Dosis única oral de ibuprofeno más codeína para el dolor posoperatorio agudo en adultos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, 6 parallel groups. Single oral dose Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 1, 2, 3, 4 hours | |
| Participants | Surgical removal of 1 to 4 impacted third molars N = 249 M = 83, F = 166 Mean age 23 years | |
| Interventions | Ibuprofen + codeine 400/60 mg, n = 41 Ibuprofen 400 mg, n = 38 Aspirin/codeine 650 mg/60 mg, n = 45 Aspirin 650 mg, n = 38 Codeine 60 mg, n = 41 Placebo, n = 46 | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Rescue medication allowed after 1 hour | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Pharmaceutical company held randomisation code and packaged bottles, which were identified by sequential code number only |
| Blinding of participants and personnel (performance bias) | Low risk | Tablets "appeared identical for every patient" |
| Blinding of outcome assessment (detection bias) | Low risk | Tablets "appeared identical for every patient" |
| Size | High risk | < 50 participants per treatment group |
| Methods | Randomised, double‐blind, 5 parallel groups. Single oral dose Medication administered when baseline pain reached a moderate to severe intensity, and ≥ 50/100 mm Pain assessed at baseline then 0.25, 0.5, 0.75, 1, 1.5, 2 hours and hourly to 12 hours | |
| Participants | Surgical removal of ≥ 3 impacted third molars (2 mandibular) N = 678 M = 271, F = 407 Mean age 20 years | |
| Interventions | Ibuprofen 400 mg + codeine 25.6 mg, n = 169 | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale PGE: standard 5‐point scale Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 Rescue medication (tramadol if < 4 hours, paracetamol/hydrocodone if 4 hours+) allowed after 1.5 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated system" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "Each treatment consisted of 2 white tablets of a similar size and was administered as a single dose taken with approximately 300 mL of water" |
| Blinding of outcome assessment (detection bias) | Low risk | "Each treatment consisted of 2 white tablets of a similar size and was administered as a single dose taken with approximately 300 mL of water" |
| Size | Unclear risk | 50 to 199 participants per treatment arm |
| Methods | RCT, DB, single oral dose, parallel groups Medication administered when baseline pain reached a moderate to severe intensity | |
| Participants | Surgical removal of impacted third molar M/F "balanced" but numbers not provided | |
| Interventions | Ibuprofen 200 mg + codeine 15 mg, n = 32 Ibuprofen 400 mg + codeine 30 mg, n = 26 Ibuprofen 800 mg + codeine 60 mg, n = 26 Aspirin 600 mg, n = 25 | |
| Outcomes | PI: non‐standard 9‐point scale Use of rescue medication Withdrawals | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4/5 Rescue medication (paracetamol) allowed after 2 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed sachets |
| Blinding of participants and personnel (performance bias) | Low risk | "aspirin specially formulated to match the other drugs" |
| Blinding of outcome assessment (detection bias) | Low risk | "aspirin specially formulated to match the other drugs" |
| Size | High risk | < 50 participants per treatment arm |
| Methods | RCT, DB, 2‐group cross‐over, multiple dose (data reported for first dose) Medication administered when baseline pain reached a moderate to severe intensity | |
| Participants | Surgical removal of impacted mandibular third molar (one at each phase of cross‐over) N = 25 | |
| Interventions | Ibuprofen 400 mg + codeine 20 mg, n = 25 (24 analysed) | |
| Outcomes | PI: standard 4‐point scale and 100 mm VAS PR: standard 5‐point scale and 100 mm VAS PGE: standard 5‐point scale Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4/5 Second dose available for inadequate pain relief | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "The white tablets were identifiable only by the patient and treatment numbers" |
| Blinding of outcome assessment (detection bias) | Low risk | "The white tablets were identifiable only by the patient and treatment numbers" |
| Size | High risk | < 50 participants per treatment arm |
| Methods | RCT, DB, 2‐group cross‐over, 2‐dose (data reported for first dose) (Due to carryover effects data analysed as parallel study using first phase only) Medication administered when baseline pain reached a moderate to severe intensity | |
| Participants | Surgical removal of impacted mandibular third molar (one at each phase of cross‐over) N = 60 | |
| Interventions | Ibuprofen 400 mg + codeine 60 mg, n = 29 | |
| Outcomes | PI: 100 mm VAS Adverse events for both doses Withdrawals | |
| Notes | Oxford Quality Score: R = 1, DB = 2, W = 1. Total = 4/5 Second dose available after 2 hours for inadequate pain relief | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "All tablets were of identical appearance" |
| Blinding of outcome assessment (detection bias) | Low risk | "All tablets were of identical appearance" |
| Size | High risk | < 50 participants per treatment arm |
| Methods | Randomised, double‐blind, 5 parallel groups. Single oral dose Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 0.5, 1, 2, 3, 4 hours | |
| Participants | Episiotomy, Caesarian section or gynaecological operations N = 195 All F Mean age 26 years | |
| Interventions | Ibuprofen + codeine 200/30 mg, n = 40 Ibuprofen + codeine 400/60 mg, n = 40 Ibuprofen 40 mg, n = 38 Codeine 60 mg, n = 37 Placebo, n = 40 | |
| Outcomes | PI: standard 4‐point scale PR: standard 5‐point scale Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 Rescue medication allowed after 1 hour | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "All unit doses were identical in appearance and packaging" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "All unit doses were identical in appearance and packaging" |
| Size | High risk | < 50 participants per treatment arm |
DB ‐ double‐blind, N ‐ number of participants in study, n ‐ number of participants in treatment arm, PGE ‐ patient global evaluation, PI ‐ pain intensity, PR ‐ pain relief, R ‐ randomised, W ‐ withdrawals
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Short abstract | |
| Participants remedicating not correctly analysed (diaries continued) | |
| Did not state which scale was used | |
| Inappropriate study design ‐ data from patients remedicating were not handled correctly | |
| No suitable (placebo or same dose of ibuprofen alone) comparator | |
| No suitable (placebo or same dose of ibuprofen alone) comparator | |
| Participants remedicating not correctly analysed (diaries continued) | |
| Medication administered preoperatively |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [2.82, 5.93] |
| Analysis 1.1  Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 1 Participants with ≥ 50% pain relief. | ||||
| 2 Participants with any adverse event Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.66] |
| Analysis 1.2  Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 2 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.01, 1.57] |
| Analysis 2.1  Comparison 2 Ibuprofen + codeine (all doses) versus same dose of ibuprofen alone, Outcome 1 Participants with ≥ 50% pain relief. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
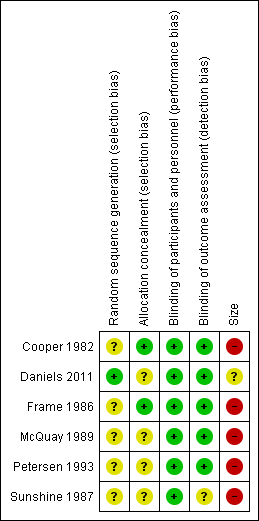
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: ibuprofen 400 mg + high dose codeine versus placebo, outcome: 2.1 Participants with ≥ 50% pain relief.
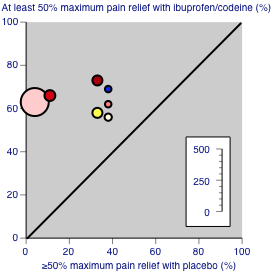
Studies comparing ibuprofen plus codeine with placebo, with the outcome of at least 50% maximum pain relief over 4‐6 hours. Colour code: white ‐ ibuprofen 200 mg + codeine 15 mg; yellow ‐ ibuprofen 200 mg + codeine 30 mg; light pink ‐ ibuprofen 400 mg + codeine 25.6 mg; medium pink ‐ ibuprofen 400 mg + codeine 30 mg; red ‐ ibuprofen 400 mg + codeine 60 mg; blue ‐ ibuprofen 800 mg + codeine 60 mg.

