자궁 절제 수술 후 감염 예방을 위한 항생제 투여
초록
배경
자궁 경부의 변형층을 포함한 절제술(자궁 경부 원추 절제술)은 자궁경부암의 위험을 낮추기 위해 자궁 경부의 전암성 병변(자궁경부 상피 내 종양 (CIN))을 치료하는 방법으로 가장 많이 이용되고있다. 자궁경부 원추 절제술은 자궁 경부에 상피결손부를 남겨 수술 후 감염의 위험이 있다. 원추 절제술(CKC)의 수술 후 감염 발생률은 36%인 반면, LLETZ(대형 전기 루프를 이용한 절제술(LEEP라고도 함))에서는 꽤 낮았다(0.8%˜14.4%). 예방적 항생제는 감염을 막을 수 있으며 이는 CKC에 종종 처방된다. 그러나 자궁 경부의 전암 병변을 외과적으로 절제하는 여성의 감염 예방을 위한 예방적 항생제 사용에 관한 공식적인 권고는 없다.
목적
자궁경부 원추 절제술 후 감염 예방을 목적으로 한 항생제의 유효성과 안전성을 평가한다.
검색 전략
검색 대상으로 한 데이터베이스는 다음과 같다: 2016년 5월까지, Cochrane Central Register of Controlled Trials (CENTRAL) (2016년 제 4호), MEDLINE, Embase, LILACS. 또한 임상시험 레지스트리를 체크하고 선택한 연구의 인용 문헌 목록, 주요 문헌과 과거의 체계적 고찰에서 관련될 수 있는 연구의 유무를 확인했다.
선정 기준
수술 방법에 관계없이 자궁경부 원추 절제술을 받은 여성에게서 예방적 항생제 투여의 유효성과 안전성을 위약 또는 무중재와 비교한 무작위대조시험(RCT)을 대상으로 하였다.
자료 수집 및 분석
Cochrane에서 요구되는 표준적인 방법론적 절차를 사용하였다. 검토자 2명이 각각 독립적으로 관련될 수 있는 임상시험을 선택하여 데이터를 추출하고 비뚤림 위험을 평가한 후 결과를 비교했으며 의견불일치는 대화를 통해 해결했다. 가능한 경우, 추가 데이터를 얻기 위해 연구자에게 연락했다.
주요 결과
검색 결과에서 특정된 370건의 임상시험(중복 제외)중 6건의 초록 및 제목을 관련가능성이 있는 임상시험으로 확인하였다. 이 6건의 임상시험 중 3건(참가자 708명)이 선택 기준을 충족했다. 대부분의 임상시험은 바이어스 위험(주로 눈가림 부족과 불완전한 데이터의 비율이 높은 데 따르는 위험)가 중간 또는 높은 수준이었다. 진행 중인 임상시험은 없었다. 대상으로 한 임상시험은 모두 검색 및 데이터 추출 시점에서 상호 검토가 있는 학술지에 발표되고 있었지만, 그 중 1건(77명)에서 평가된 결과 관련 수치 데이터를 메타분석에 통합하기에는 불충분했다.
두 군간의 비교에서 장기적으로 계속 나오는 질 분비물 혹은 자궁 경부염의 빈도(1건의 임상시험, 참가자 348명, 위험비(RR) 1.29, 95% 신뢰구간(CI) 0.72˜2.31, 근거의 질 낮음)와 심한 질 출혈의 빈도(2건의 임상시험, 참가자 638명, RR 1.21, 95% CI 0.52˜2.82, 근거의 질 매우 낮음)의 차이는 임상적으로 중요한 영향을 주는 수준에 도달하지는 않았다. 또한 항생제에 대한 부작용, 즉 구토, 설사, 두통에 있어 두 군간에 부작용의 차이는 없었다(2건의 임상시험, 참가자 638명, RR 1.69, 95% CI 0.85˜3.34, 근거의 질 매우 낮음). 두 군간의 비교에서 발열(RR 2.23, 95% CI 0.20˜24.36), 하복부 통증(RR 1.03, 95% CI 0.61˜1.72) 예상치 못한 의료기관 진료(RR 2.68, 95% CI 0.97˜7.41), 추가 자가치료(RR 1.22, 95% CI 0.56˜2.67)의 빈도에 차이는 보이지 않았다(1건의 임상시험, 참가자 290명, 근거의 질 낮음에서 매우 낮음).
연구진 결론
비뚤림 위험이 종합적으로 중간에서 높은 임상시험 3건에서 얻은 데이터는 제한적이며, 자궁 경부 원추 절제술 후 합병증을 줄이기 위해 항생제를 사용하는 것을 뒷받침하는 근거는 불충분하다. 또한, 항생제 관련 부작용에 대한 데이터는 극히 적고, 약제 내성의 발현 위험 정보는 없었다. 자궁 경부 원추 절제술의 감염 예방을 목적으로 한 항생제 투여는 임상 연구의 상황에서만 사용하여야 하며 항생제의 불필요한 처방을 피하고, 약제 내성균이 증가될 확률을 줄여야 한다.
