Medicación antimicótica oral para la onicomicosis de la uña del pie
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Design: double‐blind randomised controlled trial | |
| Participants | Number of participants randomised: not stated Number included in analysis: 45 Sex: not stated (both sexes included) Age: not stated Number completing treatment: 2 discontinuations, not clear if included in analyses. Inclusion criteria: toenail or fingernail onychomycosis Type/location/characteristics of infection: toenails and finger nails Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: Dubai Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Clinical cure and mycological cure | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Abstract only, results not separated for finger or toenails, not included in our quantitative meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[m]ulticentre, randomised, double‐blind study" Comment: no information on random sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[m]ulticentre, randomised, double‐blind study" Comment: no information on allocation concealment provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "250 mg of terbinafine was administered daily for 16 weeks, followed by 8 weeks of placebo. Griseofulvin in a dose of 1000 mg daily was administered for 24 weeks." Comment: extension of terbinafine treatment with duration with 8 weeks of placebo implied an attempt at making treatments seem identical to participants. However, no information is provided regarding blinding of personnel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[t]he overall clinical and mycological evaluation of week 48 …" Comment: no specific information regarding blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "45 patients were analysed" Comment: no info on number of participants that were initially randomised or if discontinuations are included in the analyses |
| Selective reporting (reporting bias) | Low risk | Quote: "[s]uccess was defined as a mycological cure (i.e. negative KOH preparation and culture) and the absence of clinical symptoms (paronychial inflammation, hyperkeratosis, onychomycosis" Comment: all results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: open‐label randomised controlled trial | |
| Participants | Number of participants randomised: 50 Number included in analysis: 50 Number completing treatment: 50 Sex (M/F): 24/26 Age: 16‐67 years, mean age 43 years Inclusion criteria: distal subungual toenail onychomycosis Type/location/characteristics of infection: distal subungual toenail onychomycosis Duration of infection: not stated Exclusion criteria: pregnancy or breastfeeding; psoriasis; severe liver/renal/endocrinologic impairment; concomitant therapy with rifampin, phenytoin, digoxin, oral anticoagulants and cyclosporine; hypersensitivity to azoles and terbinafine Washout period: 4 months for oral and 4 weeks for topical antifungals Setting: Turkey Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 6 months post‐treatment (9 months total) Outcomes measured: clinical outcome (on a 6‐category scale ranging from deterioration to complete improvement); mycological cure (KOH preparation and culture negative) Safety and tolerability assessed by: drug tolerability assessed every 4 weeks during 3 month | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned" Comment: method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned" Comment: method of allocation concealment not stated, unclear if any selection bias in allocating patients to particular treatments. No evidence to suggest that a robust method was used |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[a] comparative, open, prospective study" Comment: not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[a] comparative, open, prospective study" Comment: not blinded |
| Incomplete outcome data (attrition bias) | Low risk | For all 50 patients that were randomised outcome data are available |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Methods | Design: open‐label RCT | |
| Participants | Number of participants randomised: unclear, initially stated 90, but participants that did not show a response or did not attend appointments where eliminated from the studies and replaced Number included in analysis: 83 Sex: not stated but M/F ratio 3:1 Mean age: 40.5 years (range 20‐60) Number completing treatment: unclear, see above Inclusion criteria: onychomycosis in the first toe, diagnosed by clinical examination or laboratory findings Type/location/characteristics of infection: first toe only Duration of infection: not stated Exclusion criteria: pregnancy or breastfeeding, treatment in the last month prior to the study Washout period: 1 month Setting: hospital dermatology department in Mexico Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Monthly millimetric measurements of the nail | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Not included in the meta‐analysis due to methodological issues, namely the exclusion and replacement of participants that did not respond to treatment; total number of participants unclear, we have written to the author, but he has not been able to provide the needed data yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly selected to enter the study and were divided into two groups of 45 patients each." "Patients were assigned a number according to the order in which they came to the clinic and were randomly included in any of the following groups." Comment: no evidence provided of any method of random sequence generation |
| Allocation concealment (selection bias) | High risk | Quote: "[p]atients were assigned a number according to the order in which they came to the clinic and were randomly included in any of the following groups." Comment: given that order of presentation to clinic was used to assign a number and it was an open‐label study, there is no suggestion of any allocation concealment being done. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[a] comparative, open, prospective, longitudinal study" Comment: open‐label study, no blinding |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[a] comparative, open, prospective, longitudinal study with a blind evaluator was undertaken" Comment: no method of blinding the outcome assessor was detailed. |
| Incomplete outcome data (attrition bias) | High risk | Quotes: "[p]atients were eliminated from the study if they did not keep up their appointments, became pregnant during the study, did not show improvement of clinical symptoms or mycosis after 3 months of treatment, or reported side effects." "Side effects were recorded and most of the patients who were eliminated from the study were substituted with other patients." "In subgroup I/U (14 patients) one patient dropped out and in subgroup I/P (12 patients) one patient dropped out and two patients were eliminated; and in subgroup G/Is (13 patients) two patients experienced side effects and in subgroup G/U (14 patients) one patient experienced side effects. In subgroups I/Is and G/P all patients finished treatment." Comment: participants that did not respond to treatment were replaced. risk of incomplete outcome data given the replacement of participants who were eliminated or dropped out. 7 of an original 90 patients were not included in results. |
| Selective reporting (reporting bias) | Low risk | Quote: "[m]ethods: monthly millimetrical measurements of the nail were taken according to the Zaias method." Comment: results described 'cure' rates. No mention of millimetrical measurements in Results. All outcome measures presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other sources of bias seen |
| Methods | Design: open‐label comparative randomised trial | |
| Participants | Number of participants randomised: 53 Sex: not stated (both sexes included) Mean age: 39 years Number included in analysis: 43 Number completing treatment: 40 Inclusion criteria: age > 18 years, onychomycosis Type/location/characteristics of infection: distal or lateral subungual onychomycosis diagnosed by physical examination, KOH smear and culture Duration of infection: 3 months to 26 years Exclusion criteria: abnormal haematology, blood chemistries, urinalysis, liver tests; onychomycosis caused by resistant fungi; pregnancy or lactation; treatment for gastric hyperacidity; psoriasis, other serious conditions. Withdrawal criteria: serious adverse effects, development of severe disease not associated with the treatment, non‐cooperative participants, voluntary withdrawal Washout period: 3 months for antimycotic treatment Setting: hospital dermatology department in Mexico Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 9 months Outcomes measured: % of nail involvement, nail abnormalities, nail changes, nail growth, participant's evaluation of treatment, doctor's evaluation of treatment (cure vs improvement) Safety and tolerability assessed by: reporting of adverse events, LFTs | |
| Source of funding | Janssen Pharmaceutical assisted in data management and statistical analyses | |
| Conflict of interest | Clear disclosure of pharmaceutical industry involvement. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to one of the two treatment groups." Comment; method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[c]omparative, open, and prospective study" Comment: participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[c]omparative, open and prospective study" Comment: no mention that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[i]n the itraconazole treatment group 3 patients dropped out for unknown reasons and 1 patient withdrew because of headache; 23 [of 27 randomised] finished the follow‐up period." "In the terbinafine treatment group 7 patients dropped out for unknown reasons and 2 patients because of … adverse events; 17 patients [of 26 randomised] finished the follow‐up period." Comment: all participants that entered the study are accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 143 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 120 (141 for tolerability) Number completing treatment: not stated Inclusion criteria: age > 18 years, onychomycosis of feet alone or of the feet and hands, use of contraception in women of childbearing age Type/location/characteristics of infection: toenail onychomycosis, confirmed Duration of infection: 0‐71 years Exclusion criteria: pregnancy, breastfeeding Washout period: 1 month for topical or systemic therapy Setting: multicentre Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 2 and 6 months post‐treatment cessation Outcomes measured: complete cure (clinical disappearance of pathological zone of nail + mycological cure), concentration of terbinafine in toenail Safety and tolerability assessed by: basic labs, side effects | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]his study was a multicenter double‐blind study with two parallel groups" Comment: method of sequence generation not stated. Groups similar for age, sex, duration of disease, nails affected and pathogenic agent |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]his study was a multicenter double‐blind study with two parallel groups" Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]his study was a multicenter double‐blind study with two parallel groups" "The dosages were 250 mg/day for terbinafine and 1 g/day for griseofulvin. The duration of treatment in both groups was up to the longest recommended for griseofulvin i.e. a maximum of 12 months." Comment: study states that it is blinded, no mention of method, unclear if treatments appeared identical |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: mycological and clinical assessment occurred "1 month after the start of treatment, then every 2 months". Comment: blinded, but no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) | High risk | Quote: "[o]f a total of 143 patient recruited by 15 centres, 120 were assessed for effectiveness and 141 for tolerability. Two patients never took the treatment, 13 did not return after inclusion and 8 recorded negative mycology on inclusion." "As big toenails are the most difficult to cure, we calculated their data separately." Comment: adequate explanation of dropouts. However, results reported number of toenails cured, not number of participants cured. Stated what the ITT numbers of participants were, but did not state the number of participants cured |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 109 Sex: 73% male Mean age: 42.3‐46.9 years in the different treatment groups (range 18‐75) Number included in analysis: 71 Number completing treatment: not stated Inclusion criteria: age 18‐75 years Type/location/characteristics of infection: dermatophyte infection of the toenail Duration of infection: average in groups 94.2‐137.8 weeks Exclusion criteria: white superficial toenail onychomycosis, immunosuppression or immunodeficiency, hepatic enzymes > 1.5 times the upper limit of normal, marked lab abnormalities Washout period: 3 months for systemic, 1 month for topical Setting: USA multicentre Ethnicity: black participants Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 72 weeks Outcomes measured: mycologic efficacy, clinical cure (0% involvement of target toenail) Safety and tolerability assessed by: not stated | |
| Source of funding | Novartis Pharmaceuticals Corporation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry involvement (Novartis). No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised into four treatment groups" Comment: method of random sequence generation not stated. States groups have no statistically significant differences in age, sex, percentage of target toenails infected, duration of current episode of onychomycosis. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised into four treatment groups" Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Both patient and physician were blinded to the treatment for the 24‐week treatment period. Blinding was maintained through the end of the study." Comment: study is blinded, but no method stated for how terbinafine tablets and placebo tablets were made indistinguishable |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[a]ssessments were done at … weeks 4, 8, 12, and 24 during the treatment period. Follow‐up assessments were scheduled for weeks 30, 36, 42, 48, 60, and 72." Comment: Methods note that physicians were blinded for the 24‐week study period, but there is no mention that outcome assessor blinding was maintained for the follow‐up period. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[t]he efficacy analysis included all patient who were randomised to therapy, fulfilled the mycologic criteria at screening, took at least one dose of the study drug, and had at least one post‐baseline assessment." Comment: numbers of participants screened and included in the study are shown. Enough information provided to conduct an ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blind, parallel group study | |
| Participants | Number of participants randomised: 195 Sex: 64% male Mean age: 49 years Number included in analysis: 170 Number completing treatment: not stated Inclusion criteria: men and women 18 or over Type/location/characteristics of infection: clinical diagnosis of distal subungual or proximal onychomycosis and growth of dermatophytes in a mycological culture up to 12 weeks after start of treatment Duration of infection: not stated Exclusion criteria: pregnant or breastfeeding women, participants with pre‐existing renal, hepatic or gastrointestinal disease, bacterial or yeast infections of the nails or the periungual area, or psoriasis or psoriatic changes of the toenails Washout period: 3 months for systemic, 1 month for topical Setting: Germany multicentre Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 40 weeks Outcomes measured: mycological cure, area and length of the affected nail Safety and tolerability assessed by: number of adverse events, packed cell volume, haemoglobin concentration, erythrocyte and leucocyte counts, erythrocyte indices and concentration of creatinine, cholesterol, triglyceride, gamma glutamyltransferase, glutamic‐oxoacetic transaminase, glutamic‐pyruvic transaminase, alkaline phosphatase, bilirubin and potassium | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[t]he patients were randomly assigned to treatment with either terbinafine or itraconazole according to a computer generated randomisation schedule in the order of obtaining informed consent. A restricted form of randomisation was used to provide blocking over time. All centres received multiple complete blocks of length four, thus the assignments of each treatment were balanced after each block." Comment: random sequence was computer generated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[t]o keep the treatment double‐blind the patients additionally took a placebo of the comparative drug." Comment: adequate participant blinding likely to have occurred with this method |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[a]t each visit nail clippings were taken and sent to a central laboratory", "Clinical response to treatment was monitored by observing the movement of a scratch at the border between infected and normal areas on the patient's most affected nail". Comment: method blinding of outcome assessment not stated for these assessments |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a]ll the patients except two who did not have any follow up visits were included in the analyses of drug tolerance. All patients who completed the study as planned and all those who stopped treatment because of adverse events or ineffectiveness of treatment were included in the analysis of efficacy. One last result was used in patients who withdrew from the study." Comment: there is no outcome data for 25 of the original 195 participants entered into the study. 11 were excluded due to not following protocol, 3 were excluded due to concomitant disease, 7 due to adverse events. Therefore most attrition is appropriately accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 40 Sex: not stated (both sexes included) Mean age: 55.5 years and 51.6 years in the treatment groups (range 28‐80) Number included in analysis: 26 (40 for adverse events) Number completing treatment: 26 Inclusion criteria: toenail onychomycosis Type/location/characteristics of infection: lateral or distal subungual onychomycosis of the toenails positive for fungal elements on KOH and culture Duration of infection: not stated Exclusion criteria: superficial white onychomycosis, systemic mycotic disease, significant systemic illness, pregnancy and lactation, porphyria, hepatic disorders Washout period: 1 month for systemic, 2 weeks for topical Setting: USA Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 6 months Outcomes measured: clinical cure, mycological cure Safety and tolerability assessed by: side effects, monthly blood tests including LFTs and CBC | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were assigned on a randomised schedule in a double‐blind manner to one of two groups." Comment: method of sequence generation not stated. No demographic table provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were assigned on a randomised schedule in a double‐blind manner to one of two groups." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[o]ne group received ketoconazole in a dosage of 200 mg/day and placebo griseofulvin. The other group was given placebo ketoconazole and active micro size griseofulvin in a dosage of 1 gm/day." Comment: adequate blinding likely to have occurred with this double‐dummy method. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[p]atients were examined every four weeks for six months by clinical observation, photography and measurement of involved nails …" Comment: states double‐blinded, but no method of outcome assessor blinding stated. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[t]wo individuals were lost to follow‐up in the ketoconazole group", "adverse reactions severe enough to necessitate discontinuance of the trial medication occurred in four of the ketoconazole‐treated patients and in eight of the griseofulvin‐treated patients" Comment: high dropout rate, (of 40 randomised patients, 26 completed treatment and 26 were included in efficacy analysis), but all dropouts are explained and accounted for, and all 40 patients were included in adverse events analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 372 Sex: not stated (both sexes included) Mean age: not stated, but over 18 years to enter study Number included in analysis: varies for each outcome. Article states there are 372 in ITT analysis. 331 participants in mycology data; 336 participants in clinical data. Number completing treatment: 331 Inclusion criteria: age 18 or older, subungual dermatophyte infection confirmed by direct microscopy and dermatophyte culture Type/location/characteristics of infection: not stated Duration of infection: not stated Exclusion criteria: non‐dermatophytic onychomycosis, pregnancy or breastfeeding, concomitant disease or conditions interfering with absorption from the GI tract, significant kidney or liver disease, hypersensitivity to the antifungals being used, alcohol abuse, radiotherapy, clinically relevant abnormalities in values for creatinine, ALT, AST, GGT, ALP, bilirubin Washout period ‐ 4 months for oral antifungal treatment, 1 month for topical antifungal treatment Setting: Belgium multicentre Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 48 weeks Outcomes measured: mycological and clinical cure for target nail selected at start of study. Overall efficacy, overall tolerability as rated by participants and investigators. Safety and tolerability assessed by: adverse events, LFTs, kidney function tests | |
| Source of funding | Supported by an educational grant from Novartis Pharmaceuticals Corporation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding (Novartis). No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[t]he randomization list was computer‐generated in balanced blocks of four" Comment: low risk of selection bias as robust method used for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "[e]ligible patients received a numbered box containing the study medication." Comment: low risk of selection bias as allocation concealment carried out |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[a] double‐dummy technique was used: each daily dose was either a 250 mg terbinafine tablet (250 mg/day dose) and placebo capsules or two 100 mg itraconazole capsules (200 mg/day dose) and a placebo tablet. Patients were instructed to take two capsules and one tablet daily after the evening meal for 12 weeks. These instructions were printed on each medication blister pack" Comment: low risk of performance bias as robust double‐dummy method used for blinding of participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: states investigators were blinded, but details of method not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "[a] total of 372 patients (186 in each group) with dermatophyte infection confirmed by microscopy and culture were included in the intent‐to‐treat analysis." "At week 48 the percentage of patients with negative microscopy was statistically significantly higher in the terbinafine group than in the itraconazole group (77.9% [127 of 163] vs 55.4% [93 of 168])." Comment: article states there were 372 participants in the ITT analysis but 331 and 336 participants were included in mycological and clinical cure data, respectively. Reasons for non‐inclusions not clear. Number of missing participants similar across treatment groups |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: randomised double‐blind controlled trial | |
| Participants | Number of participants randomised: 297 Sex: not stated (both sexes included) Mean age: not stated, range 18‐65 years Number included in analysis: ITT population of 292 (146 in each group); 289 were available for efficacy (145 in itraconazole group and 144 in terbinafine group) Number completed treatment: 258 started the follow‐up period Inclusion criteria: age 18‐65 years, microscopically and culturally proven onychomycosis of the toenail Type/location/characteristics of infection: onychomycosis of toenail caused by dermatophyte with no evidence of a superimposed Candida infection, more than 50% of surface of at least 1 nail affected (or if lunula involved, at least 25% of surface of 1 nail affected) Duration of infection: not stated Exclusion criteria: abnormal LFTs, pregnancy/lactation, psoriasis, concurrent use of rifampicin, phenytoin, digoxin, oral anticoagulants or H2‐receptor antagonists, serious disease, previous hypersensitivity to azoles or terbinafine Washout period: 3 months for systemic antifungals, 1 month for topical antifungals Setting: Europe multicentre Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 48 weeks Outcomes measured: mycological cure, clinical evaluation, clinical response Safety and tolerability assessed by: adverse events, blood samples for haematology and biochemistry, LFTs | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients, who gave written informed consent to participate, were randomised to 12 weeks' treatment with itraconazole 200 mg daily or terbinafine 250 mg daily." Comment: method of sequence generation not stated. Demographics ‐ no significant differences between groups |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[r]andomized double‐blind comparison" Comment: study states that it is blinded, but no method stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[r]andomized double‐blind comparison", "secondary efficacy variables were investigator's global clinical evaluation of response to treatment, performed at the end of treatment and at each visit during follow‐up" Comment: study states it is blinded, but no method of assessor blinding stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 299 patients were recruited into the trial, of whom 297 were randomised to treatment. The intention‐to‐treat population comprised 292 patients, 146 in each group; 289 patients were considered evaluable for efficacy." "The intention‐to‐treat worse‐case analysis was the primary analysis for safety." Comment: dropouts accounted for (except for the 5 participants in the original 297 randomised who were not included in the 292 ITT population). Low dropout rate between ITT group and "those considered evaluable for efficacy". ITT analysis carried out for some outcomes |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 358 Sex: 77% male Mean age: 45 years Number included in analysis: 238 Number completing treatment: not stated Inclusion criteria: mycological confirmation of onychomycosis and evidence of ability of nail to grow Type/location/characteristics of infection: distal subungual onychomycosis of at least 1 great toenail (if both great toenails affected, more severe was selected for observation and testing) Duration of infection: not stated Exclusion criteria: psoriasis, proximal subungual onychomycosis, superficial white onychomycosis Washout period: 3 months for oral antifungals, 1 month for topical antifungals Setting: multicentre, USA and Canada Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 24 weeks follow‐up without treatment. Those with negative mycology and > 5 mm unaffected nail growth were followed up for an additional 48 weeks Outcomes measured: negative mycology (negative culture and KOH), length of unaffected nail,% nail involvement, participant‐ranked response to therapy (excellent, very good, slight improvement, fair, poor/no effect), recurrence rate Safety and tolerability assessed by ‐ physical exam, vital signs, lab evaluation (haematology, LFTs, bilirubin), reports of adverse events | |
