysis:
"We performed the following sensitivity analyses.
-
Best‐ and worst‐case scenarios to assess the impact of missing data, as described in the section 'Dealing with missing data'.
-
Including and excluding studies with unclear and high risks of selection bias, namely, studies with unclear or high risk for random sequence generation, allocation concealment or both.
Had sufficient data been available, we would have performed additional sensitivity analyses to include and exclude studies with methodological issues other than selection bias, such as a lack of blinding to the participants, care givers or investigators, or where blinding was unclear."
11. Under 'Subgroup analysis and investigation of heterogeneity', we added the following statement to describe the separation of comparisons into subgroups based on the solution used, in response to the referees' comments in our draft review:
"In this review, we created subgroups of comparisons based on the solution used, for example, a subgroup for chlorhexidine in aqueous solution versus povidone iodine in aqueous solution, and another subgroup for chlorhexidine in alcohol versus povidone‐iodine in aqueous solution."
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti‐Infective Agents, Local [*therapeutic use];
- Antisepsis [*methods];
- Catheter‐Related Infections [*prevention & control];
- Central Venous Catheters [*adverse effects, microbiology];
- Chlorhexidine [therapeutic use];
- Ethanol [therapeutic use];
- Povidone‐Iodine [therapeutic use];
- Randomized Controlled Trials as Topic;
- Skin [*microbiology];
Medical Subject Headings Check Words
Adult; Humans;
PICO
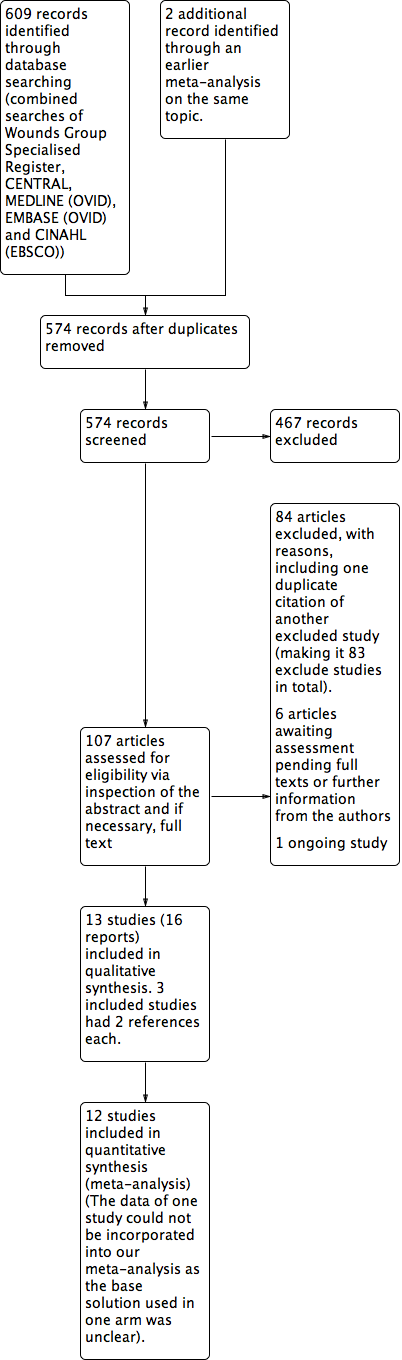
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
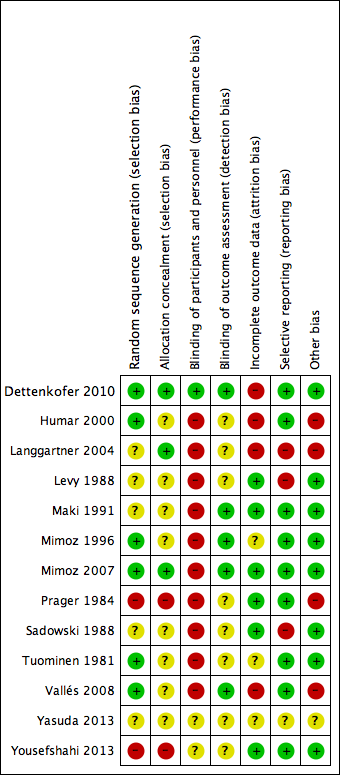
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.1 Catheter‐related BSI.

Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.3 All‐cause mortality.

Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.4 Catheter colonisation.

Comparison 1 Povidone‐iodine (in aqueous solution) versus no skin antisepsis, Outcome 1 Catheter‐related BSI.
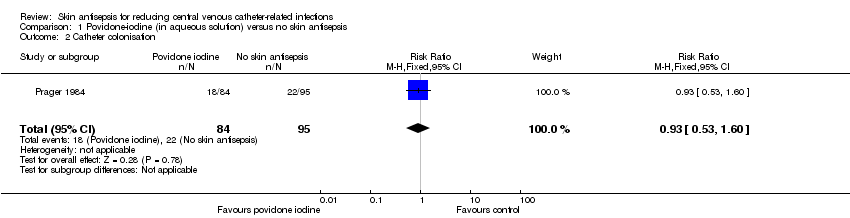
Comparison 1 Povidone‐iodine (in aqueous solution) versus no skin antisepsis, Outcome 2 Catheter colonisation.

Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 1 Septicaemia.
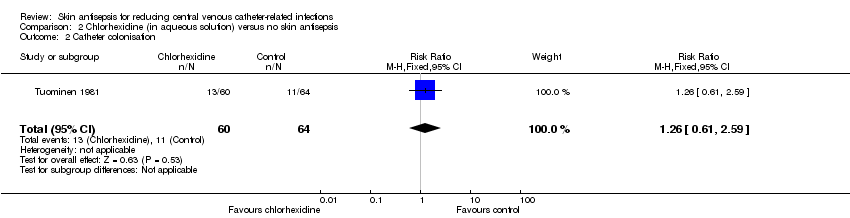
Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 2 Catheter colonisation.

Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 3 Number of patients who required antibiotics during in‐dwelling period of catheter.