PICO
쉬운 말 요약
자궁경부 절제술 후 감염 예방을 목적으로 한 예방적 항생제 투여
배경
자궁 경부의 전암 병변은 자궁 경부의 비정상적인 세포를 절제 또는 파괴하여 치료함으로써 미래의 자궁 경부암 발병 위험을 줄일 수있다. 절제술의 장점은 비정상적인 세포를 파괴하는 것이 아니라 제거하기 위해 조직을 정밀하게 검사하여 조직학적 진단을 확인하고 병변을 완전히 제거할지의 여부를 확인할 수 있다. 원추 절제를 하면 자궁 경부의 상피결손부가 발생하기 때문에 수술 후 감염 위험이 생긴다. 감염 발병 후 치료하는 것이 아니라 증상을 방지하기 위해(예방적) 수술 전에 항생제를 투여할 수 있다. 그러나 예방적 항생제 투여는 불필요하고 효과적이지 않을 가능성이 있다. 또한 항생제의 부작용도 일어날 수있다. 중요한 것은 항생제의 과용이 약제내성균을 발생시킬 우려가 높아지고 있는 것이다.
연구의 질문
자궁 경부 원추 절제술에 대한 예방적 항생제는 여성의 감염을 예방할 수 있는가? 또한 어떤 부작용이 있는가?
주요 결과
2016년 5월까지의 자료를 검색하고 고찰의 선택 기준을 충족하는 발표된 무작위대조시험 3건을 추출했다. 진행 중인 임상시험은 없었다. 선택된 3건의 임상시험 참가자들은 자궁 절제술(레이저 원추 절제술 또는 대형 루프 변형층 절제술 LLETZ 혹은 루프 전기 절제술(LEEP라고도 함))을 시행한 708명이었다. 2건의 임상시험은 항균성 질 페서리와 무치료를 비교하고 다른 1건은 경구 항생제와 위약을 비교했다. LLETZ 후 예방적으로 항생제를 투여해도 장기적으로 계속 질 분비물, 심한 질 출혈, 발열, 복부 통증, 예상치 못한 의료기관 진료 및 추가적인 자가 치료를 억제하거나 예방하는 효과는 없는 것으로 나타났다. 항생제 관련 부작용 정보는 거의 없었다. 얻어진 근거는 제한적이며, LLETZ 후 감염 예방을 위한 정기적인 항생제 투여의 시행을 뒷받침하는 것은 아니다. 항생제 내성에 대한 우려가 커지고 있기 때문에 자궁 경부 원추 절제술 후 감염 예방 목적으로 항생제를 투여하는 것은 임상시험의 상황에 한정해야 한다.
근거의 질
심한 질 출혈이나 발열, 심각한 부작용을 막기 위한 예방적 항생제 투여에 관한 근거의 질은 매우 낮고, 다른 비교 항목에서의 근거의 질도 낮았다.
Authors' conclusions
Summary of findings
| Antibiotics compared with placebo or no treatment for infection prevention after excision of the cervical transformation zone | ||||||
| Patient or population: Women undergoing excision of the cervical transformation zone for cervical neoplasia Settings: Outpatients setting, the colposcopy clinic Intervention: Prophylactic antibiotics Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| [Placebo or no treatment] | [Antibiotics] | |||||
| Number of participants who experienced prolonged vaginal discharge Follow‐up period: 2 weeks after the procedure | 103 per 1000 | 133 per 1000 | RR 1.29 (0.72 to 2.31) | 348 | ⊕⊕⊝⊝ | |
| Number of participants who had to be admitted for post‐procedure bleeding Follow‐up period: 2‐3 weeks after the procedure | 31 per 1000 | 38 per 1000 | RR 1.21 (0.52 to 2.82) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who developed fever Follow‐up period: 3 weeks after the procedure | 7 per 1000 | 16 per 1000 | RR 2.23 (0.20 to 24.36) | 290 (1 study) | ⊕⊝⊝⊝ | |
| Number of participants who experienced lower abdominal pain Follow‐up period: 3 weeks after the procedure | 163 per 1000 | 168 per 1000 | RR 1.03 (0.61 to 1.72) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who experienced any adverse effects related to antibiotics Follow‐up period: 2‐3 weeks after the procedure | 37 per 1000 | 63 per 1000 | RR 1.69 (0.85 to 3.34) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who received additional medical consultant (for any reasons) Follow‐up period: 3 weeks after the procedure | 33 per 1000 | 88 per 1000 | RR 2.68 (0.97 to 7.41) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who had additional self‐medication Follow‐up period: 3 weeks after the procedure | 72 per 1000 | 88 per 1000 | RR 1.22 (0.56 to 2.67) | 290 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on the high risk of attrition bias (high rate of incomplete participants' record charts) | ||||||
Background
Description of the condition
Cervical cancer remains a major world public health problem, as there are 500,000 new cases diagnosed and 275,000 deaths from cervical cancer occurring among women worldwide each year (Wiebe 2012). Many cervical cancers can be prevented through screening programs designed to detect and treat cervical cancer precursors. At the present, cervical intraepithelial neoplasia (CIN) grade 2 to 3, or the so‐called 'high‐grade squamous intraepithelial lesions (HSILs)' and adenocarcinoma in situ (AIS) are acknowledged as precancerous lesions of squamous cell carcinoma and adenocarcinoma of the uterine cervix, respectively (Massad 2013).