| Source of funding | Supported by Novartis pharmaceuticals cooperation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding (Novartis), several authors work for Novartis | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomised" Comment: method of random sequence generation not stated. Baseline characteristics similar for different groups |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]he 96‐week study included a double‐blind treatment phase of 24 weeks in which patients were randomly assigned to one of three parallel groups: oral terbinafine for 12 weeks then placebo for 12 weeks, oral terbinafine for 24 weeks, or placebo for 24 weeks." "This phase was followed by 24 weeks of blinded follow‐up without treatment (weeks 24 to 48)" Comment: study states participants were blinded, and all treatment groups were given therapy for the same duration; however it is unclear if placebo and terbinafine were indistinguishable |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[m]ycologic and clinical assessments were performed on the 'target' nail" Comment: study states evaluators were blinded, but no detail on method of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "[a] total of 358 patients … were enrolled." "Patients were randomly assigned to one of three parallel groups in a 2:2:1 ratio: oral terbinafine for 12 weeks (N = 142), oral terbinafine for 24 weeks (N = 145), or placebo (N = 71)." "At week 48, 70% of patients treated with terbinafine for 12 weeks and 87% of patients treated for 24 weeks exhibited both negative microscopy and negative culture versus 9% for placebo‐treated patients." No patient numbers provided for these percentages Comment: number of participants reported in outcome data is not always clear 358 participants were randomised (287 into terbinafine groups and 71 into placebo group). The number included in short‐term follow‐up data is unclear as only percentages are reported. For long‐term follow‐up, where actual patient numbers are reported, 238 participants were included in long‐term follow‐up analysis; those with new unaffected nail by week 48 were designated by protocol to be followed up for an additional 48 weeks. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: multicentre, randomised, double‐blind placebo‐controlled study | |
| Participants | Number of participants randomised: 221 Sex: not stated (both sexes included) Mean age: not stated, range 18‐70 years Number included in analysis: 214 Number completing treatment: not stated Inclusion criteria: 18‐70 years old with onychomycosis of the toenail confirmed by positive results of KOH and positive culture for a dermatophyte; AND at least 25% involvement of nail bed of a great toe. Persons with total dystrophic nail bed disease were also included. Type/location/characteristics of infection: global evaluation of cleared or marked improvement Duration of infection: not stated Exclusion criteria: onychomycosis due to moulds/Candida spp without dermatophyte, psoriasis or history thereof, baseline LFTs > 2 upper limit of normal, H2‐R blockers, antacids, rifampin, phenobarbital, phenytoin, carbamazepine, terfenadine, digoxin, hypersensitivity to azole compounds, investigational drug use within 1 month prior to screening visit, systemic antifungal therapy within 2 months or topical antifungal therapy within 2 weeks before screening visit, participation in a clinical trial for onychomycosis treatment within 6 months before screening visit, pregnant, nursing mothers. If of childbearing potential, were required to use contraception Washout period: 6 months Setting: USA (multicentre) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 9 months after treatment Outcomes measured: global evaluation based on reduction in extent of nail involvement and improvement in clinical signs as compared to baseline, KOH Ex, culture findings Safety and tolerability: well tolerated, adverse events similar to placebo | |
| Source of funding | Janssen Research Foundation | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he study participants were randomly assigned to twelve weeks of treatment with either two 100 mg capsules of itraconazole once a day or matching placebo." Method not stated. Baseline characteristics of groups similar |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he study participants were randomly assigned to twelve weeks of treatment with either two 100 mg capsules of itraconazole once a day or matching placebo." Comment: method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[t]he study participants were randomly assigned to twelve weeks of treatment with either two 100 mg capsules of itraconazole once a day or matching placebo." Comment: matching placebo method used to blind participants. Likely adequate blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[d]ouble‐blind", "Clinical success was defined as a global evaluation of cleared or markedly improved any time during the trial for the first time." Comment: no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "[t]he primary statistical analysis was for the intent‐to‐treat population, which included all patients who … received at least one dose of double‐blind medication and had at least one visit after baseline." "The proportion of placebo patients who did not complete was significantly greater in the placebo group than that in the itraconazole treatment group: 54 of 104 placebo patients discontinued the study while only 19 of 110 itraconazole‐treated patients discontinued before trial completion." Comment: unexplained discontinuations considerably higher in placebo group than treatment group, but this does not affect our analysis. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All presspecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: parallel group RCT. | |
| Participants | Number of participants randomised: 42 Sex: 75% male Mean age: 57.3 years and 55.5 years (range 23‐83) Number included in analysis: 39 Number completing treatment: 39 Inclusion criteria: mycological diagnosis (KOH test and culture for dermatophyte) of onychomycosis Type/location/characteristics of infection: distal subungual onychomycosis affecting at least 1 great toenail Duration of infection: at least 1 year Exclusion criteria: not stated Washout period ‐ not stated Setting: USA (multicentre) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 24 weeks after treatment Outcomes measured: mycology, nail growth measurement, skin tests (TRIPA‐reactivity) Safety and tolerability: not mentioned | |
| Source of funding | Supported in part by Novartis as an unrestricted, educational grant to the department. "No authors have received personal financial support for this study." | |
| Conflict of interest | "Conflict of interest: [3 authors] are or have been consultants for Novartis". | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]wo of 3 patients in each group (TRIPA‐reactive and TRIPA‐nonractive) were randomised to receive terbinafine, 1 of 3 to receive placebo." Comment: method of random sequence generation not stated. Baseline characteristics of groups similar |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]he study design was a double‐blind comparison of the effects of terbinafine with placebo tablets." Comment: states double‐blinded, but no method (e.g. double‐dummy or indistinguishable tablets) stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[m]ycologic assessment, nail growth measurements, and skin tests were repeated at week 12 (end of treatment), week 24, and week 36." Comment: states double‐blinded, but no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Table I. No of patients discontinued (before receiving treatment): 2 in terbinafine group, 1 in placebo group." Comment: number of participants discontinuing in each group is shown; low dropout number. Based on tabulated data, 39 of the 42 randomised participants were included in the analysis. ITT analysis not used |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 218 Sex: 75% male Mean age: 50.8 years (range 18‐75) Number treated: 216 Number completed: 178 Inclusion criteria: clinical and mycological diagnosis of onychomycosis and evidence of ability of nail to grow Type/location/characteristics of infection: approx 25‐75% of at least 1 great toenail (if both great toenails affected, more severe was selected for observation and testing) Duration of infection: not stated Exclusion criteria: pregnancy, lactation, nail abnormalities other than onychomycosis, liver disease, family history of long QT syndrome, clinically significant condition Washout period: 3 months for oral antifungals, 1 month for topical antifungals Setting: 23 centres in USA Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 24 or 36 weeks (up until 48 weeks after 1st dose) Outcomes measured: complete cure = negative mycology (negative culture and KOH) and clinical cure (0% nail involvement, i.e. absence of onycholysis/subungual hyperkeratosis) Treatment success = negative mycology and < 10% nail involvement Safety and tolerability assessed by: reports of adverse events, ECG, vital signs, physical exam, lab tests (LFTs, haematology) | |
| Source of funding | This study was funded by Schering‐Plough Corporation, now Merck and Co, Inc, Whitehouse Station, NJ, USA | |
| Conflict of interest | Author conflicts of interest disclosed include employee of Merck and Co | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[p]atients were randomized according to a computer‐generated randomization schedule using a central interactive voice‐ response system." Comment: robust random sequence generation method used |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[p]atients in the 24‐week treatment regimens were blinded with respect to whether they received posaconazole or identical (in terms of taste and appearance) placebo. Patients in the 12‐week posaconazole treatment regimen were blinded to treatment until week 12, when they ended therapy. Patients in the 12‐week terbinafine treatment regimen were aware of their treatment assignment." Comment: not all treatment groups blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[t]his was an investigator‐blinded study. The investigator or qualified designee performed the clinical assessments at each visit and remained blinded to all treatment arms." Comment: no mention of method of blinding, study itself was partially unblinded (see above) |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[t]he primary efficacy analysis was based on the modified intent‐to‐treat population (defined as randomised subjects who had a baseline assessment and at least one post baseline assessment available, and who had been exposed to at least one dose of study medication." Comment: low risk of attrition bias as clear subject completion numbers and intent‐to‐treat analysis all provided |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 89 Sex (M/F): 65/24 Mean age: 45 years (range 18‐71) Number included in analysis: 84 Number completed treatment: NA because there was an open component to the study after the double‐blinded comparison Inclusion criteria: culture‐proven dermatophyte infection Type/location/characteristics of infection: involving at least 1 of digits 2‐4 Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: Sweden (multicentre) Comorbidities: not stated | |
| Interventions |
Participants who did not improve after 16 weeks were entered into an open‐label study and given 250 mg/day terbinafine for 16 weeks with the study code still blinded. | |
| Outcomes | Duration of follow‐up: NA because there was an open component to the study after the double‐blinded comparison. Considered 16 weeks as this is when complete randomisation was lost Outcomes measured: mycological cure, clinical cure Safety and tolerability assessed by: side effects, LFTs | |
| Source of funding | No information given | |
| Conflict of interest | One author is an employee of the research and development laboratories from Pfizer inc | |
| Notes | There was an open‐label phase after the double‐blinded comparison, but the 'results of treatment: end point analysis' appears to describe the double‐blinded phase only, before randomisation was lost. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he patients were randomly assigned." Method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he patients were randomly assigned." Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[d]ouble‐blind", "Patients who did not improve after 16 weeks were entered into an open study and given 250 mg/day terbinafine for 16 weeks, with the study code still blinded, and then followed up for 20 weeks." States double‐blinded, but no method of blinding stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[d]ouble‐blind" States double‐blinded, no mention of blinding method |
| Incomplete outcome data (attrition bias) | Low risk | 89 patients were randomised, 84 were included in analysis efficacy analysis and 89 in adverse events analysis. Comment: dropouts are explained, low dropout rate. Enough data given to allow ITT analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 112 (99 toenail) Number included in analysis: 85 Sex (M/F): 55/30 Mean age: 44 years (range 19‐78) Number completing treatment: not clear Inclusion criteria: mycological and clinical evidence of dermatophyte infection of fingernails and toenails Type/location/characteristics of infection: toenail and fingernail infections (worst affected nail selected for assessment) Duration of infection: not stated Exclusion criteria: renal/hepatic/GI disease, psoriasis, yeast infection of nails, pregnancy, lactation Washout period: not stated Setting: 8 dermatology centres in UK Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 36 weeks after treatment Outcomes measured: mycological cure = negative microscopy and culture, clinical cure Safety and tolerability assessed by: adverse event reporting, biochemical and haematological variables | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[p]atients … were randomised (with random tables of Fisher and Yates)" Comment: method of random sequence generation adequate to minimise selection bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised in a double‐blind, placebo controlled parallel group comparison." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[p]atients were randomised in a double‐blind, placebo controlled parallel group comparison." Comment: states double‐blinded, but no method stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[p]atients were seen at monthly intervals throughout the treatment period. At each visit the mycological, biochemical and haematological investigations were repeated; compliance and the occurrence of side effects were ascertained, and the target nail was examined clinically." Comment: no mention of outcome assessor blinding method |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[o]ne hundred and twelve patients were enrolled into the study, 99 with toenail infection." Comment: data provided for both ITT analysis and per protocol analysis (Table II) |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 200 Number included in analysis: 152 Mean age: 45 years Sex (M/F): 117/35 Number completing treatment: 152 Inclusion criteria: onychomycosis confirmed by microscopy and positive culture Type/location/characteristics of infection: 'target' great toenail was the more severely affected, with > 25% involvement of nail surface and < 75% nail plate involvement Duration of infection: not stated Exclusion criteria: hypersensitivity to imidazole derivatives, onychomycosis associated with moulds without dermatophytes or Candida, elevated LFTs, serious illness, psoriasis, taking drugs that interact with itraconazole Washout period: 6 months for oral antifungals, 2 weeks for topical antifungals Setting: Canada, outpatients Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 36 weeks after treatment Outcomes measured: clinical appearance relative to baseline visit (cleared, markedly improved, slightly/moderately improved, unchanged, deterioration), mycological success = KOH and culture both negative Safety and tolerability assessed by: adverse event reports | |
| Source of funding | This study was supported by a grant from Janssen‐Ortho Inc, Canada | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding (Janssen‐Ortho). No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to treatment with either two 100‐mg capsules of itraconazole or matching placebo capsules twice daily (i.e. 400 mg daily)." Comment: method of random sequence generation not stated. Baseline characteristics not different between groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to treatment with either two 100‐mg capsules of itraconazole or matching placebo capsules twice daily (i.e. 400 mg daily)." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[t]he active and placebo formulations were packaged so that both the patient and the investigator were blinded." Comment: adequate blinding likely occurred using this method |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[d]ouble‐blind", "At the end of treatment at week 9 and at weeks 12, 24, 36 and 48, the investigator categorized the disease state of the target toenail relative to the baseline visit" Comment: no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 20 and 28 patients were excluded from the itraconazole and placebo groups, respectively, when evaluating efficacy. The reasons were (itraconazole vs placebo groups): culture negative at prescreen (0 vs 3 patients), KOH negative at prescreen (12 vs 17), culture and KOH negative at prescreen (0 vs 2), patient discontinued before week 9 (7 vs 5) and no KOH or culture data available at week 9 (1 vs 1)." Comment: reasons for dropouts explained in the text. Similar number of dropouts between groups. Sufficient data provided to complete ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: single‐blind RCT | |
| Participants | Number of participants randomised: 101 Sex (M/F): 52/49 Mean age: 53.1 years; elderly only Number included in analysis: 101 Number completing treatment: 101 Inclusion criteria: onychomycosis caused by a dermatophyte involving at least 1 big toe, in a participant aged > 60 Type/location/characteristics of infection: not stated Duration of infection: not stated Exclusion criteria: history of hypersensitivity or allergic reaction to terbinafine or azoles; intake of any medications known to interact with terbinafine or itraconazole Washout period: not stated Setting:USA and Canada (multicentre) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 18 months Outcomes measured: clinical evaluation (estimation of the nail plate area involved). Mycologic examination (microscopy, culture). Clinical efficacy (mycologic cure + either clinical cure or reduction of clinically involved nail plate to < 10%). Safety and tolerability assessed by: bloodwork (LFTs, CBC), adverse events reported by participant at each visit (investigator asked to determine potential relationship of the AE to the study drug) | |
| Source of funding | Study is stated to be non‐industry‐sponsored | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a]ll consenting, consecutive patients were randomly assigned to blocks of 6 to receive terbinafine (continuous) or itraconazole (pulse) therapy." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]erbinafine (continuous) therapy was 250 mg/day administered for 12 weeks. Itraconazole pulse therapy, 200 mg twice daily given for 1 week with 3 weeks off between successive pulses, was administered for 3 pulses. Subjects were asked to take the medications after a meal." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[s]ingle‐blind", "the clinical evaluation was performed in a single‐blinded manner so that the evaluator was not aware of the randomization order or therapy being received by the patient." Comment: high risk of performance bias as participants and personnel likely not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[t]he clinical evaluation was performed in a single‐blinded manner so that the evaluator was not aware of the randomisation order or therapy being received by the patient." Comment: likely adequate blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[t]here were a total of 101 patients with 50 receiving terbinafine continuous treatment and 51 patients administered itraconazole (pulse) therapy" Comment: low risk of attrition bias as outcomes of all patients included in analysis, with no exclusions |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: single‐blinded RCT | |
| Participants | Number of participants randomised: 59 Sex (M/F): 48/11 Mean age: 68 years Number included in analysis: 59 Number completing treatment: 56 Inclusion criteria: age > 18 years Type/location/characteristics of infection: toenail onychomycosis caused by S brevicaulis spp Distal and lateral onychomycosis, moderate‐severe disease of target nail Duration of infection: not stated Exclusion criteria: allergy to any of the drugs in the study, medications known to interact with study medications, immunocompromise, pregnancy and lactation Washout period: 6 months for oral and 2 weeks for topical Setting: USA and Canada (multicentre) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: clinical cure, mycological cure Safety and tolerability assessed by: side effects, LFTs, CBC, RFTs | |
| Source of funding | Study is stated to be non‐industry‐sponsored | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[e]ligible patients with onychomycosis due to S. brevicaulis were randomly divided to receive treatment with griseofulvin, ketoconazole, itraconazole, terbinafine and fluconazole". "At baseline, for the 5 treatment groups, there was no significant difference in the mean age of the patients or mean severity of disease". Comment: no method of random sequence generation stated. Baseline characteristics between groups appear similar from the text, but no tabulated data provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[e]ach consecutive patient who fulfilled the inclusion criteria was considered for the study". Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[s]ingle‐blinded", "Treatment with the oral agents was given as follows: griseofulvin 600 mg twice daily for 12 months, ketoconazole 200 mg daily for 4 months, itraconazole 3 pulses with each pulse consisting of 200 mg twice daily for 1 week on, 3 weeks off, terbinafine 250 mg daily for 12 weeks, and fluconazole 150 mg/day for 12 weeks." Comment: study states that it is single‐blinded, but does not state any methods for blinding participants or personnel |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[s]ingle‐blinded", "When the patients were seen at the follow‐up, at month 12 from the start of treatment, the efficacy parameters were CC (clinical cure) and MC (mycological cure)". Comment: study states that it is single‐blinded, but does not state any methods for blinding outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[i]n this study a total of 59 patients with S. brevicaulis onychomycosis of the toes were evaluated". "Patients were randomised to the following groups: griseofulvin (11 patients), ketoconazole (12 patients), itraconazole (12 patients), terbinafine (12 patients) and fluconazole (12 patients)." Comment: low risk of attrition bias as outcomes for all randomised participants reported, with no exclusions |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: single‐blind, randomised, prospective study | |
