Contenido relacionado
Revisiones y protocolos relacionados
Apiwat Aue-aungkul, Chumnan Kietpeerakool, Siwanon Rattanakanokchai, Khadra Galaal, Teerayut Temtanakitpaisan, Chetta Ngamjarus, Pisake Lumbiganon | 25 enero 2021
Ofrat Beyar‐Katz, Yaakov Dickstein, Sara Borok, Liat Vidal, Leonard Leibovici, Mical Paul | 3 junio 2017
Marc Arbyna, Lan Xua, Cindy Simoens, Pierre PL Martin‐Hirsch | 9 mayo 2018
Anat Gafter‐Gvili, Abigail Fraser, Mical Paul, Liat Vidal, Theresa A Lawrie, Marianne D van de Wetering, Leontien CM Kremer, Leonard Leibovici | 18 enero 2012
Pierre PL Martin-Hirsch, Andrew Bryant | 4 diciembre 2013
Esther van der Heijden, Alberto D Lopes, Andrew Bryant, Ruud Bekkers, Khadra Galaal | 6 enero 2015
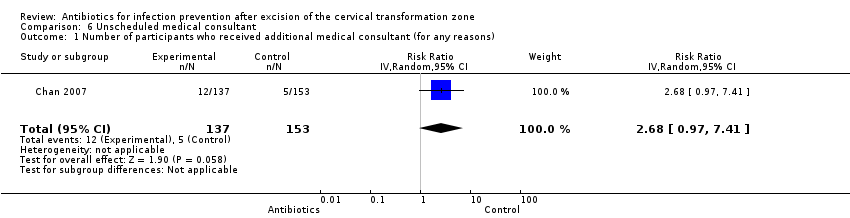
Chumnan Kietpeerakool, Apiwat Aue‐aungkul, Khadra Galaal, Chetta Ngamjarus, Pisake Lumbiganon | 12 febrero 2019
Ceder H van den Bosch, Job van Woensel, Marianne D van de Wetering | 7 octubre 2021
Lise J Estcourt, Simon J Stanworth, Carolyn Doree, Patricia Blanco, Sally Hopewell, Marialena Trivella, Edwin Massey | 29 junio 2015
C. William Helm, Douglas J Lorenz, Nicholas J Meyer, William WR Rising, Judith L Wulff | 6 junio 2013
Respuestas clínicas Cochrane
Jane Burch, Sera Tort | 1 junio 2017