Excision of the cervical transformation zone, an area where the majority of abnormalities occur, is the most commonly used approach to treat cervical precancerous lesions. The major advantage of excisional treatment is that the affected area is removed, so the excised specimen can be sent for detailed pathological examination to determine the severity of the lesion and ensure that abnormal lesion has been completely removed. The common surgical techniques for excision of the cervical transformation zone include large loop excision of the transformation zone (LLETZ), cold‐knife conization (CKC), and laser conization (LC). The terms LLETZ and loop electrosurgical excision procedure (LEEP) both have been used to describe a technique to excise the transformation zone using a fine wire loop with an electrical current (diathermy). This technique is also referred to as loop diathermy excision, loop biopsy, and loop cone. Current evidence obtained from a Cochrane review conducted to evaluate the effectiveness of various surgical techniques for treating cervical intraepithelial neoplasia concludes that there is no single method that is a superior surgical technique for treating cervical intraepithelial neoplasia (Martin‐Hirsch 2013).
Genital infections, as defined by cervical tenderness, fever, or wound infection requiring antibiotic therapy, have been reported to be as high as 36% after CKC (Janthanaphan 2009) and may have serious complications such as sepsis from lung and liver abscesses (Treszezamsky 2010). LLETZ has a lower incidence of infectious complications ranging from 0.8% to 14.4% (Kietpeerakool 2007; Lopez 1994; Mints 2006; Mitchell 1998; Prendiville 1989; Takac 1999). The incidence of infectious complications after excision of the cervical transformation zone would be much higher, if delayed vaginal bleeding (occurring after 24 hours of the procedure) and offensive discharge are also included (Chamot 2010). However, excisional procedures are often associated with prolonged vaginal discharge, which is not necessarily infective, but may be due to the healing processes and oedema secondary to the procedure.
Description of the intervention
The American College of Obstetricians and Gynecologists (ACOG) recommends single‐dose antibiotic prophylactic protocols using cephalosporin, ampicillin, gentamicin, metronidazole or antibiotics in the quinolone group prior to some major surgical procedures (ACOG 2009). However, there are no formal recommendations regarding the use of prophylactic antibiotics for LC, CKC or LLETZ, which are outpatient treatments that could be considered as contaminated procedures, since the vagina is not sterile. Therefore, the use of prophylactic antibiotics may be considered (Chamot 2010).
How the intervention might work
The excision of the transformation zone leaves a raw surface on the cervix, where vaginal flora or introduced bacteria may initiate infections. Theoretically, prophylactic antibiotics would prevent these infections by eliminating these bacteria at an early stage. Several studies have shown that prophylactic antibiotics can reduce the risk of infections among women undergoing intrauterine device insertion, surgical abortion, and repair of severe perineal tear (Buppasiri 2014; Grimes 1999; Sawaya 1996).
Why it is important to do this review
Standard clinical practice guidelines regarding the effectiveness and safety of prophylactic antibiotics for infection prevention after excision of the cervical transformation zone are lacking. A previous systematic review conducted to determine the safety of LLETZ identified that information regarding the use of prophylactic antibiotic therapy for infection prevention was inconsistently reported. Routine antibiotic therapy was described in only three out of 16 included studies (Chamot 2010). This systematic review, looking at the use of prophylactic antibiotics for the prevention of infection following the excision of the cervical transformation zone aims to evaluate the best and current evidence for the use of prophylactic antibiotics in this setting.
Objectives
To evaluate the effectiveness and safety of antibiotics for infection prevention following excision of the cervical transformation zone.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We did not include quasi‐randomised trials as these may have been subjected to increased bias.
Types of participants
Women of any age undergoing excision of the cervical transformation zone for cervical intraepithelial neoplasia (CIN), irrespective of surgical techniques.
Types of interventions
Prophylactic antibiotics (irrespective of regimens) versus placebo or no treatment.
Classification of antibiotics were as follows (Marjoribanks 2004).
-
Cephalosporins
-
Penicillins
-
Macrolides
-
Fluoroquinolones
-
Sulfonamides
-
Tetracyclines
-
Aminogylocosides
-
Glycopeptides
-
Antiprotozoals
-
Combination drugs
Antibiotic regimen includes the following.
-
Administration route (for example intravenous, intramuscular, oral, vaginal)
-
Number of doses (for example single versus multiple doses)
Types of outcome measures
Primary outcomes
-
Infectious complications (defined as any documented sites of infection identified by cultivation or clinical symptoms and signs, or both), measured as the proportion of participants who developed each of the following within eight weeks of surgery: cervicitis, endometritis, pelvic inflammatory disease, prolonged vaginal discharge, severe vaginal bleeding, lower abdominal pain, fever, and additional antibiotic treatment.
-
Antibiotic resistance
Secondary outcomes
-
Adverse effects related to antibiotics: classified according to Common Terminology Criteria for Adverse Events (CTCAE 2010) as follows: gastrointestinal disorders (nausea, vomiting, anorexia, diarrhoea); immune system disorders (allergic reaction, anaphylaxis) blood and lymphatic system disorders (leucopenia, anaemia, thrombocytopenia, neutropenia); skin (stomatitis, mucositis, alopecia, allergy); nervous system disorders; genitourinary; and other side effects not categorised above
-
Unscheduled medical consultant (for any reasons).
-
Additional self‐medication.