| Participants | Number of participants randomised: 190 (IIT: 93, TTT: 97). 14 participants who did not meet inclusion/exclusion criteria did not commence the study ‐ 176 started treatment (IIT: 81, TTT: 95) Number included in analysis: 165 (IIT: 75, TTT: 90) Number completing treatment: 165 (IIT: 75, TTT: 90) Sex (M/F): IIT 33/42, TTT 60/30 Mean age: 35.6 years (range 25‐53) Inclusion criteria: at least 18 years old, clinical diagnosis of distal and lateral onychomycosis of the toes. Dermatophyte had to be aetiologic organism Type/location/characteristics of infection: distal and lateral onychomycosis of the toes Duration of infection: not stated Exclusion criteria: those who had received oral antifungal therapy within the previous 3 months or applied topical antifungal to the feet during the previous 1 month, proximal subungual or white superficial onychomycosis, onychomycosis caused by Candida or nondermatophyte moulds, participants taking medications known to interact with itraconazole or terbinafine, individuals with concomitant nail disease such as psoriasis or lichen planus. Prengnancy, lactation, inadequate contraception, history of renal disease, participants with baseline liver function tests (ALT, AST, alkaline phosphatase, total bilirubin) elevated to more than twice the upper limit of normal Washout period: not stated Setting: 3 outpatient dermatology offices in North America Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 72 weeks Outcomes measured: mycological cure rate (negative light microscopy and culture), clinical cure (nail plate appeared completely normal), effective therapy (mycological cure and outgrowth of at least 5mm new clinically unaffected nail plate) and complete cure (mycological and clinical cure simultaneously), recurrence rate Safety and tolerability assessed by: adverse effect reporting; liver enzymes changes are stated but whether they were tested for routinely is unclear | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[r]andomised", "Patients were assigned to one arm of the study or the other in balanced blocks of 6 at each centre". "The baseline characteristics of age, race, duration of onychomycosis, causative organism, and percentage of toenail involved were similar, with no statistically significant difference between the 2 treatment groups". Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he nature of the treatment was discussed with each patients, and informed consent was obtained". Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]he study was single‐blinded with the evaluator of the primary and secondary outcome measures not being aware of the randomization order or the treatment being administered to the patient". Comment: high risk of performance bias as participants and personnel likely not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[t]he study was single‐blinded with the evaluator of the primary and secondary outcome measures not being aware of the randomization order or the treatment being administered to the patient". Comment: likely adequate blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 190 patients were found to have dermatophyte toe onychomycosis after initial screening for the study and were randomised (IIT: 93, TTT: 97). Fourteen patients were found to violate inclusion/exclusion criteria or decided not to start therapy after randomization but before start of treatment. The number of patients who received intervention therapy were IIT 81 and TTT 95. At the end of week 72, there were 75 patients in the IIT group who were regarded as having completed the study with 6 withdrawals. In the TTT group, the corresponding numbers were 90 and 5 patients, respectively." Comment: low number of exclusions with reasons for exclusion stated in the text. Enough data provided to perform an ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 151 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 135 Number completing treatment: 135 Inclusion criteria: onychomycosis confirmed by direct microscopy and/or fungal culture Type/location/characteristics of infection: distal subungual onychomycosis of 1 great toenail (min area 25%), and at least 2 mm proximal nail clear Duration of infection: not stated Exclusion criteria: conditions known to produce abnormal‐appearing nails (psoriasis), proximal subungual onychomycosis, white superficial onychomycosis, allergy to azole drugs, use of drugs that prolong QT interval, abnormal LFTs Washout period: 3 months for oral antifungals, 2 weeks for topical antifungals Setting: 10 dermatology practices in USA, Canada, France Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 36 weeks after treatment Outcomes measured: effective cure (mycological and clinical cure or > 30% improvement), percentage of nail plate infected, length of unaffected nail, mycological examination of cultures, concentrations of ravuconazole in plasma and in toenails Safety and tolerability assessed by: adverse event reports, haematology, serum chemistry, urinalysis | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[u]pon subject enrolment, subject number and treatment assignment were obtained from a central call‐in randomization system". "Age, sex and race distribution was similar between the four treatment groups." Comment: likely robust randomisation using this method. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Low risk | Quote: "[u]pon subject enrolment, subject number and treatment assignment were obtained from a central call‐in randomization system". Comment: allocation concealment likely achieved using the method above, as patients very unlikely unable to preempt treatment allocation prior to study enrolment |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[m]edication was administered in a double‐dummy fashion. Placebo tablets were identical to the treatment drug tablets. Neither subject nor evaluating physician was aware of which treatment group the subject had been assigned to." Comment: blinding of participants and personnel likely adequate using this double‐dummy method |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[n]either subject nor evaluating physician was unaware of which treatment group the subject had been assigned to". Comment: outcome assessment likely blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[o]ne hundred and fifty‐one subjects were randomised by 10 investigators in three countries … Of these 151 subjects, 148 received at least one dose of the study medication, and 135 were evaluable at the end of the study." "Of the 16 subjects who were randomised to treatment but were not evaluable at the test of cure visit, three were not treated because of withdrawal of consent or inability to comply with protocol requirements. Of the 13 treated subjects who were randomised to treatment but not evaluable at the test of cure visit, three had no test of cure evaluation performed, and 10 subjects did not complete the study". Comment: low number of dropouts, with reasons for exclusion explained Sufficient data provided to complete ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 70 Sex: not stated (both sexes included) Mean age: not stated Number treated: 70 Number completed: 64 Inclusion criteria: clinical and mycological diagnosis of onychomycosis with type I or type II diabetes Type/location/characteristics of infection: dermatophyte infection of at least 1 great toenail with 10% or more involvement Duration of infection: not stated Exclusion criteria: pregnancy, breastfeeding, malignancy other than basal cell carcinoma or squamous cell carcinoma, abnormal liver function tests, uncontrolled renal/hepatic disease, immunosuppressant treatment Washout period: 12 months for oral antifungals, 4 weeks for topical antifungals Setting: USA (2 private practices) Comorbidities: type 1 or type 2 diabetes | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 48 weeks Outcomes measured: comple/e cure = negative mycology (negative culture and KOH) and effective cure (0% nail involvement, i.e. absence of onycholysis/subungual hyperkeratosis). Treatment success = negative mycology and <10% nail involvement Safety and tolerability assessed by: reports of adverse events and LFTs | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[p]atients meeting inclusion/exclusion criteria with dermatophyte onychomycosis of the target great toenails were allocated through computer‐generated block randomization in blocks of 10 in a ratio of 1:1 to one of the two treatment groups". Comment: robust randomisation likely achieved with the above method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[r]andomization was concealed and performed by someone other than the investigators assessing the outcome measures." Comment: no method of allocation concealment stated and unclear whether allocation was concealed from participants as well as investigators |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[s]ingle‐blind", "evaluator‐blind", "Randomisation was concealed and performed by someone other than the investigators assessing the outcome measures. During the treatment period, the designated evaluators remained blinded." Comment: high risk of performance bias as participants likely not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[d]uring the treatment period, the designated evaluators remained blinded." Comment: outcome assessor blinding likely adequate |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[s]eventy patients were enrolled; this was the intention‐to‐treat population. Six patients withdrew consent, one from the itraconazole and five from the terbinafine treatment arms. The patient from the itraconazole group withdrew because of gastric side effects. The remaining five patients withdrew for reasons unrelated to the study medication". "Missing data was entered with the last observation carried forward method". "All subjects who received at least one dose of the treatment medication were included in the analysis." Comment: small number of dropouts, with reasons for exclusion detailed in the text. Anlysis was performed using the last observation carried forward method. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: randomised, evaluator‐blind, comparator‐controlled trial | |
| Participants | Number of participants randomised: 142 Sex: not stated (both sexes included) Mean age: 51 years (range 23‐98) Number treated: 142 Number completed: 105 Inclusion criteria: clinical and mycological (positive potassium hydroxide (KOH] and culture) Type/location/characteristics of infection: dermatophyte infection of at least 1 great toenail with 20% or more involvement Duration of infection: not stated Exclusion criteria: pregnancy, breastfeeding, malignancy other than basal cell carcinoma or squamous cell carcinoma, abnormal liver function tests, uncontrolled renal/hepatic disease, immunosuppressant treatment Washout period: 12 months for oral antifungals, 4 weeks for topical antifungals Setting: Canada (2 outpatient clinics) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 72 weeks Outcomes measured: mycological cure rates (negative KOH and culture) and effective cure rate (simultaneous mycological cure and ≤ 10% nail plate involvement) Safety and tolerability assessed by: reports of adverse events and LFTs | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[d]ata from a Canadian study of continuous terbinafine and intermittent itraconazole was compared to an intermittent terbinafine regimen using a similar protocol to the randomised study." "Patients attending one of two Southwestern Ontario (Canada) dermatology clinics who met the treatment criteria were provided with an intermittent terbinafine regimen (TOT)". Comment: patients not randomised as all patients received the same treatment. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[d]ata from a Canadian study of continuous terbinafine and intermittent itraconazole was compared to an intermittent terbinafine regimen using a similar protocol to the randomised study." "Patients attending one of two Southwestern Ontario (Canada) dermatology clinics who met the treatment criteria were provided with an intermittent terbinafine regimen (TOT)". Comment: all patients received the same treatment, so allocation concealment likely not performed |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]he study medications were obtained by subjects from a local pharmacy by prescription and self‐administered." Comment: high risk of performance bias as participants unlikely to be blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[a]ll nail samples were processed by a local mycology laboratory with the laboratory staff blinded to the treatment arm and the treatment time‐point." Comment: outcome assessment blinding likely adequate |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[i]n the TOT group, one patient did not continue follow‐up to week 48 (reason unknown). Another patient completed treatment despite an AE of restlessness, but was then lost to follow‐up. An additional 12 patients were lost to follow‐up between weeks 48 and 72 for reasons related to therapy." Comment: reasons for dropouts explained. Sufficient data to perform ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other clear bias seen |
| Methods | Design: RCT | |
| Participants | Number of participants randomised: 137 Sex (M/F): 60/77 Mean age: 48.8 years Number included in analysis: 137 Number completing treatment: 130 Inclusion criteria: age 18‐65 years, clinical diagnosis of onychomycosis affecting at least ⅓ of a nail, confirmed by positive potassium hydroxide (KOH) wet mount and culture for a dermatophyte Type/location/characteristics of infection: affecting at least ⅓ of the fingernail or toenail Duration of infection: not stated Exclusion criteria: systemic antifungal therapy within the previous 6 months, topical antifungal therapy within the previous month, pregnancy, lactation, any concomitant disease that could affect study outcome, participants on medications known to interact with either study agent, history of allergy to terbinafine or azole drug, discontinuation of previous therapy with terbinafine or an azole drug due to adverse effects Washout period: not stated Setting: Finland; 6 centres Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 60 weeks Outcomes measured: primary efficacy endpoint was mycological cure (negative direct microscopy of KOH wet mount and negative culture for a dermatophyte), clinical evaluation based on 4‐point rating scare (complete cure, minimal symptoms, slight improvement or failure) Safety and tolerability assessed by: reporting of adverse events, physical examination, tolerability of treatment rated on a 5‐point scale (very good, good, moderate, poor, or very poor) by both participant and investigator | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]atients were randomly divided into three groups to receive active treatment with either terbinafine 250 mg daily for 12 weeks, fluconazole 150 mg once weekly for 12 weeks, or fluconazole 150 mg once weekly for 24 weeks". "Patients in all three treatment groups were well matched for age, duration of infection and species of dermatophyte." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly divided into three groups to receive active treatment with either terbinafine 250 mg daily for 12 weeks, fluconazole 150 mg once weekly for 12 weeks, or fluconazole 150 mg once weekly for 24 weeks." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[d]ouble‐blind, double‐placebo", "To maintain blinding, patients in group A also received placebo capsules once weekly for weeks 1‐24; patients in group B also received a placebo tablet daily for weeks 1‐12 and a placebo capsule once weekly for weeks 13‐24; patients in group C also received a placebo tablet daily for weeks 1‐12." Comment: likely adequate blinding of participants achieved |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[d]ouble‐blind". "Mycological evaluation comprised of direct microscopy of a KOH wet mount and culture for a dermatophyte." "Clinical outcome was evaluated separately by the patient and physician." Comment: study states that it is double‐blind; no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 137 patients with mycologically confirmed toenail or fingernail onychomycosis were included in the study. Of these, 130 were evaluable at the 60‐week follow‐up visit." "Seven patients withdrew from the study. Of these, one withdrew due to taste disturbance with terbinafine; one for constipation and depression; one for raised serum alanine aminotransferase level; and one for nausea and diarrhoea. In addition, one subject from each group was withdrawn because of protocol violations." Comment: low number of dropouts, with reasons for exclusion explained. Sufficient data for ITT analysis provided |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: double‐blind study | |
| Participants | Number of participants randomised: 90 Number included in analysis: 74 Sex (M/F): 53/21 Mean age: not stated Number completing treatment: 64 Inclusion criteria: dermatophyte infection, no further criteria given Type/location/characteristics of infection: any location including 20 infected toenails Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: UK Comorbidities: not stated | |
| Interventions |
Both doses were doubled if not sufficient effect after 3 months of treatment | |
| Outcomes | Cure, no further info available | |
| Source of funding | Janssen Pharmaceutical Ltd, UK, supplied the medication ketoconazole | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | This study is not included in the quantitative meta‐analysis as there are a number of different dermatophyte infections included, and extraction on participant level for onychomycosis only is not possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]here were no significant differences in the age, sex, weight or height distribution of either group." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[a] total of 90 patients entered the study. Of these, 16 subsequently failed to attend and so results of treatment in 74 were available for assessment. Thirty‐seven patients were receiving ketoconazole or griseofulvin respectively." Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]he merits of oral ketoconazole and griseofulvin in dermatophytosis have been compared in a double‐blind study on 74 patients with 152 infected sites." Comment: study title states that it is double‐blind, but no method of blinding of participants and personnel described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[d]ouble‐blind". "Patients' symptoms and clinical improvement were analysed using the Wilcoxon test to compare successive time points for each treatment and using the Mann‐Whitney U‐test to compare results of treatments at each visit." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 90 patients entered the study. Of these 16 subsequently failed to attend and so results of treatment in 74 were available for assessment". "Ten patients dropped out of the trial during the study period, seven in the ketoconazole and three in the griseofulvin group. In only one case was this due to a side effect, diarrhoea, attributed to ketoconazole. A further patient taking ketoconazole needed cimetidine for a duodenal ulcer and antifungal therapy was withdrawn." Comment: of the 16 patients who were enrolled but excluded from analysis, 10 were accounted for. There is low risk of attrition bias as the majority of attrition is explained. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 195 Number treated: 195 Sex: 56% male Mean age: 50 years (range 19‐93) Number completed: 171 (24 lost due to protocol violations and no show at follow‐up visits) Inclusion criteria: distal subungual onychomycosis confirmed by culture and wet mount Duration of infection: not described Exclusion criteria: < 18 years, pregnant, breastfeeding, preexisting renal, hepatic or gastrointestinal disease, psoriasis or psoriatic nail changes, bacterial or yeast infections nail Washout period: 3 months oral medication and 1 month topical medication Setting: 22 centres in Germany Comorbidities: no | |
| Interventions |
| |
| Outcomes | Duration of follow‐up:48 weeks and 72 weeks Outcomes measured: negative culture, growth of healthy nail, nail score Safety and tolerability assessed by: adverse event monitoring, transaminase monitoring | |
| Source of funding | This study was supported in part by Sandoz AG, Nurnberg, Germany | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a] total of 195 patients, from 22 centres, were included in the study and were randomised to receive either 250 mg/d of terbinafine (N = 97) or 1000 mg/d of micronized griseofulvin (N = 98)." "Patients' characteristics were comparable for both treatment groups." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised and assigned to one of two treatment groups". Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]he study consisted of a 48‐week double‐blind treatment phase and a 24‐week double‐blind follow‐up phase." Comment: study claims that it is double‐blind; no method of participant or personnel blinding stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[d]ouble‐blind". "Examination for fungi included identification by microscopic evaluation of potassium hydroxide preparation and mycological culture. The clinical response to treatment was monitored by observance of the outgrowth of a scratch mark placed at the border between infected and normal area on each patient's most involved nail, excluding that of the little toe." Comment: study claims that it is double‐blind, no method of outcome assessment blinding stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 195 patients, from 22 centres, were included in the study and were randomised … Fourteen patients in the terbinafine group and 10 patients in the griseofulvin group were excluded from the evaluation of drug efficacy, mainly because of protocol violations in terms of intake of study drugs or non‐appearance for control visits." Comment: small number of dropouts with all exclusions accounted for |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blind parallel group RCT | |
| Participants | Number of participants randomised: 179 Sex: not stated (both sexes included) Mean age: 40.4 years Number treated: 174 Number completed: 135 Inclusion criteria: clinical and mycological diagnosis of onychomycosis Type/location/characteristics of infection: toe with distal subungual onychomycosis Duration of infection: not stated Exclusion criteria: pregnancy, breastfeeding, systemic diseases/conditions that might affect the study and therapy with concomitant drugs that might interfere with the metabolism of the drugs being studied Washout period: 3 months for oral antifungals, 1 month for topical antifungals Setting: South America (multicentre) Comorbidities: none | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 52 weeks Outcomes measured: efficacy = negative mycology (negative culture and KOH) and clinical cure (level of onycholysis / subungual hyperkeratosis/paronychial inflammation). Treatment success = effectively cured participant (> 50% improvement) + mycological cure Safety and tolerability assessed by: reports of adverse events, LFTs, haematology | |
| Source of funding | The study drugs were supplied by Sandoz Basle | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]wo randomised groups of patients with toe distal sub‐ungual onychomycosis received ..." Comment: study claims to be randomised, no method of random sequence generation stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]wo randomised groups of patients with toe distal sub‐ungual onychomycosis received either one tablet (250 mg) of terbinafine pulse itraconazole placebo or two tablets (100 mg each) of itraconazole plus terbinafine placebo, once a day for 4 months." Comment: unclear whether patients or personnel could anticipate patient allocation prior to enrolment |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[t]wo randomised groups of patients with toe distal sub‐ungual onychomycosis received either one tablet (250 mg) of terbinafine pulse itraconazole placebo or two tablets (100 mg each) of itraconazole plus terbinafine placebo, once a day for 4 months." Comment: likely adequate blinding of participants and personnel achieved using this double‐dummy method |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[c]linical assessments at entry and during the visits scheduled in the treatment and post‐treatment phases were performed for onycholysis, hyperkeratosis and paronychial inflammation." "Mycological evaluation … was performed at entry and after the 2nd, 4th, 6th, 8th, 10th and 12th months." Comment: no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "from 179 recruited patients … 6 were excluded from the efficacy analysis as they were only examined at the entry visit and dropped from the study. In the itraconazole group, 2 patients discontinued for unknown reasons. In the terbinafine group, 1 patient discontinued as the nail fell off. Thirty‐nine patients … did not complete the study and were excluded from the final analysis of efficacy at the 12th month. The reasons were protocol violation (18 patients), loss of follow‐up after the 4th month (17 patients) and side effects before the 4th month (4 patients, all in the itraconazole group." Comment: of the initial 179 patients, there were 39 dropouts. These exclusions were accounted for, and most (175 patients) were evaluated for adverse effects. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 73 Number included in analysis: 68 Sex (M/F): 50/18 Mean age: 48 years (range 18‐70) Number completing treatment: not stated. 68 had data beyond the baseline visit Inclusion criteria: T rubrum‐positive onychomycosis of the great toenail. Type/location/characteristics of infection: positive onychomycosis of the great toenail Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: USA Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 12 weeks, then monitoring for relapse Outcomes measured: healthy nail growth,% of nail area involved, signs of onychomycosis, investigator's global evaluation, mycological evaluation Safety and tolerability assessed by: LFTs, urinalysis, urine pregnancy tests | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]hirty‐six of the patients were randomised to receive itraconazole 200 mg daily for 12 weeks; and 37 patients received placebo." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomly assigned to treatment with 100‐mg capsules once daily of itraconazole or placebo". Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[d]ouble‐blind". "Patients were randomly assigned to treatment with 100‐mg capsules once daily of itraconazole or placebo to be taken with a meal at the same time each day for 12 weeks". Comment: states study was double‐blinded, no method of binding participants or personnel (e.g. double‐dummy or matching placebo) stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[d]ouble‐blind". "[Patients] were … evaluated by investigators at wk 4, 8, and 12 for healthy nail growth, percent of nail area involved, signs of onychomycosis, the investigator's global evaluation, and mycologically." Comment: states study was double‐blinded, no method of outcome assessor blinding stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[o]f the 76 patients enrolled in the trial, 68 had data beyond the baseline visit and were included in the results of initial effectiveness". "All 73 patients were included in the safety analysis". Comment: all dropouts accounted for. Sufficient information provided to complete intention‐to‐treat analysis. Safety data reported for most patients enrolled in study |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: open‐label, randomised, parallel‐group study | |
| Participants | Number of participants randomised: 61 Sex (M/F): 27/34 Mean age: 47 years Number included in analysis: 51 Number completing treatment: 61 (10 in the itraconazole group received an additional pulse and were not included in efficacy analysis) Inclusion criteria: distal and lateral subungual onychomycosis with ≥ 50% nail plate involvement; positive KOH test or positive culture; mycologic examination anonymous for the clinician; mycologic evaluation at baseline then every 3rd mo for 9mo after treatment; and lab blood parameters within normal limits. Type/location/characteristics of infection: positive onychomycosis of the toenails Duration of infection: not stated Exclusion criteria: systemic antimycotic therapy within 3 months prior; use of any antifungal agent during trial (incl top); pregnancy/breastfeeding/lack of reliable contraception; use of cisapride, astemizole, terfenadine, simvastatin, lovastatin, phenytoin, triazolam, or rifampin; serious diseases affecting the liver or kidneys; or psoriasis Washout period: not stated Setting: Czech Republic Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: every 3rd month for 9 months after treatment Outcomes measured: affected nails/participants, global clinical parameter, KOH test, culture Safety and tolerability assessed by: reported adverse effects (cephalgia, exanthem, urticaria, diarrhoea, fatigue, dyspepsia, bloating, gryphosis of mycotic nails, obstipation, weight gain, flatulence, myositis) | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a] total of 61 patients were randomly assigned treatment and entered into the study." "Patients were similar in gender and age." Comment: method of random sequence generation not stated. Baseline characteristics similar between treatment groups |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[a] total of 61 patients were randomly assigned treatment and entered into the study." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]his open, randomised, parallel‐group study represents a comparative clinical evaluation of therapeutic efficacy and tolerability of oral itraconazole pulse therapy (400 mg twice daily, 1 week/month, 3 pulses) and continuous terbinafine therapy (250 mg/day, 98 days)." Comment: participants and personnel likely unblinded as study states that it was an open study. Hence, there is a high risk of performance bias, especially as one treatment was pulse therapy and the other was continuous. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[o]pen". "Patients were evaluated every third month for 9 months after treatment". Comment: outcome assessors likely unblinded as study states that it was an open‐label study |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 61 patients were randomly assigned treatment and entered into the study. In the itraconazole arm, 10 patients received an additional pulse and were not included in the efficacy analysis." Comment: all dropouts explained and accounted for. Sufficient data provided for ITT analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 1381 (3:3:1 for intervention 1, 2, and 3, respectively) Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: not stated, only percentages given Number completing treatment: not stated Inclusion criteria: no details given, conference abstract only Type/location/characteristics of infection: no details given, conference abstract only Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: USA and Canada (multicentre) Comorbidities: not stated | |