Comparison 3 Alcohol versus no skin antisepsis, Outcome 1 Catheter colonisation.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 1 Catheter‐related BSI.
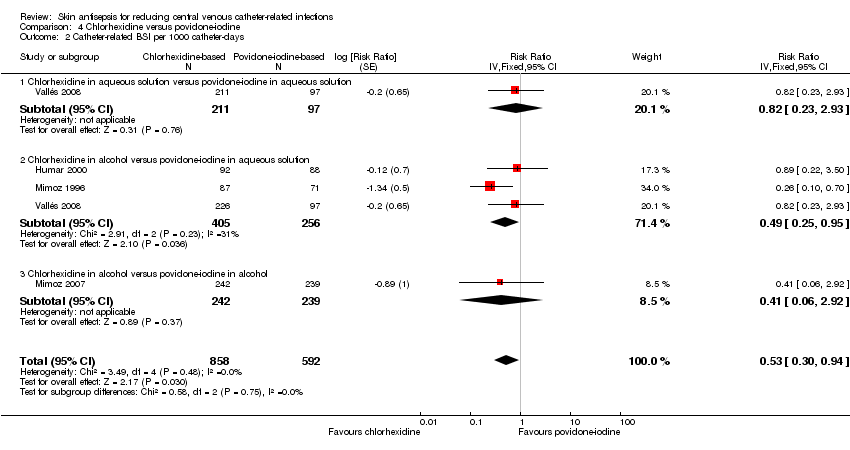
Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 2 Catheter‐related BSI per 1000 catheter‐days.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 3 All‐cause mortality.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 4 Catheter colonisation.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 5 Catheter colonisation per 1000 catheter‐days.
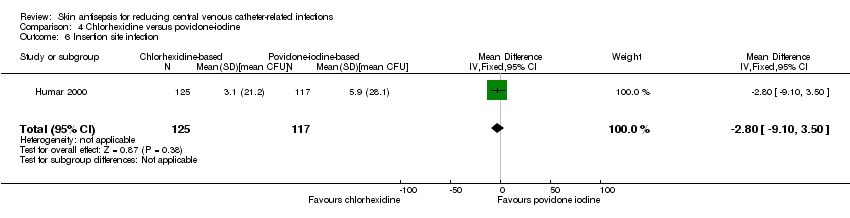
Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 6 Insertion site infection.
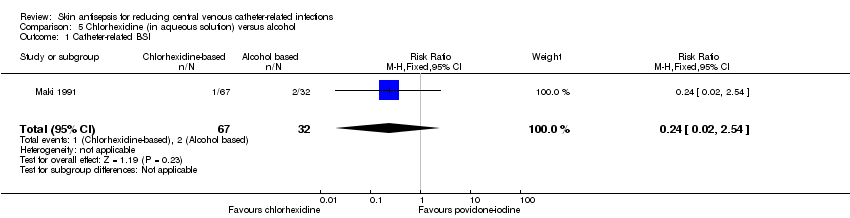
Comparison 5 Chlorhexidine (in aqueous solution) versus alcohol, Outcome 1 Catheter‐related BSI.
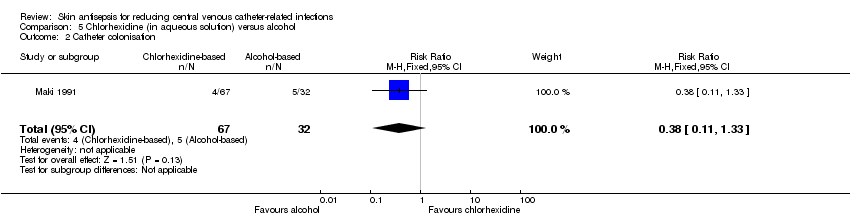
Comparison 5 Chlorhexidine (in aqueous solution) versus alcohol, Outcome 2 Catheter colonisation.

Comparison 6 Povidone‐iodine (in aqueous solution) versus alcohol, Outcome 1 Catheter‐related BSI.

Comparison 6 Povidone‐iodine (in aqueous solution) versus alcohol, Outcome 2 Catheter colonisation.
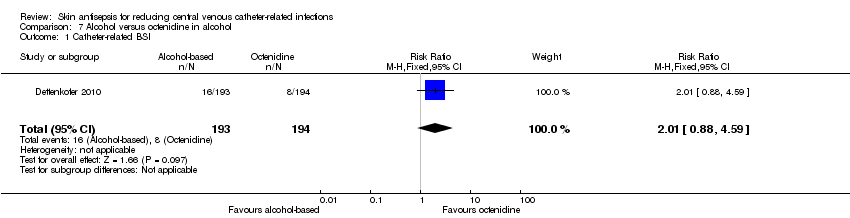
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 1 Catheter‐related BSI.

Comparison 7 Alcohol versus octenidine in alcohol, Outcome 2 Catheter‐related BSI per 1000 catheter‐days.
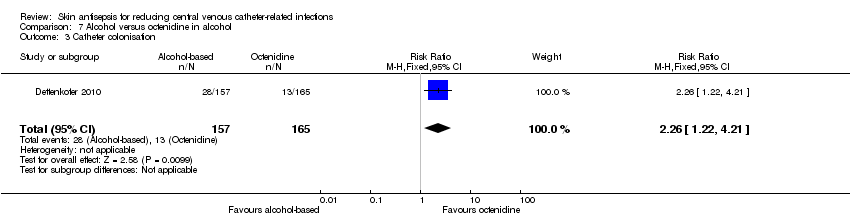
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 3 Catheter colonisation.
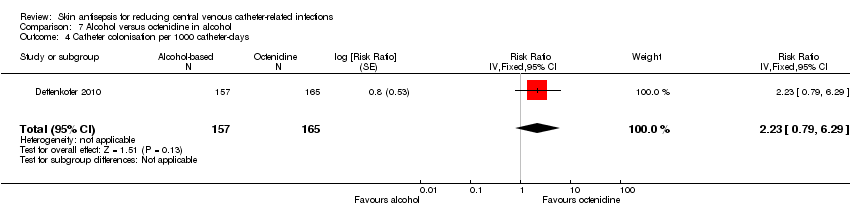
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 4 Catheter colonisation per 1000 catheter‐days.