As the association between vaginal bleeding, offensive vaginal discharge and secondary infection following excision of the cervical transformation zone has been observed in previous reports (Chan 2007; Doyle 1992), we chose severe vaginal bleeding and prolonged vaginal discharge as the surrogates for infectious complication. We did not include urinary tract infections and quality of life (QoL) in this review as these were not directly related to excision of the cervical transformation zone.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library, MEDLINE (1946 to May week 1, 2016), Embase (1980 to 2016, week 19), and LILACS (1970 to May, 2016).
Appendix 1, Appendix 2, Appendix 3, and Appendix 4 display the search strategies for CENTRAL, MEDLINE, Embase and LILACS, respectively.
Searching other resources
Unpublished and grey literature
We searched the following sources for ongoing trials.
-
ISRCTN registry, metaRegister of Controllled Trials (mRCT http://www.isrctn.com/page/mrct).
-
Physicians Data Query (https://www.cancer.gov/publications/pdq).
-
Clinical trials.gov http://www.clinicaltrials.gov.
-
International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/).
-
World Health Organization (WHO) International Clinical Trial Registry (http://apps.who.int/trialsearch/Default.aspx).
We searched electronic databases including Greynet.org (http:// www.greynet.org), the Ohio College Library Center (OCLC) WorldCat Dissertations and Theses (WorldCatDissertations; https://www.oclc.org/support/services/firstsearch/documentation/dbdetails/details/WorldCatDissertations.en.html) and Index to theses (ProQuest Dissertations & Theses: UK & Ireland) to identify the possible relevant conference abstracts and proceedings.
Handsearching
We handsearched reports of conferences from the following sources.
-
Gynecologic Oncology (Annual Meeting of the Society of Gynecologic Oncology)
-
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society)
-
Annual Meeting of the European Society of Medical Oncology (ESMO)
-
Annual Meeting of the British Gynaecological Cancer Society (BGCS)
-
Biennial Meeting of the Asian Society of Gynecologic Oncology (ASGO)
-
Biennial Meeting of Asia and Oceania Federation of Obstetrics and Gynaecology (AOFOG)
-
Biennial Meeting of the European Society of Gynaecologic Oncology (ESGO)
We also checked the citation lists of the included studies and key textbooks for potentially relevant references. We searched for papers in all languages and would have had them translated, if necessary.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to Endnote, and duplicates were removed. Two review authors (CK and BC) independently examined the remaining references. We excluded those studies that did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. We (CK and BC) independently assessed the eligibility of retrieved papers. We resolved disagreements by discussion and, if there was no consensus, by appeal to a third review author (JT or PL). We documented the reasons for exclusion (see Excluded studies and Characteristics of excluded studies).
Data extraction and management
We (CK and BC) abstracted data independently onto a data abstraction form specifically designed for the review. We resolved differences between review authors by discussion or by appeal to a third reviewer (JT or PL), if necessary. The same two review authors independently assessed the quality of the trials and abstracted data using forms specifically designed for the review, according to Cochrane guidelines (Higgins 2011).
Trial characteristics
-
Method of randomisation
-
Method of allocation concealment
-
Presence or absence of blinding of participants, clinicians and outcome assessors to treatment allocation
-
Number of participants randomised
-
Number of withdrawals (participants excluded from analysis or lost to follow‐up) and reasons
-
Whether an intention‐to‐treat (ITT) analysis was done
-
Duration, timing and location of the study
Characteristics of the study participants
-
Type of an excision of the cervical transformation zone undergone (either by CKC, laser conization, LEEP, LLETZ, or any other excision technique)
-
Inclusion criteria
-
Exclusion criteria
-
Whether the groups of participants were well balanced with regard to prognostic factors
Intervention used
-
Type of antibiotics used
-
Dose
-
Route
-
Single or multiple doses given
-
Duration of course of antibiotics
-
Number and timing of doses
Nature of comparison
-
No treatment, or placebo
-
Route of administration
-
Drug regimen
Outcomes
-
Methods used to measure genital infections
-
Methods used to evaluate adverse effects
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of the included studies according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which recommends the explicit reporting of the following individual elements for RCTs.
-
Selection bias: random sequence generation and allocation concealment.
-
Performance bias: blinding of participants and personnel (participants and treatment providers).
-
Detection bias: blinding of outcome assessment.
-
Attrition bias: incomplete outcome data.
-
Reporting bias: selective reporting of outcomes.
-
Other bias
Two review authors (CK, BC) applied the ’Risk of bias’ tool independently, and we resolved differences by discussion or by appeal to a third review author (JT or PL). We judged each item as being at high, low or unclear risk of bias as set out in the criteria shown in Appendix 5 (Higgins 2011). We provided a quote from the study report or a statement as justification for our judgement. We summarised results in a ’Risk of bias’ summary figure.
Measures of treatment effect
We used the following measures of the effect of treatment.
-
For dichotomous data (e.g. adverse events or infections), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio (RR) with 95% confidence intervals (CIs).
-
For continuous data, we planned to use the mean difference (MD) or standardized mean difference (SMD) with 95% CIs.
Where possible RRs of individual studies were combined for meta‐analysis using RevMan 2014 software.
Dealing with missing data
We did not impute missing outcome data for the primary outcome and we attempted to contact study authors to obtain missing data. If data were missing to the extent that we could not have included the study in the analysis, we had planned to present the results in a narrative way.