| Interventions |
Follow‐up 40 weeks after treatment, 52 weeks in total | |
| Outcomes | Complete cure of the big toenail (clinical cure and mycological cure) | |
| Source of funding | Commercial support: 100% is sponsored by Stiefel Laboratories | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | Conference abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a] total of 1381 subjects were randomised (3:3:1) to treatment and received the study drug." Comment: no method of random sequence generation stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[a] total of 1381 subjects were randomised (3:3:1) to treatment and received the study drug." Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[t]his randomised, multicenter, parallel group, placebo‐controlled, evaluator‐blinded study was designed to compare the efficacy of itraconazole given as one 200‐mg tablet QD with itraconazole given in two 100‐mg capsules QD for 12 weeks of treatment and 40 weeks of follow‐up." Comment: study states that it was placebo controlled. Likely adequate blinding of participants and personnel achieved with this method. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[t]his randomised, multicenter, parallel group, placebo‐controlled, evaluator‐blinded study was designed to compare the efficacy of itraconazole given as one 200‐mg tablet QD [4 times daily] with itraconazole given in two 100‐mg capsules QD for 12 weeks of treatment and 40 weeks of follow‐up." Comment: study states that it was evaluator‐blinded. Likely adequate blinding of outcome assessors achieved with this method |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "[a] total of 1381 subjects were randomised ..." "The proportions of subjects (intent to treat population) with complete cure at week 52 were greater in the active treatment groups (22.3% in the itraconazole 200‐mg tablet group and 21.7% in the itraconazole 100‐mg capsule group) compared with the placebo groups (1.0%). Comment: study states that intent to treat population was used for analysis. Outcome data presented as percentages only, with number of participants per group not stated in the text. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: controlled open‐label trial | |
| Participants | Number of participants randomised: 120 Number included in analysis: 108; 1 participant suffered exclusively from fingernail involvement Number completing treatment: 109 Sex (M/F): 56/53 Mean age: 46 years Inclusion criteria: suggestive clinical appearance, positive KOH preparation, and a dermatophyte cultured on Kimmig's agar within 3 months of commencing treatment Type/location/characteristics of infection: positive toenail onychomycosis Duration of infection: not stated Exclusion criteria: severe additional diseases (e.g. impaired liver and kidney function, lupus erythematosus, porphyria), systemic antifungal treatment within the previous 4 weeks, age < 18 years, pregnant/lactating women, and those not using suitable contraceptive measures Washout period: not stated Setting: Germany Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 4 week interval evaluation with treatment for up to 18 months Outcomes measured: clinical status, mycological status, adverse reactions Safety and tolerability assessed by: AEs, glutamic‐pyruvic transaminase, GGT, total and low‐density lipoprotein cholesterol levels | |
| Source of funding | Janssen GmbH, Neuss, Germany, supplied the study medication | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | No clear other bias seen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he patients were assigned a study number in the order of their agreement to take part in the study". "The study number served for the random assignment of the patients to the three treatment groups (1:1:1 ratio)." Comment: method of random sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he patients were assigned a study number in the order of their agreement to take part in the study". "The study number served for the random assignment of the patients to the three treatment groups (1:1:1 ratio)." Comment: method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]he study described here was a controlled open trial" Comment: study states that it was an open trial with no mention of blinding in the Methods; therefore, there is a high risk of performance bias. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[t]he study described here was a controlled open trial" Comment: study states that it was an open‐label trial with no mention of blinding in the Methods; therefore, there is a high risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a] total of 120 patients … were asked to take part in the study." "Discontinuation of treatment because of side effects was necessary in different proportions of the various treatment groups." "Results are reported for all subjects enrolled in the study except for those who dropped out before the completion of baseline parameters (intention‐to‐treat analysis)." Comment: outcome data provided for 108 of the original 120 randomised participants. Small number of dropouts, with reasons for exclusions explained. ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: controlled trial | |
| Participants | Number of participants randomised: 174 Sex (M/F): 137/37 Mean age: not stated Number included in analysis: 174 Number completing treatment: 174 Inclusion criteria: 5y history of toenail dermatomycosis Type/location/characteristics of infection: positive toenail onychomycosis Duration of infection: minimum 5 years Exclusion criteria: not stated Washout period: not stated Setting: not stated Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 3 years after treatment Outcomes measured: mycological culture and microscopy Safety and tolerability assessed by: not stated | |
| Source of funding | No informations available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Conference abstract only, not a full article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he patients … were randomly divided into two groups" Comment: no method of random sequence generation stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he patients … were randomly divided into two groups" Comment: no method of allocation concealment stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]he mycological efficacy and recurrences of the onychomycosis … were invested during three years of observation." Comment: no mention of blinding of participants or personnel; therefore, there is a unclear risk of performance bias |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[t]he mycological efficacy and recurrences of the onychomycosis … were invested during three years of observation." Comment: no mention of blinding of outcome assessment; therefore, there is a high risk of detection bias. |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "[t]he patients (n = 174) … were randomly divided into two groups; the first group (N = 67) treated by terbinafine …; the second group (N = 107) received itraconazole". Comment: numbers of patients randomised into each group provided. Outcome data likely presented as a percentage of the number of randomised patients (i.e. ITT analysis); however, no details regarding presence or absences of dropouts provided. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blinded study | |
| Participants | Number of participants randomised: 29 Sex (M/F): 17/12 Mean age: 36.3 years (range 14‐76) Number included in analysis: 29 Number completed treatment: 28 Inclusion criteria: unclear Type/location/characteristics of infection: dermatophyte infection of the toenail Duration of infection: not stated Exclusion criteria: other systemic illnesses or on other therapy Washout period: not stated Setting: Italy Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 6 months post‐treatment (10 months for intervention 1, 15 months for intervention 2) Outcomes measured: mycological (positive Sabourad culture) and clinical cure (method of evaluation not stated) Safety and tolerability assessed by: drug tolerability, full blood count, and liver enzymes assessed every 4 weeks during treatment | |
| Source of funding | Help from pharmaceutical company was provided (Sandoz) | |
| Conflict of interest | Clear disclosure of pharmaceutical industry involvement. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method given |
| Allocation concealment (selection bias) | Unclear risk | No details of method given |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[i]n a comparative double‐blind study...". No other details given. Comment: the differing duration of treatment and follow‐up ("14 were treated with terbinafine 250 mg/day for 4 months and 15 with griseofulvin 1 g/day for 9 months") adds confusion as to how the study could be double‐blinded despite authors describing it as such. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[i]n a comparative double‐blind study..." Comment: apart from stating that the study is double‐blind, there is no detail of method or explicit statement about blinding in the text. The differing duration of follow‐up adds confusion as to how the study could be double‐blinded despite authors describing it as such. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[o]f 29 patients affected by toenail onychomycosis ... 11 of the 14 patients with toenail onychomycosis (78.5%) treated with terbinafine with completely cured." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 97 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 96 Number completing treatment: not stated Inclusion criteria: not stated Type/location/characteristics of infection: target toenail, not specifically stated Duration of infection: not stated Exclusion criteria: not stated Washout period: not stated Setting: USA (multicentre) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 72 weeks after treatment Outcomes measured: negative mycology (culture and KOH microscopy), zero nail involvement, overall efficacy according to participant and investigator (5‐point scale of excellent, very good, good, fair, poor) Safety and tolerability assessed by: adverse event reporting | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Paper has no Methods section | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind, placebo‐controlled, multicenter study". Comment: states double‐blinded, but no method stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "clinical and mycologic evaluations of a target toenail were performed " Comment: method not stated other than as outlined in quote above |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "97 subjects were randomised." Comment: attrition not accounted for in data analysis, but only 1 participant (of 96 total in that group) appears to have been lost to follow‐up for the outcome of clinical cure, and 3 participants (of 94 total in that group) appear to have been lost to follow‐up for mycology. Overall, attrition represents very small proportion of total subjects. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias reported |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 386 Sex: not stated (both sexes included) Mean age: not stated, range 18‐70 years Number included in analysis: 331 (from table II) Number completing treatment: 245 (141 dropouts) Inclusion criteria: mycologically confirmed (KOH test and positive culture) onychomycosis of toenail Type/location/characteristics of infection: distal subungual onychomycosis of toenail, with a target toenail being a large toenail with > 25% involvement and > 2 mm healthy nail at nail fold Duration of infection Exclusion criteria: pregnancy, lactation, hypersensitivity to azoles, significant systemic disease, diabetes mellitus requiring medication, concomitant use of interfering drugs, HIV positivity, liver disease, positive fungal culture for non‐dermatophytes, psoriasis, lichen planus, anatomical abnormalities of toe, regular heavy alcohol intake Washout period: 3 months for oral antifungals, 2 weeks for topical antifungals Setting: USA (multicentre); 24 centres Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 9 months total treatment plus 6 month additional blinded follow‐up for those with clinical cure or improvement Outcomes measured: clinical response compared to baseline (classified as cure, improvement or failure), mycologic evaluation (KOH, fungal culture). Clinical success = clinical cure or area involved < 25%. Post‐treatment cure and relapse. Quality of life questionnaire. Safety and tolerability assessed by: adverse event reporting, lab tests, vital signs, weight | |
| Source of funding | Sponsored by pharmaceutical industry | |
| Conflict of interest | "For the evaluation of efficacy at the end of treatment and at the 6‐month follow‐up, clinical success was arbitrarily defined by the sponsor of the study." Comment: industry sponsored and input unclear | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]atients were randomised to four double‐blind treat‐ment groups" Comment: no further information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[m]ulticenter, randomised, double‐blind, parallel, placebo‐controlled trial" Comment: no further information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Method not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[o]f the 384 subjects, 243 (221 fluconazole, 22placebo) entered the posttherapy follow‐up phase. Fifty‐two of the 386 randomised subjects were excluded from the efficacy analysis for non‐efficacy–related reasons, including protocol violations,withdrawal of consent, adverse events leading to early discontinuation, or loss to follow‐up" Comment: all participants are accounted for, although large number of dropouts in placebo group |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Unclear risk | Quote: "[t]he evaluation of efficacy at the end of treatment and at the 6‐month follow‐up, clinical success was arbitrarily defined by the sponsor of the study" |
| Methods | Design: randomised, multicentre, parallel group, placebo‐controlled study | |
| Participants | Number of participants randomised: 1381 Sex (M/F): 1034/347 Mean age: 47.4 years (range 16‐75) Number included in analysis: 1381 Number completing treatment: 1169 Inclusion criteria: clinical diagnosis of distal and/or lateral subungual onychomycosis affecting > 1 great toenail. > 25% to 75% nail involvement and > 2 mm of nail length uninvolved. Positive potassium hydroxide (KOH) microscopic examination and a culture positive for a dermatophyte from the target toenail Duration of infection: not specified Exclusion criteria: currently or within the previous 24 weeks participated in an investigational trial involving systemic treatment of onychomycosis of the fingernail or toenail, those having used any topical onychomycosis treatments in the 2 weeks prior to screening, and those with onychomycosis due to a Candida sp without the presence of a dermatophyte Setting: 62 sites in 7 countries (USA, Canada, South Africa, the Dominican Republic, Ecuador, Honduras and Panama) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 52 weeks Outcomes measured: clinical and mycological cure, % nail involvement, total number of fingernail and toenails with onychomycosis over time, proportion of participants with no signs or symptoms of tinea pedis over time Safety and tolerability assessed by: recordings of adverse events and concomitant medications, clinical laboratory tests, electrocardiograms and audiology assessments | |
| Source of funding | Funding for this research was provided by Stifel, a GSK company | |
| Conflict of interest | Authors have served as consultants for Stiefel, a GSK company and L Bulger is an employee of Stiefel | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization schedule was generated by QST consultations, stratified by investigational site and utilized a block size of 7" Comment: clear description of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote "schedule was generated by QST consultations" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[b]ecause the tablets and capsules differed obviously in appearance and dosing regimen, a designated individual at each site who was not involved with the evaluation of the patients dispensed, collected and accounted for the study drugs and thus was unblinded... Patients were instructed not to discuss the appearance or dosing regiment of their assigned study drugs with the investigator/evaluators." Comment: no ability to verify this actually happened |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[b]ecause the tablets and capsules differed obviously in appearance and dosing regimen, a designated individual at each site who was not involved with the evaluation of the patients dispensed, collected and accounted for the study drugs and thus was unblinded ... Patients were instructed not to discuss the appearance or dosing regiment of their assigned study drugs with the investigator/evaluators." Comment: unclear degree to which investigators/evaluators would be blinded given that patients and a single individual could inform them of details that could introduce detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "of the 118 patients 111 completed part 1, 56 in the terbinafine and 55 placebo" |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: open‐label parallel group trial | |
| Participants | Number of participants: 200 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 140 Number completing treatment: 140 Inclusion criteria: finger or toenail onychomycosis confirmed on culture Duration of infection: not specified Exclusion criteria: not specified Setting: SCB Medical College, Cuttack, India Comorbidities: not stated | |
| Interventions |
Both for a period of 4 pulses | |
| Outcomes | Cure (not further defined) | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | Unable to separate toenail from fingernail results, not included in pooled analysis, only abstract available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a]ll patients were assigned individual identification numbers and were divided randomly and equally into two groups (A and B) using a table of random numbers" Comment: no clear information given on method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "using a table of random numbers" Comment: no further information given |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "randomised, single‐blind, longitudinal, clinical comparative study ... The drugs were bought by the physician and dispensed to the patients in unmarked packets " Comment: personnel were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[t]he drugs were bought by the physician and dispensed to the patients in unmarked packets" Comment: personnel/physicians were not blinded |
| Incomplete outcome data (attrition bias) | High risk | "10 (16%) patients in Group A and 12 (20%) patients in Group B could not complete the one‐year follow up period and were excluded from the analysis of the results." Comment: large proportion of patients unable to complete follow‐up; reason for being unable to complete 1‐year follow‐up not given |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other sources of bias seen |
| Methods | Design: parallel group single blind RCT | |
| Participants | Number of participants: 61 Sex (M/F): 29/32 Mean age: 44 years (range 18‐70) Number included in analysis: 36 Number completing treatment: 51 (some dropped out in follow‐up periods after completing treatment) Inclusion criteria: outpatients with a clinical diagnosis of onychomycosis of finger or toenails caused by dermatophytes and proven by culture Duration of infection: not specified Exclusion criteria: participants were excluded from the study if they were under 18 or over 70 years of age (although a patient that was "seven years old" was included), pregnant or lactating, had advanced liver disease, or used concomitantly rifampicin, the contraceptive pill, anticoagulant agents or antacid treatment Setting: 5 dermatological centres in Finland Comorbidities: not stated | |
| Interventions |
Treatment duration 6‐9 months depending on clinical condition of the nail(s) | |
| Outcomes | Mycological cure, negative culture and clinical assessment of the nail | |
| Source of funding | Orion Pharmaceutics | |
| Conflict of interest | Lead author works at Orion Pharmaceutics | |
| Notes | Unable to extract data on participant level for toenails, not included in pooled analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[m]edication was delivered in identical‐looking sealed plastic containers" Quote: "[s]ingle‐blind study" Comment: unclear who was blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[s]ingle blind study" Comment: unclear if outcome assessor ("investigator") was the one that was blinded |
| Incomplete outcome data (attrition bias) | High risk | Quote: "84% completed the treatment ... 10% ..." and "24% discontinued the study. " Comment: large number of patients discontinued without clear reasons given |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other sources of bias seen |
| Methods | Design: randomised, double‐blind, comparative study | |
| Participants | Number of participants: 90 Sex: not stated (both sexes included) Mean age: 47.6 years (range 20‐80) Number included in analysis: 57 Number completing treatment: 57 Inclusion criteria: outpatients with a clinical diagnosis of onychomycosis of finger or toenails caused by non‐dermatophytes and proven by culture Duration of infection: 1 month‐20years Exclusion criteria: pregnant women, breastfeeding mothers, patients with known renal or liver impairment, congestive cardiac failure Setting: dermatology clinic at the General Hospital Chillaw, Sri Lanka and Base Hospital Homagama, Sri Lanka Comorbidities: not stated | |
| Interventions |
In divided doses for 7 days per month (1 week on and 3 weeks off monthly pulses). 2 pulses were prescribed for fingernails and 3 pulses for toenails | |
| Outcomes | Clinical and mycological cure. Clinical cure was defined as complete absence of all the clinical signs of onychomycosis. Mycological cure was defined as negative direct microscopy and culture | |
| Source of funding | No information available | |
| Conflict of interest | Authors did not declare any conflict of interest | |
| Notes | Data for toenails only provided after communication with the lead author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the treatment options were documented separately and packed in covered opaque envelopes consecutively numbered according to the randomisation schedule as to have a ratio of 1:1 |
| Allocation concealment (selection bias) | Low risk | Quote: "[t]he allocation sequence was concealed from the researcher enrolling and assessing the participants" Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[t]he participants and the investigator (outcome assessor) were blind to the type of therapy " Comment: participants and personnel were blinded to therapy |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[t]he participants and the investigator (outcome assessor) were blind to the type of therapy. Same investigator performed clinical assessment on all the participants at each visit until cure" Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Some differences in loss to follow‐up (7/43 in itraconazole and 14/47 in terbinafine defaulted to other treatments before the end of the trial) |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other biases identified |
| Methods | Design: parallel group RCT | |
| Participants | Number of participants randomised: 362 Sex: not stated (both sexes included) Mean age: not stated; range 18‐70 years Number included in analysis: 355 ITT Number completing treatment: 313 Inclusion criteria: mycological diagnosis and a positive culture for dermatophytes Type/location/characteristics of infection: 25% involvement of the target nail with at least 2 mm of healthy nail from the nail fold to the proximal onychomycotic border Duration of infection: not stated Exclusion criteria: pregnancy, lactation, hypersensitivity to azoles, significant systemic disease, diabetes mellitus, immunosuppression, renal or hepatic dysfunction, fungal culture positive for non‐dermatophytes, drugs that may interfere with azoles Washout period: 3 months for oral antifungals, 2 weeks for topical antifungals Setting: USA (multicentre) Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 6 months after treatment Outcomes measured: clinical (visual; % of nail involved, distance from nail fold, signs/symptoms of onychomycosis) and mycologic (microscopic and microbiologic) evaluations Safety and tolerability: adverse event reporting, blood and urine specimens (haematology, blood chemistry, urinalysis), vital signs, body weight, use of concomitant medications | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]his study followed a randomised, double‐blind, fixed‐dose, parallel‐group, placebo‐controlled multi‐center design ... Patients were randomly assigned to one of the following four treatment regimens" Comment: not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]his study followed a randomised, double‐blind,fixed‐dose, parallel‐group, placebo‐controlled multi‐center design". Comment: study claims to be double‐blind, no further details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[t]his study followed a randomised, double‐blind,fixed‐dose, parallel‐group, placebo‐controlled multi‐center design " Comment: stated multiple times that remained double‐blinded on follow‐up visits, no details given |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis included. All participants are accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No clear other bias seen |
| Methods | Design: prospective, randomised, double‐blind, double‐dummy, multicentre, parallel‐group study | |