Studies comparing ibuprofen plus codeine with same dose of ibuprofen, with the outcome of at least 50% maximum pain relief over 4‐6 hours. Colour code: darker yellow ‐ ibuprofen 200 mg + codeine 20 mg versus ibuprofen 200 mg; lighter yellow ‐ ibuprofen 400 mg + codeine 60 mg versus ibuprofen 400 mg.
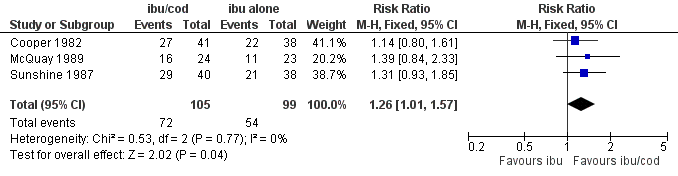
Forest plot of comparison: ibuprofen + codeine (all doses) versus same dose of ibuprofen alone, outcome: 3.1 Participants with ≥ 50% pain relief.
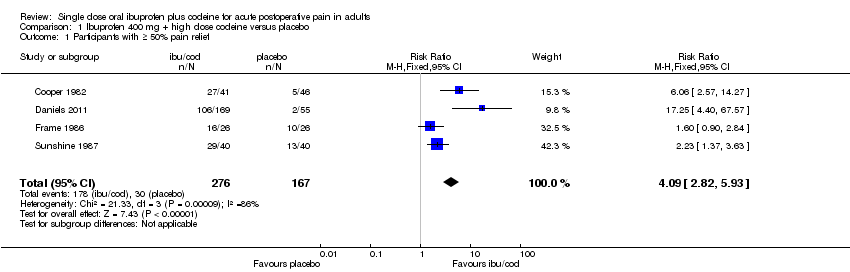
Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 1 Participants with ≥ 50% pain relief.

Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 2 Participants with any adverse event.

Comparison 2 Ibuprofen + codeine (all doses) versus same dose of ibuprofen alone, Outcome 1 Participants with ≥ 50% pain relief.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [2.82, 5.93] |
| 2 Participants with any adverse event Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.01, 1.57] |