Assessment of heterogeneity
We assessed heterogeneity using visual inspection of the forest plots. We also assessed statistical heterogeneity in each meta‐analysis using the I² statistic and Chi² test. We regarded heterogeneity as substantial if the I² statistic value was greater than 50%, or there was a low P value (< 0.10) in the Chi² test for heterogeneity (Deeks 2001; Higgins 2011). If there was substantial statistical heterogeneity, we planned to carry out subgroup analyses to assess differences between the included studies. However, if there had been clinical, methodological or considerable statistical heterogeneity (I² greater than 75%) across included studies, we had planned to use a narrative approach to data synthesis.
Assessment of reporting biases
We had planned to examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects, such as publication bias, if more than 10 studies were included. If asymmetry had been suggested by a visual assessment, we had planned to perform sensitivity analysis to investigate whether it affected the pooled results. (Sterne 2011).
Data synthesis
Where feasible, the results were pooled in a meta‐analyses. We used the random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986). We performed statistical analysis using RevMan 2014.
-
For dichotomous outcomes, we calculated the RR for each study as well as for the pooled outcome.
-
For continuous outcomes, we calculated and pooled the mean difference between the treatment arms if all trials measured the outcome on the same scale; otherwise we pooled the standardised mean differences.
We prepared a ’Summary of findings’ table to present the results of the meta‐analysis, based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schunemann 2011). For assessments of the overall certainty of evidence for each outcome that includes pooled data from RCTs only, we downgraded the evidence from 'high certainty' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. The following outcomes were included in the 'Summary of findings' table:
-
prolonged vaginal discharge;
-
postoperative bleeding;
-
fever;
-
abdominal pain;
-
adverse effect related to antibiotic;
-
additional medical support;
-
additional self‐medication.
We presented the results of the meta‐analysis for the relevant outcomes and adverse events as outlined in the ’Types of outcome measures’ section.
Subgroup analysis and investigation of heterogeneity
As we found only a few trials, we did not perform any subgroup analyses. However, we considered factors such as type of excisional techniques (e.g. CKC, LC, or LLETZ/LEEP) and antibiotic regimen prescribed in the interpretation of review findings. In future updates, we will perform subgroup analysis based on these factors, if feasible.
Sensitivity analysis
As we found only three trials, we did not perform any sensitivity analysis. In future updates, if statistical heterogeneity is detected and there are sufficient trials included (greater than 10), we will conduct sensitivity analyses to examine the possible contribution of other clinical or methodological differences between the trials, specifically:
-
trials with adequate methodology versus those with poor methodology;
-
trials which seem to differ from the others in their clinical criteria for defining genital infections.
Results
Description of studies
Results of the search
We ran a broad search that yielded 390 references from the combined searches. We checked the reference lists and handsearched journals and congress abstracts, which identified two additional references. We did not identify any ongoing trials. After de‐duplication, we screened 370 references and excluded 364 references that obviously did not meet the inclusion criteria. Of the six studies that potentially met the review inclusion, we excluded three studies after reviewing the full texts (Doyle 1992; Gerli 2012; Minorchio 1990) (see Excluded studies and Characteristics of excluded studies). Figure 1 displays the PRISMA flowchart for study selection.

Study flow diagram.
Included studies
We found three studies that met the inclusion criteria (Chan 2007; Foden‐Shroff 1998; Gornall 1999). See 'Characteristics of included studies' for details of each included study.
Foden‐Shroff 1998 was conducted in UK from July 1994 to August 1996. Participants enrolled in the antibiotic group received ofloxacin 400 mg, once daily for five consecutive days, starting immediately after the procedure. Participants assigned to the control group received an identical placebo. All participants received a pictorial chart to record the amount of vaginal discharge and adverse events for two weeks after the procedure. Reported outcomes included: prevalence of prolonged vaginal discharge; postoperative vaginal bleeding requiring hospitalisation; and adverse events. From the 500 participants recruited, only 348 participants' charts were suitable for analyses (70% of total group).
Gornall 1999 was conducted in the UK. The study period was not reported. The participants allocated to the intervention group received Sultrin®, which is antimicrobial vaginal pessary containing sulphatiazole 3.4%, sulphacetamide 2.8%, and sulphabenzamide 3.7%, twice daily for five days. Participants assigned to the control group received no treatment. All participants received patient charts to record of their symptoms and requirement of unscheduled medical visits and additional antibiotic treatment, if any during the first four weeks after the procedure. Study outcomes were: (1) severity of vaginal bleeding, discharge, and pain; and (2) the unscheduled medical visits and additional antibiotic therapy. From the 100 participants recruited, 77 participants' charts were available for analyses, corresponding to a rate of 77%.
Chan 2007 was conducted in Hong Kong between May 2003 and August 2006. Of 321 participants complying with inclusion/exclusion criteria; 157 were randomly allocated to receive antibiotics and 164 who were assigned to the control group did not receive any medication. The intervention was an antimicrobial vaginal pessary containing 100 mg tetracycline and 50 mg amphotericin B (Talsutin®), given twice daily for 14 days, starting on the day of large loop excision of the transformation zone (LLETZ). All participants received a diary to record the daily amount of vaginal bleeding, vaginal discharge, and lower abdominal pain for three weeks after the procedure. Study outcomes were: (1) prevalence of vaginal bleeding that required admission to hospital, additional medical consultation, additional self‐medication, fever; (2) severity of vaginal bleeding, pain, and lower abdominal pain. The authors excluded 23 participants (12 in antibiotic group and 11 in control group) because they did not return the diary. In addition, the authors excluded eight participants assigned to antibiotic group due to non‐compliance with treatment, leaving 290 charts for final analyses (approximately 90% of total group).