| Participants | Number of participants randomised: 843 Sex: 58% male Mean age: 50.1 years (range 18‐75) Number included in analysis: 496 (this is the ITT population) Number completing treatment: not stated Inclusion criteria: men and women aged 18‐75 years with clinical diagnosis of distal subungual or total dystrophic onychomycosis of the toenails, confirmed by positive mycological culture and microscopy Type/location/characteristics of infection: only participants with dermatophyte infections were included, all were required to have involvement of a great toenail Duration of infection: not stated Exclusion criteria: pregnant and lactating women, people receiving drugs known or believed to interact with either of the study agents, people with conditions that might lead to altered absorption, metabolism or excretion of study agents, systemic antifungal therapy within 12 months prior to screening visit, or topical antifungal therapy within the 4 weeks prior to screening visit, diagnosis of immunodeficiency disorder, psoriasis or mucocutaneous candidiasis, radiotherapy, chemotherapy or immunosuppressive therapy within 12 weeks of the start of study, alanine transaminase and/or aspartate transaminase levels more than 1‐5 times the upper limit of the normal range and/or serum creatinine level above 300 μmol/L Washout period: 12 months for systemic, 4 weeks for topical Setting: participants were recruited from 35 centres in Finland, Germany, Iceland, Italy, the Netherlands and the UK Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 56 weeks (treatment phase till week 16, follow‐up phase till week 72) Outcomes measured: mycological cure, clinical cure, % nail involvement, efficacy as rated by participants Safety and tolerability assessed by: number and type of adverse events | |
| Source of funding | Novartis provided support and funding | |
| Conflict of interest | Dr Sigurgeirsson has received funds for research and fees for speaking and organising educational meetings from several pharmaceutical companies, including Novartis Pharma. Professor Evans has received funds for research and also fees for speaking and consulting from a number of pharmaceutical companies, including Novartis Pharma and Janssen Pharmaceuticals. Dr Billstein is an employee of Novartis Pharmaceuticals Corporation, USA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "[b]oth the investigators and the participants remained blinded throughout the entire 72‐week study" Comment: participants and personnel were blinded to study group |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[b]oth the investigators and the participants remained blinded throughout the entire 72‐week study". Comment: it is clearly stated that investigators, including those assessing clinical cure, were blinded for the entire 72‐week study. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[e]fficacy results are based on the number of observed cases in the ITT population at 72 weeks". Comment: ITT performed |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: randomised, double‐blind, placebo‐controlled, parallel group study | |
| Participants | Number of participants randomised: 582 Sex (M/F): 441/141 Mean age: 48.6 years (range 19‐74) Number included in analysis: 582 Number completing treatment: 482 Inclusion criteria: distal subungual onychomycosis affecting at least 1 great toenail (target toenail) with > 25% nail involvement, > 2 mm of unaffected toenail at the proximal end, and microscopic (KOH, calcofluor) and culture confirmation of dermatophytes. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and total bilirubin levels that were < 1.5 times the upper normal limit. Baseline ECG normal or clinically insignificant. Duration of infection: not specified Exclusion criteria: women who were pregnant, trying to become pregnant or breastfeeding, receipt of an investigational drug within 4 weeks before the first dose of the study product, an investigational drug treatment for onychomycosis within 6 months before the first dose of the study product; schedule receipt of any other investigational drug during the study; receipt of any known substrate of the 3A4 isozyme of cytochrome P450 (CYP3A4) with QT prolongation potential; concomitant use of prohibited medications, a history of any condition that could possibly affect drug absorption (e.g. gastrectomy), uncontrolled diabetes, clinically significant peripheral vascular disease or circulatory impairment, any major illness within 30 days before screening, ECG abnormalities deemed clinically significant. Washout period: 4 weeks Setting: 26 centres in the USA, 3 in Canada and 1 in Iceland Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 52 weeks Outcomes measured: mycological and clinical cure, adverse events Safety and tolerability assessed by: clinical laboratory indicators, vital signs and physical examination results and ECG measurements | |
| Source of funding | Supported by Stiefel, a GSK company | |
| Conflict of interest | Dr Sigurgeirsson was a sponsored investigator on this study and a member of an advisory board that assisted in the planning and design of the study. He also has served as a consultant and investigator for and received honoraria from Arpedia, Celtic, deCode, Galderma, Novartis, Prostrakan, Stiefel, TLT, Topica, and Vertex. Dr van Rossem, Mr Malahias, and Ms Raterink are employees of Stiefel, a GSK company | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "independently randomized (with a 1:1:1:1:1 schedule) using a computer‐generated schedule to 1 of the 5 study groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Quote "Investigators, study centre personnel, patients, study monitors, and statisticians were unaware of the assigned study treatment", "placebo‐matched capsules" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "[i]nvestigators, study centre personnel, participants, study monitors, and statisticians were unaware of the assigned study treatment." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | High completion rate (82%‐98%) and very low loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blind study | |
| Participants | Number of participants randomised: 20 Sex (M/F): 18/2 Mean age: 40.5 years (range 14‐65) Number included in analysis: 17 Number completing treatment: the study defines 'completing treatment' as treatment till cure, rather than stipulating a timeframe. 9 participants in the ketoconazole group were treated for 8‐12 months (mean 10.6), 5 participants in the griseofulvin group were treated for 12 months Inclusion criteria: culturally proven onychomycosis caused by dermatophytes severe enough to indicate systemic treatment Duration of infection: 1‐30 years (mean 9.3) Exclusion criteria: not specified Washout period: not specified, but 15 participants in the study had received prior treatment with griseofulvin for less than 3 months without side effects Setting: not explicitly stated, but the author is based at Rigshospital, Copenhagen, Denmark, and acknowledges technical assistance from the Dermatological Department of this hospital. Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: 'cure' defined as clinical and mycological cure Safety and tolerability assessed by: laboratory tests, including haemoglobin, leucocyte count, platelet estimate, creatinine, cholesterol and alanine‐aminotransferase | |
| Source of funding | Ketoconazle tablets were supplied by Janssen Pharmaceutica, Beerse, Belgium and griseofulvin tablets by Leo, Haelsingborg, Sweden | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | This a 2‐part study. The first part assesses responsiveness of infection of various body parts to ketoconazole. The details above apply to the second part of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: study claims to be randomised, but does not state method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: not stated how allocation was concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: study claims to be double‐blind, but no further details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[i]n the double‐blind study ... on a randomised basis" Comment: study claims to be double‐blind, but no further details |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blind, controlled, multicentre study | |
| Participants | Number of participants randomised: 148 Sex: not stated (both sexes included) Mean age: not stated Number included in analysis: 147 Number completing treatment: 127 Inclusion criteria: age of 18 years or older Type/location/characteristics of infection: proven modest to severe dermatophyte infection of 1 or both great toenails (positive microscopy and culture) Duration of infection: not stated Exclusion criteria: impaired liver and kidney function, pregnant or lactating women Washout period: 1 month for both topical and systemic treatment Setting: Denmark (multicentre) 15 dermatology clinics and 5 hospital departments Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: mycological cure, clinical cure, % of nail unaffected, degree of subungual keratosis Safety and tolerability assessed by: number and type of side effects | |
| Source of funding | Supported by Sandoz Ag Basle | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | Those who had no improvement or deterioration (terbinafine or placebo group) were treated with further 3 months of terbinafine ‐ these participants are not included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[a]fter 1‐month wash‐out period with no topical or systemic treatment the patients were randomised to receive ..." Comment: study stated to be randomised, but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]he investigation was carried out as a double‐blind, controlled, multi‐centre ..." Comment: method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]he investigation was carried out as a double‐blind, controlled, multi‐centre ..." Comments: no further information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[t]he investigation was carried out as a double‐blind, controlled, multi‐centre ..." Comments: no further information provided |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other clear bias seen |
| Methods | Design: open‐label, randomised study | |
| Participants | Number of participants randomised: 63 Sex (M/F): 31/32 Mean age: 47.3 years (range 27‐60) Number included in analysis: 60 (57 with toenail infection) Number completing treatment: 60 (57 with toenail infection) Inclusion criteria: not stated Type/location/characteristics of infection: toenails, fingernails or both Duration of infection: not stated Exclusion criteria: systemic antifungal agents in previous 6 weeks, participant with severe liver, renal or cardiovascular disease and pregnant women Washout period: not stated, but participants who has systemic antifungal therapy in previous 6 weeks were excluded Setting: Italy Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 10 months (for toenail infection) Outcomes measured: mycological cure, presence of nail deformity Safety and tolerability: number of participants who reported adverse side effects | |
| Source of funding | This study was partially supported by Novartis Farma SpA Italy and by the University of Bologna ‐ funds for selected research topics | |
| Conflict of interest | Clear disclosure of pharmaceutical industry funding. No details regarding individual author conflict of interest statements provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]he experimental design was open and randomised. Patients were assigned sequentially to treatment." Comment: unclear if random sequence generation was adequate |
| Allocation concealment (selection bias) | High risk | Quote: "[t]he experimental design was open and randomised. Patients were assigned sequentially to treatment." Comment: likely allocation was not concealed |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "[t]he experimental design was open" Comment: study was not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "[t]he experimental design was open" Comment: study was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[a]ll participants who started treatment were considered able to be evaluated even if they withdrew the first day because of adverse events (intention to treat)." Comment: ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other source of bias seen |
| Methods | Design: double‐blind RCT | |
| Participants | Number of participants randomised: 20 Sex: not stated for toenail subgroup (both sexes included) Mean age: not stated Number included in analysis: 20 Number completing treatment: 20 Inclusion criteria Type/location/characteristics of infection: toenail or fingernail onychomycosis caused by T rubrum or T mentagrophytes Duration of infection: not stated Exclusion criteria: antimycotic therapy within 1 month of start of study, pregnancy or serious concurrent disease Washout period: not explicitly stated, by participants with antimycotic therapy within 1 month of start of study were excluded Setting: not stated, study authors are all from Copenhagen Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 12 months Outcomes measured: cure (defined as clinical and mycological cure), marked improvement (defined as positive microscopy and negative culture), and improvement (50% clinical improvement compared to baseline and positive mycology) Safety and tolerability: side effects reported | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blind study ... randomised basis" Comment: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "double‐blind study ... randomised basis" Comment: method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[f]or each patient, 12 boxes were prepared, each containing blister packs" Comment: blister packs were used, but it was not clear whether any visual differences remained between treatments |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double‐blind study ... randomised basis" Comment: method not stated |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other risks of bias identified |
| Methods | Design: RCT | |
| Participants | Number of participants randomised: 118 Sex: 58% male Mean age: not stated Number included in analysis: 118 ITT Number completing treatment: 111 Inclusion criteria: distal or total dermatophyte onychomycosis of at least 1 toenail confirmed by mycological culture Type/location/characteristics of infection: distal or total dermatophyte onychomycosis, at least 1 toenail Duration of infection: not stated Exclusion criteria: renal, hepatic, cardiovascular or gastrointestinal disease, psoriasis, pregnancy, lactation, inadequate contraception, if non‐dermatophyte was considered to be primary pathogen, if participant used topical or oral antifungal agent within 2 or 6 weeks, respectively Washout period: not stated Setting: 13 centres in Australia and New Zealand Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 48 weeks from start of treatment Outcomes measured: clinical assessment (no signs of infection or considerable, minor or no improvement) and mycology (microscopy and mycological culture) Safety and tolerability: adverse event reporting, biochemical, haematologic studies, urinalysis and clinical examination | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: method not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: study claims to be double‐blind, but no method stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "[t]his was a randomised, double‐blind, 48‐week study." Comment: no mention of method |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other risks of bias identified |
| Methods | Design: open‐label, randomised study | |
| Participants | Number of participants randomised: 72 Sex: 50% male Mean age: 45.8 years (range 17‐70) Number included in analysis: 49 Number completing treatment: assumed 49 (no discontinuations reported) Inclusion criteria: unclear Type/location/characteristics of infection: distal or distolateral subungual toenail onychomycosis, not more than 75% involvement of nail plate, confirmed by microscopy or culture Duration of infection: not specified Exclusion criteria: any systemic disease Washout period: 1 month for topical antifungal therapies or topical steroids, 2 months for systemic antifungal therapy Setting: 2 research centres in Seoul, Korea Comorbidities: not stated | |
| Interventions |
| |
| Outcomes | Duration of follow‐up: 96 weeks Outcomes measured: mycological cure, clinical cure, adverse events, subject acceptance Safety and tolerability: adverse events reporting, measurement of alanine aminotransferase, aspartate aminotransferase and gamma‐glutamyl transpeptidase | |
| Source of funding | No information available | |
| Conflict of interest | No conflict of interest identified | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[p]articipants were randomly selected" to their treatment group Comment: no method is given |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[p]articipants were randomly selected" to their treatment group Comment: method not stated |
| Blinding of participants and personnel (performance bias) | High risk | Comment: blinding is not mentioned in this study, and participants were given 300 mg of itraconazole daily for 1 week every 4 weeks or 250 mg terbinafine daily for 12 weeks. Because of these factors, it is possible that blinding could have been broken. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: blinding is not mentioned in this study |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: because the nail involvement was statistically different between groups, 21 of the initial 70 randomised participants were excluded, and the outcome data are unavailable. No systemic differences between withdrawals between groups |
| Selective reporting (reporting bias) | Low risk | All results presented as set out in the Methods. All prespecified outcomes appear to be reported. |
| Other bias | Low risk | No other risks of bias identified |
AE: adverse event; ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate transaminase; CBC: complete blood count; ECG: electrocardiogram; GGT: gammaglutamyl transferase; GI: gastrointestinal; IIT: itraconazole‐itraconazole‐terbinafine (3 pulses total); ITT: intention‐to‐treat; KOH: potassium hydroxide; LFT: liver function test; NA: not applicable;RCT: randomised controlled trial; RFT: renal function test; TRIPA: trichophytin antigen; TTT: terbinafine × 3 pulses.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study compares itraconazole to 'palliative care': trimming, soaking and cleaning. No placebo group | |
| This is a dose‐finding study with no comparisons between different drugs or between drug and placebo | |
| This is a dose‐finding study with no comparisons between different drugs or between drug and placebo | |
| This is a dose‐finding study with no comparisons between different drugs or between drug and placebo | |
| This is a dose‐finding study with no comparisons between different drugs or between drug and placebo | |
| This is a dose‐finding study with no comparisons between different drugs or between drug and placebo | |
| This is a dose‐finding study with no comparisons between different drugs or between drug and placebo | |
| Pharmacokinetic study of drug concentrations in healthy nails | |
| Dose‐finding with no comparison between different drugs or a placebo. Also, focuses on pharmacokinetics and nail plate concentrations of drug (not clinical or mycological cure) | |
| Looked at tinea pedis | |
| Not an RCT: no comparison group, only a treatment group | |
| This is a dose‐finding study (continuous vs pulse) with no comparisons between different drugs or between drug and placebo | |
| This is a dose‐finding study (continuous vs pulse) with no comparisons between different drugs or between drug and placebo | |
| Looked at efficacy of topical adjunct to griseofulvin | |
| Study assesses efficacy of adjuncts (amorolfine and pentoxifylline) to itraconazole and does not compare 2 different anti‐fungal agents | |
| Dose finding for terbinafine, no placebo group | |
| Letter to the editor, not an RCT | |
| Dose finding for terbinafine, no placebo group | |
| This is a dose‐finding study for itraconazole with no comparisons between different drugs or between drug and placebo | |
| Dose finding for terbinafine, no placebo group | |
| Dose finding for terbinafine, no placebo group | |
| Dose finding for terbinafine, no placebo group | |
| This is a dose‐finding study for terbinafine (continuous vs intermittent) with no placebo group | |
| Dose finding (continuous vs pulse) for terbinafine, no placebo group | |
| This is a dose‐finding study for itraconazole pulse therapy | |
| Dose finding for terbinafine, no placebo group | |
| Fungal skin infections, not nail infections |
RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 15 | 2168 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.72, 0.95] |
| Analysis 1.1  Comparison 1 Azole versus terbinafine, Outcome 1 Clinical cure. | ||||
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.96] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 9 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.00] |
| 2 Mycological cure Show forest plot | 17 | 2544 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.68, 0.88] |
| Analysis 1.2  Comparison 1 Azole versus terbinafine, Outcome 2 Mycological cure. | ||||
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 8 | 1287 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.64, 0.93] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 9 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.95] |
| 3 Adverse events Show forest plot | 9 | 1762 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.17] |
| Analysis 1.3  Comparison 1 Azole versus terbinafine, Outcome 3 Adverse events. | ||||
| 4 Recurrence rate Show forest plot | 5 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.68, 1.79] |
| Analysis 1.4 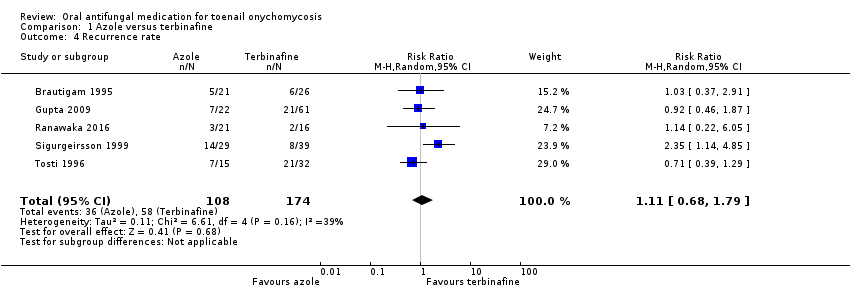 Comparison 1 Azole versus terbinafine, Outcome 4 Recurrence rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 8 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 6.00 [3.96, 9.08] |
| Analysis 2.1  Comparison 2 Terbinafine versus placebo, Outcome 1 Clinical cure. | ||||
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 5.60 [3.66, 8.55] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 26.01 [3.69, 183.44] |
| 2 Mycological cure Show forest plot | 8 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 4.53 [2.47, 8.33] |
| Analysis 2.2  Comparison 2 Terbinafine versus placebo, Outcome 2 Mycological cure. | ||||
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 4.60 [2.26, 9.36] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 7.79 [0.42, 144.44] |
| 3 Adverse events Show forest plot | 4 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.47] |
| Analysis 2.3  Comparison 2 Terbinafine versus placebo, Outcome 3 Adverse events. | ||||
| 4 Recurrence rate Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.38] |
| Analysis 2.4  Comparison 2 Terbinafine versus placebo, Outcome 4 Recurrence rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 9 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 22.18 [12.63, 38.95] |
| Analysis 3.1  Comparison 3 Azole versus placebo, Outcome 1 Clinical cure. | ||||
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 7 | 2695 | Risk Ratio (M‐H, Random, 95% CI) | 23.89 [11.99, 47.64] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 2 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 19.11 [7.21, 50.65] |
| 2 Mycological cure Show forest plot | 9 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 5.86 [3.23, 10.62] |
| Analysis 3.2 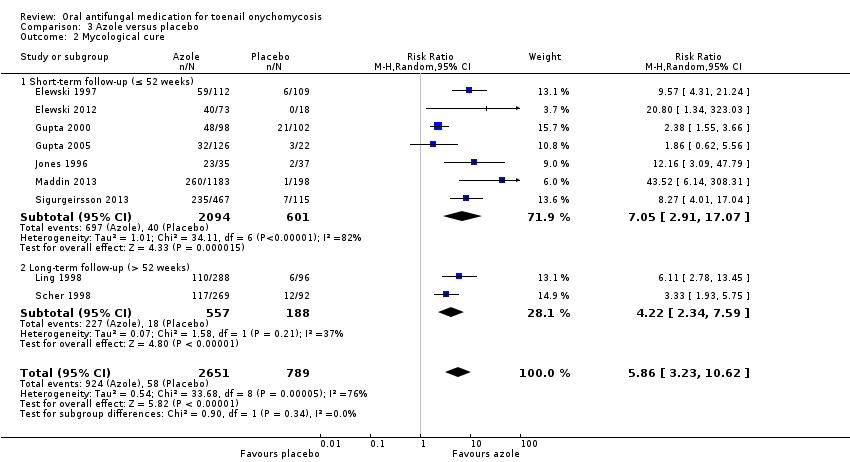 Comparison 3 Azole versus placebo, Outcome 2 Mycological cure. | ||||
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 7 | 2695 | Risk Ratio (M‐H, Random, 95% CI) | 7.05 [2.91, 17.07] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 2 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 4.22 [2.34, 7.59] |
| 3 Adverse events Show forest plot | 9 | 3441 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| Analysis 3.3  Comparison 3 Azole versus placebo, Outcome 3 Adverse events. | ||||
| 4 Recurrence rate Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.29, 1.07] |
| Analysis 3.4  Comparison 3 Azole versus placebo, Outcome 4 Recurrence rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 5 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.45, 1.96] |
| Analysis 4.1 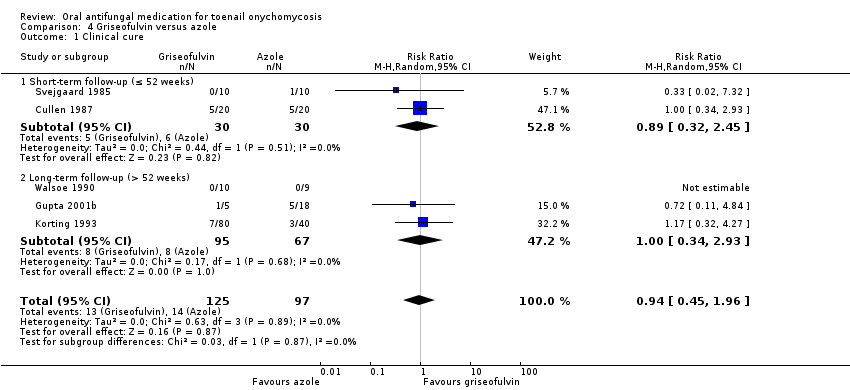 Comparison 4 Griseofulvin versus azole, Outcome 1 Clinical cure. | ||||
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.32, 2.45] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.34, 2.93] |
| 2 Mycological cure Show forest plot | 5 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.50, 1.51] |
| Analysis 4.2  Comparison 4 Griseofulvin versus azole, Outcome 2 Mycological cure. | ||||
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.52, 1.76] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.16, 2.10] |
| 3 Adverse events Show forest plot | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.56, 3.73] |
| Analysis 4.3  Comparison 4 Griseofulvin versus azole, Outcome 3 Adverse events. | ||||
| 4 Recurrence rate Show forest plot | 1 | 7 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.26, 61.76] |
| Analysis 4.4  Comparison 4 Griseofulvin versus azole, Outcome 4 Recurrence rate. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 4 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.72] |
| Analysis 5.1  Comparison 5 Griseofulvin versus terbinafine, Outcome 1 Clinical cure. | ||||
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.39] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.71] |
| 2 Mycological cure Show forest plot | 5 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.90] |
| Analysis 5.2  Comparison 5 Griseofulvin versus terbinafine, Outcome 2 Mycological cure. | ||||
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.20] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.09] |
| 3 Adverse events Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.15, 3.82] |
| Analysis 5.3  Comparison 5 Griseofulvin versus terbinafine, Outcome 3 Adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 1 Clinical cure. | ||||
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Long‐term follow‐up (> 52 weeks) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 2 Mycological cure. | ||||
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Long‐term follow‐up (> 52 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.3 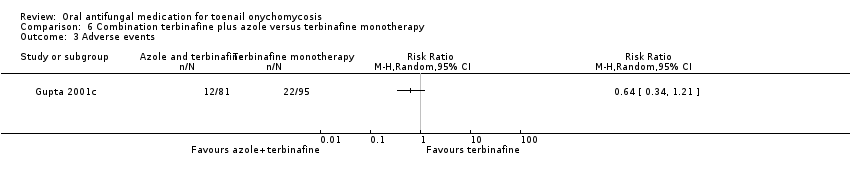 Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 3 Adverse events. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 3 Azole versus terbinafine, outcome: 3.1 Clinical cure.
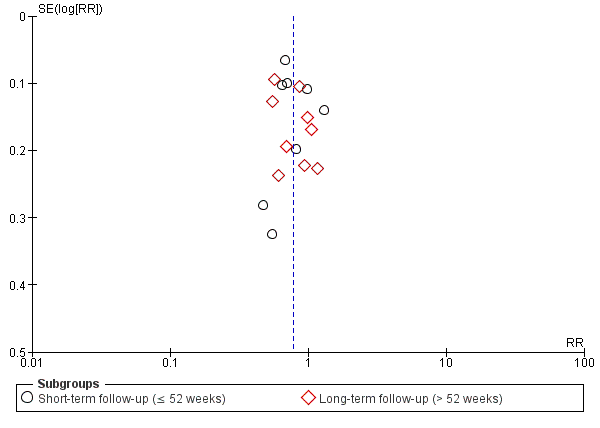
Funnel plot of comparison: 3 Azole versus terbinafine, outcome: 3.2 Mycological cure.
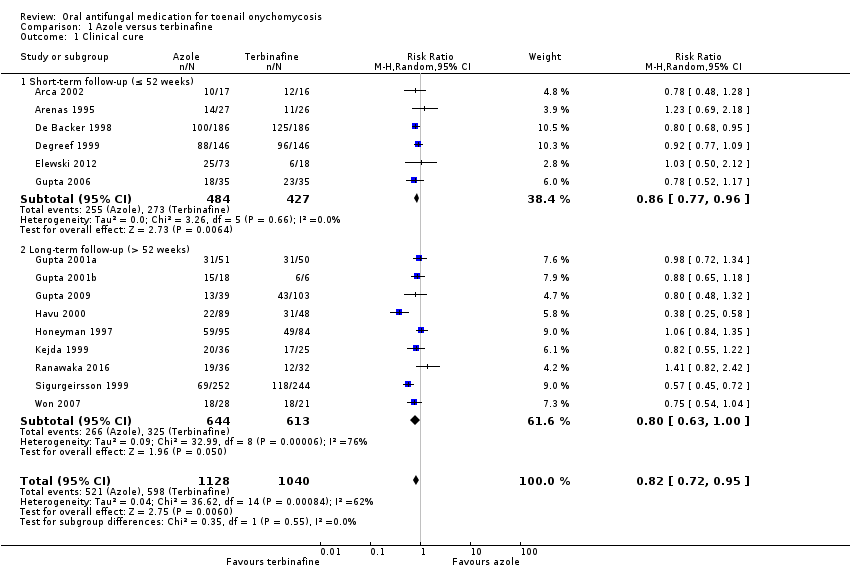
Comparison 1 Azole versus terbinafine, Outcome 1 Clinical cure.

