Antibióticos para la prevención de infecciones después de la escisión de la zona de transformación cervical
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009957.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 enero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer ginecológico, neurooncología y otros cánceres
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Chumnan Kietpeerakool: developed and completed the review
Bandit Chumworathayi: conceived the review question, developed, co‐ordinated the review.
Jadsada Thinkamrop: performed part of the editing of the review and advised on part of the review.
Butsakorn Ussahgij: searched for potential eligible studies and advised on part of the review
Pisake Lumbiganon: co‐ordinated the development of the review, edited the review, and advised on the review.
All authors approved the final version of the review prior to submission.
Sources of support
Internal sources
-
Department of Obstetrics and Gynaecology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.
-
Cochrane Thailand, Thailand.
External sources
-
Thailand Research Fund (Distinguished Professor Award), Thailand.
Declarations of interest
CK: None known
BC: None known
JT: None known
BU: None known
PL: None known
Acknowledgements
We thank Gail Quinn, Clare Jess and Tracey Harrison for their rigorous contribution to the editorial process; Jo Platt and Jane Hayes for designing the search strategies and running the search; and Jo Morrison for clinical and editorial advice. We thank the referees for many helpful suggestions and comments, some of these include Khadra Galaal and Jennifer Cove.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 21 | Antibiotics for infection prevention after excision of the cervical transformation zone | Review | Chumnan Kietpeerakool, Bandit Chumworathayi, Jadsada Thinkhamrop, Butsakorn Ussahgij, Pisake Lumbiganon | |
| 2012 Jul 11 | Antibiotics for infection prevention after excision of the cervical transformation zone | Protocol | Bandit Chumworathayi, Banchong Udomthavornsuk, Jadsada Thinkhamrop, Pisake Lumbiganon | |
Differences between protocol and review
Types of interventions
In the review, we additionally stated the details of antibiotic regimen as "Antibiotic regimen includes the followings: administration route (for example intravenous, intramuscular, oral, vaginal); number of doses (for example, single versus multiple doses).
Types of outcome measures
In the review protocol, we stated that primary outcome was genital infections occurring within seven days of excision of the cervical transformation zone including (a) cervix: cervicitis 24 hours following the procedure as defined by one or more of the symptoms and signs; delayed bleeding; redness; pus or offensive discharge; positive gram stain; positive culture; (b) uterus or adnexa: pelvic inflammatory disease (PID) assessed by vaginal examination for all women 24 hours after the procedure, found to have clinical evidence of PID (cervical excitation, pelvic organ tenderness, or both); and (c) sepsis or other serious infection or infectious complications, either with or without fever, defined as body temperature higher than 38o Celsius on at least two occasions more than four hours apart, 24 hours after the procedure. Secondary outcome was adverse effects of antibiotics including adverse drug reactions, which were defined as any medical complications related to drug metabolisms; allergy, liver failure, renal failure, etc. according to the CTCAE version 4 (CTCAE 2010) and drug interactions.
In attempts to cover a broad range of outcome measures for infectious complications following excision of the cervical transformation zone, we revised this section in this review as follows; Primary outcomes: (1) Infectious complications (defined as any documented sites of infection identified by cultivation or clinical symptoms and signs, or both), measured as the proportion of participants who developed each of the following within eight weeks of surgery: cervicitis, endometritis, pelvic inflammatory disease, prolonged vaginal discharge, severe vaginal bleeding, lower abdominal pain, fever, and additional antibiotic treatment; (2) Antibiotic resistance.
Secondary outcomes included (1) Adverse effects‐related to antibiotics: classified according to Common Terminology Criteria for Adverse Events (CTCAE 2010) as follows: gastrointestinal disorders (nausea, vomiting, anorexia, diarrhoea); immune system disorders (allergic reaction, anaphylaxis) blood and lymphatic system disorders (leucopenia, anaemia, thrombocytopenia, neutropenia); skin (stomatitis, mucositis, alopecia, allergy); nervous system disorders; genitourinary; and other side effects not categorised above; (2) unscheduled medical consultant (for any reasons); and (3) additional self‐medication .
Searching other source
In the review, we added Index to theses (ProQuest Dissertations & Theses: UK & Ireland) and the WHO International Clinical Trial Registry (http://apps.who.int/trialsearch/Default.aspx) as additional databases to be searched. In addition, we added the lists of conferences used for searching abstracts and proceedings
Data synthesis
We additionally stated in the review that "we prepared a ’Summary of findings’ table to present the results of the meta‐analysis, based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schunemann 2011). We presented the results of the meta‐analysis for the primary outcome and adverse events as outlined in the 'Types of outcome measures’ section".
Assessment of reporting biases
As there was only three trials that met our inclusion criteria, we were unable to construct funnel plots to determine the possibility of publication bias as previously stated in the review protocol. In future update of this review, we will construct funnel plots corresponding to meta‐analyses of the primary outcome to assess the possibility of publication bias if we identify a sufficient number of included studies (i.e. more than 10). We will also carry out sensitivity analyses to investigate the effect on the pooled results if the funnel plots are asymmetrical.
Subgroup analysis and investigation of heterogeneity
All three included trials in this review relate primarily to cervical transformation zone excision using electrical wire loop. Therefore, subgroup analyses according to the types of excisional techniques as mentioned in the review protocol was not feasible.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti-Bacterial Agents [adverse effects, *therapeutic use];
- Bacterial Infections [*prevention & control];
- Postoperative Complications [*prevention & control];
- Postoperative Hemorrhage [etiology];
- Precancerous Conditions [*surgery];
- Randomized Controlled Trials as Topic;
- Uterine Cervical Dysplasia [*surgery];
- Uterine Cervical Neoplasms [*surgery];
Medical Subject Headings Check Words
Female; Humans;
PICO

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prolonged vaginal discharge, Outcome 1 Number of participants who experienced prolonged vaginal discharge.