See 'Characteristics of included studies' for full details of the included studies. Although all three included studies had been published in peer‐reviewed journals at the time of the search and data extraction, numerical data regarding the outcome measured in Gornall 1999 was insufficient for meta‐analyses. Attempts to obtain additional data from the investigators were not successful, since the authors did not reply to our inquiries. We therefore did not pool the results of this study and instead presented them in a narrative format.
Excluded studies
After excluding non‐relevant and duplicated records, we retrieved six possibly eligible studies for more detailed evaluation. Of the six potentially eligible studies that we assessed in full‐text format, we excluded three references for the following reasons (see 'Characteristics of excluded studies). One trial was excluded because it was a non‐randomised study. In addition, the treatment used in this study was cervical ablation, rather than excision, so it did not meet the review inclusion criteria (Minorchio 1990). Another reference was a randomised study evaluating effectiveness and safety of two preparations of vaginal antiseptic suppositories after cervical ablation using CO2 laser, thus it did not comply to the review inclusion criteria (Gerli 2012). The third was a randomised study, but it was excluded because the intervention that was evaluated in this study was a Monsel’s solution, rather than prophylactic antibiotics (Doyle 1992).
Risk of bias in included studies
Allocation
In two included studies (Chan 2007; Foden‐Shroff 1998), the participants were randomly allocated to the comparison groups with adequate allocation concealment. We therefore determined both trials as having low risk of bias. The other included study (Gornall 1999) used sealed envelopes for treatment allocation, but no details of randomisation were given. We determined this to indicate unclear risk of bias for random sequence generation and low risk of bias for allocation concealment (Figure 2).

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Blinding
In Foden‐Shroff 1998, the authors provided the study medications containing either antibiotics or placebo with the same appearance and packaging. We determined this to indicate low risk of bias
In the remaining two included studies (Chan 2007; Gornall 1999), participants assigned to the control group did not have a placebo control, so they were aware about the treatment allocated. We therefore judged both trials as having high risk of bias (Figure 2).
Incomplete outcome data
In the studies of Foden‐Shroff 1998 and Gornall 1999 the rates of incomplete data were high (30% and 23%, respectively). Therefore we judged both studies as having high risk of bias. In Chan 2007 90% of participants had complete data, indicating low risk of bias (Figure 2).
Selective reporting
Although the study protocols of included studies were not available (Chan 2007; Foden‐Shroff 1998; Gornall 1999), the authors did report the expected relevant outcomes, so we judged this domain to be at a low risk of bias (Figure 2).
Other potential sources of bias
The analyses performed in all three included studies (Chan 2007; Foden‐Shroff 1998; Gornall 1999) did not follow an intention‐to‐treat basis. Additionally, in Gornall 1999, there were no data regarding the review inclusion and exclusion criteria applied. The authors also did not provide the data regarding the baseline characteristics of participants and number of participants allocated to each comparison group, which are mandatory for determining treatment outcomes and assessing the quality of study methodology. So, we deemed this domain to have a high risk of bias (Figure 2).
Effects of interventions
See: Summary of findings for the main comparison
See: Differences between protocol and review
Primary outcomes
Infectious complications
Prolonged vaginal discharge
Only Foden‐Shroff 1998 reported the incidence of prolonged vaginal discharge after cervical excision in the two comparison groups. Participants receiving prophylactic antibiotics had a higher incidence of prolonged vaginal discharge after LLETZ compared to those who were assigned to placebo group (13.3% versus 10.3%, respectively; Table 1). However, there was no statistical difference (one study; 348 participants; risk ratio (RR), 1.29; 95% confidence interval (CI) 0.72 to 2.31; low‐quality evidence). See summary of findings Table for the main comparison; Analysis 1.1.
| Study | Antibiotic group | Placebo or no treatment |
| Number of participants who experienced prolonged vaginal discharge | ||
| 23/173 (13.3%) | 18/175 (10.3%) | |
| Number of participants who had to be admitted for post‐procedure bleeding | ||
| 2/173 (1.2%) | 3/175 (1.7%) | |
| 9/137 (6.6%) | 7/153 (4.6%) | |
| Number of participants who experienced lower abdominal pain | ||
| 23/137 (16.8%%) | 25/153 (16.3%) | |
| Number of participants who developed fever | ||
| 2/137 (1.5%) | 1/153 (0.7%) | |
| Number of participants who experienced adverse events | ||
| 20/173 (11.6%) | 12/175 (6.9%) | |
| 0/137 (0%) | 0/153 (0%) | |
| Number of participants who required unscheduled medical consultation | ||
| 12/137 (8.8%) | 5/153 (3.3%) | |
| Number of participants reported to have additional self‐medication | ||
| 12/137 (8.8%) | 11/153 (7.2%) |
Severe vaginal bleeding
All included studies reported the incidence of severe vaginal bleeding requiring hospital admission. However, numerical data regarding this outcome measured in the study Gornall 1999 was insufficient for meta‐analyses. In Foden‐Shroff 1998, only 1.2% of participants assigned to the antibiotic group experienced severe vaginal bleeding requiring hospitalisation, which was comparable to the 1.7% reported for participants in the placebo group (Table 1). In Chan 2007, participants receiving prophylactic antibiotics had a higher incidence of severe vaginal bleeding requiring hospitalisation compared to those who were assigned to placebo group (6.6% versus 4.6%, respectively; Table 1). After combining data from Foden‐Shroff 1998 and Chan 2007, there was no difference (638 participants; RR 1.21; 95% CI 0.52 to 2.82; very low‐quality evidence). See summary of findings Table for the main comparison; Analysis 2.1.