Comparison 1 Azole versus terbinafine, Outcome 2 Mycological cure.

Comparison 1 Azole versus terbinafine, Outcome 3 Adverse events.

Comparison 1 Azole versus terbinafine, Outcome 4 Recurrence rate.

Comparison 2 Terbinafine versus placebo, Outcome 1 Clinical cure.
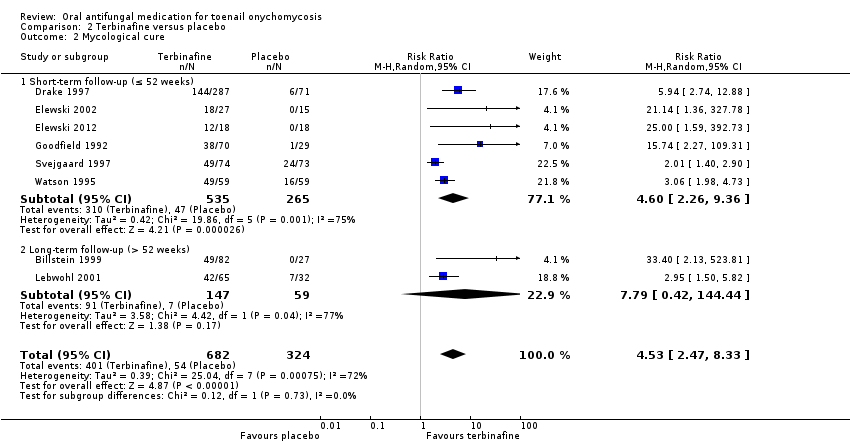
Comparison 2 Terbinafine versus placebo, Outcome 2 Mycological cure.