Comparison 7 Alcohol versus octenidine in alcohol, Outcome 5 Skin colonisation.

Comparison 7 Alcohol versus octenidine in alcohol, Outcome 6 Adverse effects.
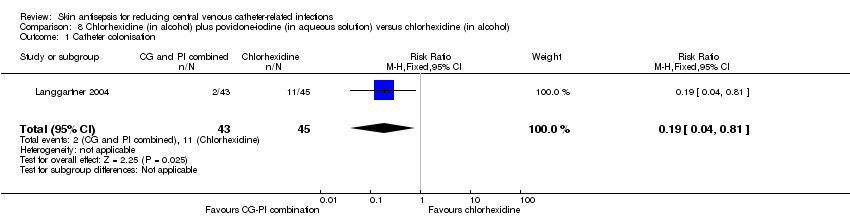
Comparison 8 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine (in alcohol), Outcome 1 Catheter colonisation.
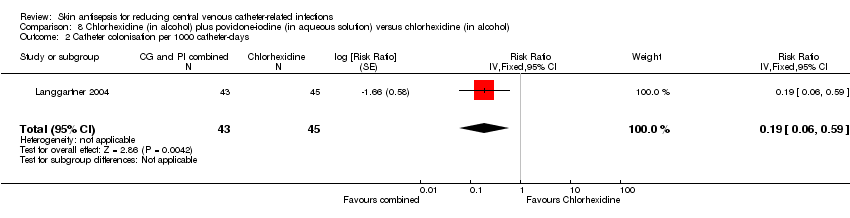
Comparison 8 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine (in alcohol), Outcome 2 Catheter colonisation per 1000 catheter‐days.

Comparison 9 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution), Outcome 1 Catheter colonisation.

Comparison 9 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution), Outcome 2 Catheter colonisation per 1000 catheter‐days.