In Gornall 1999, of the 77 participants, two assigned to the control group were admitted because of excessive vaginal bleeding. None of the participants who received antibiotics had severe vaginal bleeding (Table 2).
| Outcomes reported in Gornall 1999 | Sultrin (sample size = n) | Control (sample size = 77‐n) | 95% confidence interval | P value |
| Mean duration of bleeding (days) | 15.2 | 11.2 | ‐7.7 to ‐0.2 | 0.04 |
| Mean duration of discharge (days) | 16.4 | 13.1 | ‐7.2 to 0.7 | 0.11 |
| Mean duration of pain (days) | 7.7 | 5.7 | ‐5.4 to 1.5 | 0.26 |
| Number of participants who received additional antibiotic therapy | 2 | 7 | Not reported | Not reported |
| Number of participants who had to be admitted for postoperative bleeding | 0 | 2 | Not reported | Not reported |
Numbers of the participant in each comparison group were not reported.
Fever
Only Chan 2007 reported the incidence of fever following excision of the cervical transformation zone and demonstrated no difference between the groups (one study; 290 participants; RR, 2.23; 95% CI 0.20 to 24.36; very low‐quality of evidence). See summary of findings Table for the main comparison: Analysis 3.1.
Lower abdominal pain
The incidence of postoperative lower abdominal pain was reported in one of three included studies (Chan 2007). Approximately 16.8% of participants assigned to the antibiotic group experienced lower abdominal pain following LLETZ, which was comparable to the 16.3% reported for participants in the control group (Table 1) (one study; 290 participants; RR, 1.03; 95% CI 0.61 to 1.72: low‐quality evidence). See summary of findings Table for the main comparison; Analysis 4.1.
Additional antibiotic treatment
Gornall 1999 reported that, of 77 participants available for the study analyses, two and seven participants assigned to the antibiotic and control group, respectively, received additional antibiotics. Because of missing data regarding the number of participants in each comparison group, the incidence of the requirement of additional antibiotic treatment among the two comparison groups and the relative effect of prophylactic antibiotics, could not be determined (Table 2). Foden‐Shroff 1998 and Chan 2007 did not report the requirement of additional antibiotic treatment in their studies.
Other infectious complications
The included studies did not report the incidence of cervicitis, endometritis, and pelvic inflammatory disease.
Antibiotic resistance
No information on antibiotic resistance was reported in any of the included studies.
Secondary outcomes
Adverse effects
No reported data on the rate of adverse events were available for Gornall 1999 (Table 2). In Chan 2007, no participants reported antibiotic adverse events (Table 1). Foden‐Shroff 1998 reported that 11.6% of participants who received antibiotic experienced adverse events, including nausea, vomiting, diarrhoea, and headache, which was higher than the 6.9% reported in participants assigned to placebo group. When we pooled the data from these two included studies (Chan 2007; Foden‐Shroff 1998), no difference was demonstrated (638 participants; RR 1.69; 95% CI 0.85 to 3.34; very low‐quality evidence). See summary of findings Table for the main comparison: Analysis 5.1.
Unscheduled medical consultation
Only Chan 2007 reported the rate of unscheduled medical consultation in the two comparison groups. Participants receiving antimicrobial vaginal pessary had a higher rate of unscheduled medical consultation than those who were assigned to the control arm (8.8% versus 3.3%, respectively), but this difference was not significant (one study, 290 participants; RR 2.68, 95% CI 0.97 to 7.41; low‐quality evidence). See summary of findings Table for the main comparison; Analysis 6.1.
Additional self‐medication
The rate of additional self‐medication was reported in one study (Chan 2007). Approximately 8.8% of participants assigned to antibiotic group reported to have self‐medication which was comparable to the 7.2% reported for participants in the control group (one study; 290 participants; RR 1.22; 95%CI 0.56 to 2.67; very low‐quality evidence; summary of findings Table for the main comparison; Analysis 7.1).
Discussion
Summary of main results
We found three studies that met our inclusion criteria. However, numerical data regarding the outcome measured in one small study was insufficient for inclusion in the meta‐analyses. Evidence from this review indicates that there were no differences between women receiving prophylactic antibiotics and those receiving a placebo or no treatment in terms of prolonged vaginal discharge, severe vaginal bleeding, fever, lower abdominal pain, requirement of unscheduled medical consultation, and self‐medication. In addition, the number of adverse events did not differ between the two comparison groups. None of the three included studies reported data on antibiotic resistance. However, it is unknown whether these symptoms were due to infection, since samples for microbiological culture were not routinely taken in these studies and symptoms were largely self‐reported.