Comparison 2 Excessive vaginal bleeding, Outcome 1 Number of participants who had to be admitted for postoperative bleeding.

Comparison 3 Fever, Outcome 1 Number of participants who developed fever.

Comparison 4 Lower abdominal pain, Outcome 1 Number of participants who experienced lower abdominal pain.

Comparison 5 Adverse effects, Outcome 1 Number of participants who experienced any adverse effects related to antibiotics.
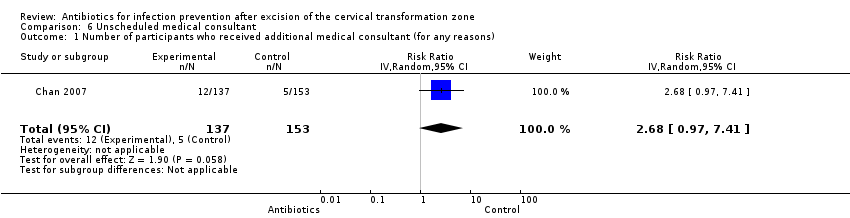
Comparison 6 Unscheduled medical consultant, Outcome 1 Number of participants who received additional medical consultant (for any reasons).

Comparison 7 Additional self‐medication, Outcome 1 Number of participants who had additional self‐medication.
| Antibiotics compared with placebo or no treatment for infection prevention after excision of the cervical transformation zone | ||||||
| Patient or population: Women undergoing excision of the cervical transformation zone for cervical neoplasia Settings: Outpatients setting, the colposcopy clinic Intervention: Prophylactic antibiotics Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| [Placebo or no treatment] | [Antibiotics] | |||||
| Number of participants who experienced prolonged vaginal discharge Follow‐up period: 2 weeks after the procedure | 103 per 1000 | 133 per 1000 | RR 1.29 (0.72 to 2.31) | 348 | ⊕⊕⊝⊝ | |
| Number of participants who had to be admitted for post‐procedure bleeding Follow‐up period: 2‐3 weeks after the procedure | 31 per 1000 | 38 per 1000 | RR 1.21 (0.52 to 2.82) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who developed fever Follow‐up period: 3 weeks after the procedure | 7 per 1000 | 16 per 1000 | RR 2.23 (0.20 to 24.36) | 290 (1 study) | ⊕⊝⊝⊝ | |
| Number of participants who experienced lower abdominal pain Follow‐up period: 3 weeks after the procedure | 163 per 1000 | 168 per 1000 | RR 1.03 (0.61 to 1.72) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who experienced any adverse effects related to antibiotics Follow‐up period: 2‐3 weeks after the procedure | 37 per 1000 | 63 per 1000 | RR 1.69 (0.85 to 3.34) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who received additional medical consultant (for any reasons) Follow‐up period: 3 weeks after the procedure | 33 per 1000 | 88 per 1000 | RR 2.68 (0.97 to 7.41) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who had additional self‐medication Follow‐up period: 3 weeks after the procedure | 72 per 1000 | 88 per 1000 | RR 1.22 (0.56 to 2.67) | 290 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on the high risk of attrition bias (high rate of incomplete participants' record charts) | ||||||
| Study | Antibiotic group | Placebo or no treatment |
| Number of participants who experienced prolonged vaginal discharge | ||
| 23/173 (13.3%) | 18/175 (10.3%) | |
| Number of participants who had to be admitted for post‐procedure bleeding | ||
| 2/173 (1.2%) | 3/175 (1.7%) | |
| 9/137 (6.6%) | 7/153 (4.6%) | |
| Number of participants who experienced lower abdominal pain | ||
| 23/137 (16.8%%) | 25/153 (16.3%) | |
| Number of participants who developed fever | ||
| 2/137 (1.5%) | 1/153 (0.7%) | |
| Number of participants who experienced adverse events | ||
| 20/173 (11.6%) | 12/175 (6.9%) | |
| 0/137 (0%) | 0/153 (0%) | |
| Number of participants who required unscheduled medical consultation | ||
| 12/137 (8.8%) | 5/153 (3.3%) | |
| Number of participants reported to have additional self‐medication | ||
| 12/137 (8.8%) | 11/153 (7.2%) |
| Outcomes reported in Gornall 1999 | Sultrin (sample size = n) | Control (sample size = 77‐n) | 95% confidence interval | P value |
| Mean duration of bleeding (days) | 15.2 | 11.2 | ‐7.7 to ‐0.2 | 0.04 |
| Mean duration of discharge (days) | 16.4 | 13.1 | ‐7.2 to 0.7 | 0.11 |
| Mean duration of pain (days) | 7.7 | 5.7 | ‐5.4 to 1.5 | 0.26 |
| Number of participants who received additional antibiotic therapy | 2 | 7 | Not reported | Not reported |
| Number of participants who had to be admitted for postoperative bleeding | 0 | 2 | Not reported | Not reported |
| Numbers of the participant in each comparison group were not reported. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced prolonged vaginal discharge Show forest plot | 1 | 348 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.72, 2.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had to be admitted for postoperative bleeding Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.52, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who developed fever Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.23 [0.20, 24.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced lower abdominal pain Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.61, 1.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced any adverse effects related to antibiotics Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.85, 3.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who received additional medical consultant (for any reasons) Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.68 [0.97, 7.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had additional self‐medication Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.56, 2.67] |