Comparison 2 Terbinafine versus placebo, Outcome 3 Adverse events.

Comparison 2 Terbinafine versus placebo, Outcome 4 Recurrence rate.
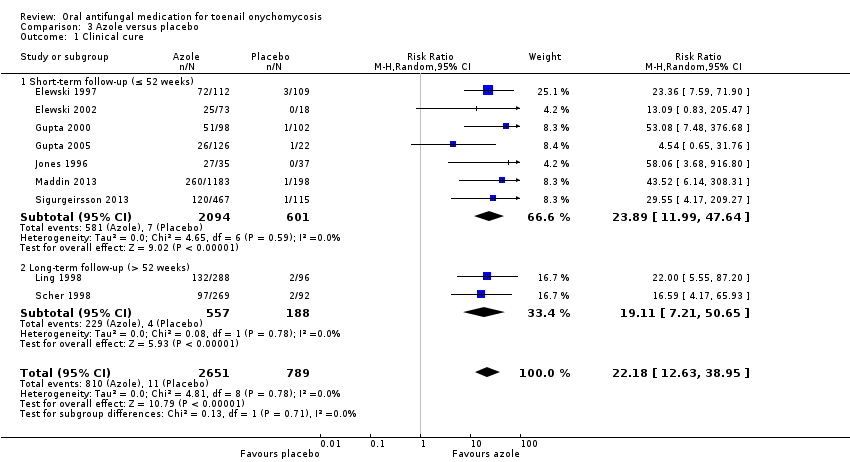
Comparison 3 Azole versus placebo, Outcome 1 Clinical cure.