Comparison 10 Sanosil (hydrogen peroxide and silver) versus water as adjunct to chlorhexidine 2% aqueous bath plus povidone‐iodine aqueous 10% scrub, Outcome 1 Catheter colonisation.
| Chlorhexidine compared to povidone‐iodine for patients with a central venous catheter | |||||
| Patient or population: patients with a central venous catheter | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Povidone‐iodine | Chlorhexidine | ||||
| Catheter‐related BSI ‐ overall comparison between chlorhexidine and povidone‐iodine (during in‐patient stay) | Study population | RR 0.64 | 1436 | ⊕⊝⊝⊝ | |
| 64 per 1000 | 41 per 1000 | ||||
| Moderatea | |||||
| 46 per 1000 | 29 per 1000 | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | Study population | RR 0.64 | 452 | ⊕⊝⊝⊝ | |
| 86 per 1000 | 55 per 1000 | ||||
| Moderate | |||||
| 84 per 1000 | 54 per 1000 | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | Study population | RR 0.77 | 503 | ⊕⊝⊝⊝ | |
| 70 per 1000 | 54 per 1000 | ||||
| Moderate | |||||
| 69 per 1000 | 53 per 1000 | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in alcohol versus povidone‐iodine in alcohol | Study population | RR 0.4 | 481 | ⊕⊕⊕⊝ | |
| 42 per 1000 | 17 per 1000 | ||||
| Moderate | |||||
| 42 per 1000 | 17 per 1000 | ||||
| Primary BSI or clinical sepsis | No studies under this comparison assessed this outcome. | ||||
| All‐cause mortality ‐ Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | Study population | RR 1.15 | 213 | ⊕⊕⊝⊝ | |
| 236 per 1000 | 271 per 1000 | ||||
| Moderate | |||||
| 236 per 1000 | 271 per 1000 | ||||
| All‐cause mortality ‐ Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | Study population | RR 0.8 | 222 | ⊕⊕⊝⊝ | |
| 236 per 1000 | 189 per 1000 | ||||
| Moderate | |||||
| 236 per 1000 | 189 per 1000 | ||||
| Mortality attributable the CVC‐related infections. | No studies under this comparison assessed this outcome. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a'Moderate risk' was calculated from the median control event rate for each outcome. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.37, 2.61] |
| 2 Catheter colonisation Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Septicaemia Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.31, 27.31] |
| 2 Catheter colonisation Show forest plot | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.61, 2.59] |
| 3 Number of patients who required antibiotics during in‐dwelling period of catheter Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 4 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.99] |
| 1.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 2 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.32, 1.28] |
| 1.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.39, 1.53] |
| 1.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.13, 1.24] |
| 2 Catheter‐related BSI per 1000 catheter‐days Show forest plot | 4 | 1450 | Risk Ratio (Fixed, 95% CI) | 0.53 [0.30, 0.94] |
| 2.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 308 | Risk Ratio (Fixed, 95% CI) | 0.82 [0.23, 2.93] |
| 2.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 3 | 661 | Risk Ratio (Fixed, 95% CI) | 0.49 [0.25, 0.95] |
| 2.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (Fixed, 95% CI) | 0.41 [0.06, 2.92] |
| 3 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.72, 1.83] |
| 3.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.48, 1.34] |
| 4 Catheter colonisation Show forest plot | 5 | 1533 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.12, ‐0.03] |
| 4.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 2 | 452 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.17, ‐0.02] |
| 4.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 3 | 600 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 4.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.17, ‐0.04] |
| 5 Catheter colonisation per 1000 catheter‐days Show forest plot | 5 | 1547 | Risk Ratio (Fixed, 95% CI) | 0.64 [0.50, 0.81] |
| 5.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 308 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.40, 1.20] |
| 5.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 4 | 758 | Risk Ratio (Fixed, 95% CI) | 0.64 [0.48, 0.85] |
| 5.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (Fixed, 95% CI) | 0.53 [0.24, 1.17] |
| 6 Insertion site infection Show forest plot | 1 | 242 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐9.10, 3.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.02, 2.54] |
| 2 Catheter colonisation Show forest plot | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.08] |
| 2 Catheter colonisation Show forest plot | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2.1 Povidone‐iodine in aqueous solution versus alcohol | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.49, 3.14] |
| 2.2 Povidone‐iodine‐impregnated adherent film versus alcohol | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.51, 160.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.88, 4.59] |
| 2 Catheter‐related BSI per 1000 catheter‐days Show forest plot | 1 | 387 | Risk Ratio (Fixed, 95% CI) | 2.18 [0.54, 8.77] |
| 3 Catheter colonisation Show forest plot | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.22, 4.21] |
| 4 Catheter colonisation per 1000 catheter‐days Show forest plot | 1 | 322 | Risk Ratio (Fixed, 95% CI) | 2.23 [0.79, 6.29] |
| 5 Skin colonisation Show forest plot | 1 | 365 | Mean Difference (IV, Fixed, 95% CI) | 79.00 [32.76, 125.24] |
| 6 Adverse effects Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.81] |
| 2 Catheter colonisation per 1000 catheter‐days Show forest plot | 1 | 88 | Risk Ratio (Fixed, 95% CI) | 0.19 [0.06, 0.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 2 Catheter colonisation per 1000 catheter‐days Show forest plot | 1 | 95 | Risk Ratio (Fixed, 95% CI) | 0.17 [0.05, 0.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.68, 1.72] |