Comparison 3 Azole versus placebo, Outcome 2 Mycological cure.
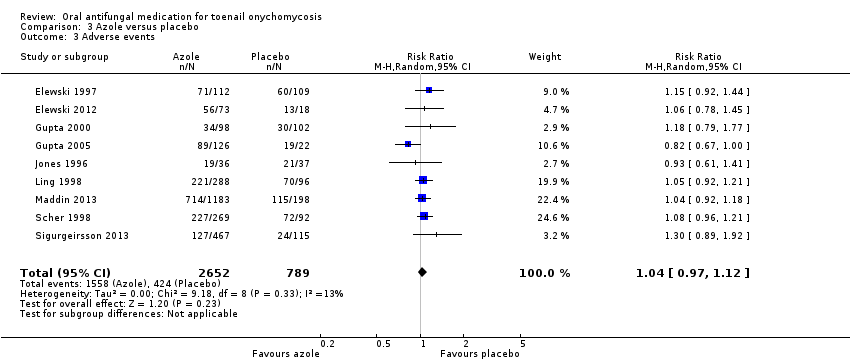
Comparison 3 Azole versus placebo, Outcome 3 Adverse events.
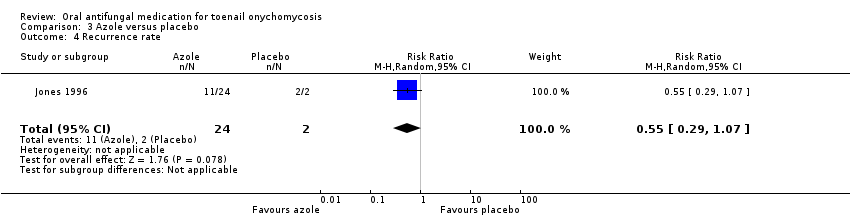
Comparison 3 Azole versus placebo, Outcome 4 Recurrence rate.

Comparison 4 Griseofulvin versus azole, Outcome 1 Clinical cure.

Comparison 4 Griseofulvin versus azole, Outcome 2 Mycological cure.

Comparison 4 Griseofulvin versus azole, Outcome 3 Adverse events.

Comparison 4 Griseofulvin versus azole, Outcome 4 Recurrence rate.

Comparison 5 Griseofulvin versus terbinafine, Outcome 1 Clinical cure.
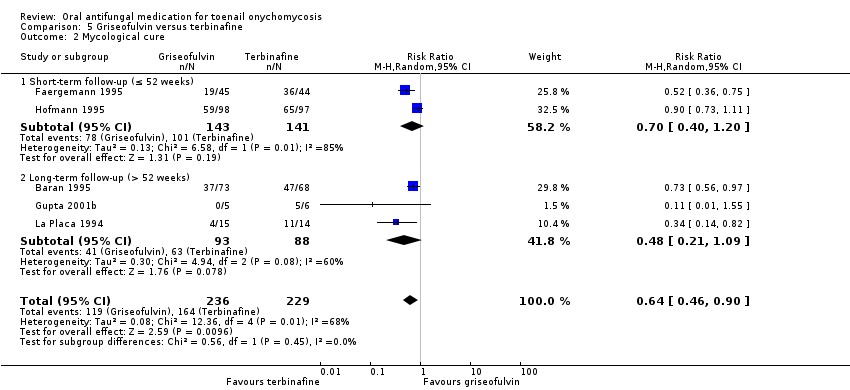
Comparison 5 Griseofulvin versus terbinafine, Outcome 2 Mycological cure.

Comparison 5 Griseofulvin versus terbinafine, Outcome 3 Adverse events.
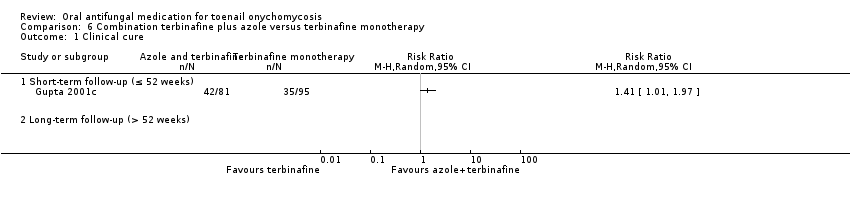
Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 1 Clinical cure.

Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 2 Mycological cure.

Comparison 6 Combination terbinafine plus azole versus terbinafine monotherapy, Outcome 3 Adverse events.
| Azole compared to terbinafine for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with terbinafine | Risk with azole | ||||
| Clinical cure | Study population | RR 0.82 | 2168 | ⊕⊕⊕⊝ | |
| 575 per 1000 | 471 per 1000 | ||||
| Mycological cure | Study population | RR 0.77 | 2544 | ⊕⊕⊕⊝ | |
| 682 per 1000 | 525 per 1000 | ||||
| Adverse events | Study population | RR 1.00 | 1762 | ⊕⊕⊕⊝ | |
| 346 per 1000 | 346 per 1000 | ||||
| Recurrence rate | Study population | RR 1.11 | 282 | ⊕⊕⊝⊝ | |
| 333 per 1000 | 370 per 1000 | ||||
| Quality of life | None of the studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level for risk of bias because of large number of unblinded studies, lack of description of randomisation process and allocation concealment for most studies. | |||||
| Terbinafine compared to placebo for toenail onychomycosis | |||||
| Patient or population: patients with confirmed toenail onychomycosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with placebo | Risk with terbinafine | ||||
| Clinical cure | Study population | RR 6.00 | 1006 | ⊕⊕⊕⊕ | |
| 62 per 1000 | 370 per 1000 | ||||
| Mycological cure | Study population | RR 4.53 | 1006 | ⊕⊕⊕⊕ | |
| 167 per 1000 | 755 per 1000 | ||||
| Adverse events | Study population | RR 1.13 | 399 | ⊕⊕⊕⊝ | |
| 429 per 1000 | 484 per 1000 | ||||
| Recurrence rate | 667 per 1000 | 33 per 1000 (7 to 253) | RR 0.05 (0.01 to 0.38) | 35 | ⊕⊕⊝⊝ |
| Quality of life | Not addressed by any of the trials | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aLarge number of unblinded studies and studies with poor description of blinding and randomisation but large effect estimate; therefore, this outcome was not downgraded for risk of bias as the quality of evidence was considered to be high because of the large effect observed. | |||||
| Azole compared to placebo for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with placebo | Risk with azole | ||||
| Clinical cure | Study population | RR 22.18 (12.63 to 38.95) | 3440 | ⊕⊕⊕⊕ | |
| 14 per 1000 | 309 per 1000 (176 to 543) | ||||
| Mycological cure | Study population | RR 5.86 | 3440 | ⊕⊕⊕⊕ | |
| 74 per 1000 | 431 per 1000 | ||||
| Adverse events | Study population | RR 1.04 | 3441 | ⊕⊕⊕⊝ | |
| 537 per 1000 | 559 per 1000 | ||||
| Recurrence rate | Study population | RR 0.55 (0.29 to 1.07) | 26 (1 RCT) | ⊕⊕⊝⊝ | |
| 1000 per 1000 | 550 per 1000 (290 to 1000) | ||||
| Quality of life | None of the studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aLarge number of unblinded studies and studies with poor description of blinding and randomisation, but large effect estimate; therefore, this outcome was not downgraded for risk of bias as the quality of evidence was considered to be high because of the large effect observed. | |||||
| Griseofulvin compared to azole for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with azole | Risk with griseofulvin | ||||
| Clinical cure | Study population | RR 0.94 | 222 | ⊕⊕⊕⊝ | |
| 144 per 1000 | 136 per 1000 | ||||
| Mycological cure | Study population | RR 0.87 | 222 | ⊕⊕⊕⊝ | |
| 186 per 1000 | 161 per 1000 | ||||
| Adverse events | Study population | RR 2.41 | 143 | ⊕⊕⊕⊝ | |
| 276 per 1000 | 665 per 1000 | ||||
| Recurrence rate | Study population | RR 4.00 | 7 | ⊕⊝⊝⊝ | |
| 0 per 1000 | 0 per 1000 | ||||
| Quality of life | None of the studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by one level due to risk of bias (about half of the studies were not blinded). | |||||
| Griseofulvin compared to terbinafine for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with terbinafine | Risk with Griseofulvin | ||||
| Clinical cure | Study population | RR 0.32 | 270 | ⊕⊕⊝⊝ | |
| 561 per 1000 | 179 per 1000 | ||||
| Mycological cure | Study population | RR 0.64 | 465 | ⊕⊕⊝⊝ | |
| 716 per 1000 | 458 per 1000 | ||||
| Adverse events | Study population | RR 2.09 | 100 | ⊕⊕⊝⊝ | |
| 160 per 1000 | 334 per 1000 | ||||
| Recurrence rate | No studies addressed recurrence rate. | ||||
| Quality of life | No studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by two levels due to risk of bias (two studies not blinded; other studies at unclear risk for blinding of participant and outcome assessor). | |||||
| Combination terbinafine plus azole compared to terbinafine monotherapy for toenail onychomycosis | |||||
| Patient or population: participants with confirmed toenail onychomycosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with terbinafine monotherapy | Risk with combination terbinafine plus azole | ||||
| Clinical cure | Study population | RR 1.41 (1.01 to 1.97) | 176 | ⊕⊝⊝⊝ | |
| 368 per 1000 | 519 per 1000 (732 to 726) | ||||
| Mycological cure | Study population | RR 1.41 (1.08 to 1.83) | 176 | ⊕⊝⊝⊝ | |
| 474 per 1000 | 668 per 1000 | ||||
| Adverse events | Study population | RR 0.64 | 176 | ⊕⊕⊝⊝ | |
| 232 per 1000 | 148 per 1000 | ||||
| Recurrence rate | No studies addressed recurrence rate. | ||||
| Quality of life | No studies addressed quality of life. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded by three levels due to risk of bias (two levels: single non‐blinded study) and imprecision (single study). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 15 | 2168 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.72, 0.95] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.96] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 9 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.00] |
| 2 Mycological cure Show forest plot | 17 | 2544 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.68, 0.88] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 8 | 1287 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.64, 0.93] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 9 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.95] |
| 3 Adverse events Show forest plot | 9 | 1762 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.17] |
| 4 Recurrence rate Show forest plot | 5 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.68, 1.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 8 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 6.00 [3.96, 9.08] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 5.60 [3.66, 8.55] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 26.01 [3.69, 183.44] |
| 2 Mycological cure Show forest plot | 8 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 4.53 [2.47, 8.33] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 6 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 4.60 [2.26, 9.36] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 7.79 [0.42, 144.44] |
| 3 Adverse events Show forest plot | 4 | 399 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.47] |
| 4 Recurrence rate Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 9 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 22.18 [12.63, 38.95] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 7 | 2695 | Risk Ratio (M‐H, Random, 95% CI) | 23.89 [11.99, 47.64] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 2 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 19.11 [7.21, 50.65] |
| 2 Mycological cure Show forest plot | 9 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 5.86 [3.23, 10.62] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 7 | 2695 | Risk Ratio (M‐H, Random, 95% CI) | 7.05 [2.91, 17.07] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 2 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 4.22 [2.34, 7.59] |
| 3 Adverse events Show forest plot | 9 | 3441 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.12] |
| 4 Recurrence rate Show forest plot | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.29, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 5 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.45, 1.96] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.32, 2.45] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.34, 2.93] |
| 2 Mycological cure Show forest plot | 5 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.50, 1.51] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.52, 1.76] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.16, 2.10] |
| 3 Adverse events Show forest plot | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.56, 3.73] |
| 4 Recurrence rate Show forest plot | 1 | 7 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.26, 61.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 4 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.72] |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.39] |
| 1.2 Long‐term follow‐up (> 52 weeks) | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.71] |
| 2 Mycological cure Show forest plot | 5 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.90] |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.20] |
| 2.2 Long‐term follow‐up (> 52 weeks) | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.09] |
| 3 Adverse events Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.15, 3.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Short‐term follow‐up (≤ 52 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Long‐term follow‐up (> 52 weeks) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Short‐term follow‐up (≤ 52 weeks) | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Long‐term follow‐up (> 52 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