Overall completeness and applicability of evidence
This review included three randomised controlled trials (RCTs) evaluating prophylactic antibiotic in 708 participants who had undergone large loop excision of the transformation zone (LLETZ) procedure in the UK and Hong Kong. The interventions evaluated in the included studies were different antibiotic regimens to prevent infection following excision of the cervical transformation zone. Primary outcomes of this review were signs and symptoms suggesting infectious complications including: cervicitis; endometritis; pelvic inflammatory disease; prolonged vaginal discharge; severe vaginal bleeding; lower abdominal pain; fever; and additional antibiotic treatment received. Secondary outcomes included adverse events; antibiotic resistance; and incidence of unscheduled medical consultation and self‐medication. However, the included studies did not report the incidence of cervicitis, endometritis, pelvic inflammatory disease, and antibiotic resistance.
It is important to note that the excisional method used in all three included studies was LLETZ. Therefore, the same results may not be applicable to other excisional techniques. In addition, the two studies included in the review that tested drugs targeting Chlamydia trachomatis took place in a population with a prevalence of chlamydial infection that varied from 2% to 10% (Chan 2007; Foden‐Shroff 1998). The other antimicrobial used was vaginal pessary, which was a preparation used against Haemophilus (Garnerella) vaginalis (Gornall 1999).Therefore, generalisation of the results to different settings and different antibiotics or a combination of antibiotics may be limited.
Quality of the evidence
The greatest threat to the validity of the included study is likely to be the risk of bias (see Figure 2). In all included studies, treatment outcomes were measured using self‐reported data from participants. However, participants assigned to the control groups of two included studies did not receive a placebo. Thus, these participants were unblinded for treatment allocated, resulting in a high risk of performance and detection biases (Chan 2007; Gornall 1999). Another limitation for this review was the potential risk of attrition bias. Lack of data for final analyses were considerable, greater than 20% in two included studies (Foden‐Shroff 1998; Gornall 1999). In addition, analyses carried out in all included studies did not follow an intention‐to‐treat principle. Data from one study involving 77 participants were also limited. The authors of this study did not provide additional information. Baseline characteristics of participants, and number of participants allocated to each comparison group, which are necessary for assessing the effects of treatment and quality of study methodology, were not available (Gornall 1999).
Another major limitation of this review is the small number of included studies, which has the potential to affect the accuracy in determining statistical heterogeneity and thus we used random‐effects model for all meta‐analyses.
We assessed the quality of evidence using the GRADE approach for each outcome (see summary of findings Table for the main comparison). Based on the concerns regarding the risk of the potential bias, we downgraded the evidence to low quality for the incidences of prolonged vaginal discharge, lower abdominal pain, unscheduled medical consultant, and self‐medication. We downgraded the evidence to very low quality for severe vaginal bleeding, postoperative fever, and adverse events due to the potential biases and sparseness of data. We found no available evidence on the potential risk of antibiotic resistance, one of the most serious health threats, following the use of antibiotic prophylaxis for excision of the cervical transformation zone.
Potential biases in the review process
With assistance from the Information Specialist, Cochrane Gynaecological, Neuro‐oncology & Orphan Cancer Group, we made every attempt to include global studies including a thorough search of the grey literature, conference proceedings, and ongoing trials. However, as there were only three studies that met the review inclusion criteria, there remains the possibility that there may be other unpublished trials of intervention that the review authors did not discover. This means that the review authors may unwittingly have perpetuated a publication bias. Another source of potential biases is our inability to obtain relevant incomplete data leading to the exclusion of one small study (Gornall 1999) from the meta‐analysis.
None of the review authors have any links to drug companies or a financial interest in the prescription of the drug under assessment, nor were they involved in the conduct of the included study. Thus, there were no issues associated to bias secondary to conflicts of interests in this review.
Agreements and disagreements with other studies or reviews
According to the surgical wound classification system, an excision of the cervical transformation zone is considered as a clean‐contaminated procedure. Although ACOG (ACOG 2009) and the National Institute for Health and Care Excellence (NICE 2008) generally recommend prophylactic antibiotics for clean‐contaminated procedures, findings from this review show that there is not sufficient evidence to support routine antibiotics for infection prevention after LLETZ procedure. This conclusion is in broad agreement with previously published reviews (Craciunas 2014; Morrill 2013).
Antibiotic resistance is one of the biggest threats to global health and requires urgent, co‐ordinated action across all healthcare sectors (Del Mar 2012; WHO 2015).The misuse of antibiotics accelerates the emergence of drug‐resistant bacteria (WHO 2015). As noted in previous reports in a variety of clinical settings, administration of antibiotic prophylaxis can be a risk factor for antibiotic resistance (Bitsori 2014; McMurray 2015; Minami 2014). Therefore, the potential benefits and possible harms of prophylactic antibiotics should be carefully assessed and considered, particularly for the risk of developing antibiotic resistance. However, there has been little discussion of potential risk of antibiotic resistance after administration of prophylactic antibiotics in previous reports (Buppasiri 2014; Grimes 1999; Morrill 2013; Sawaya 1996). In this review, none of the included studies reported data on antibiotic resistance.

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prolonged vaginal discharge, Outcome 1 Number of participants who experienced prolonged vaginal discharge.

Comparison 2 Excessive vaginal bleeding, Outcome 1 Number of participants who had to be admitted for postoperative bleeding.

Comparison 3 Fever, Outcome 1 Number of participants who developed fever.

Comparison 4 Lower abdominal pain, Outcome 1 Number of participants who experienced lower abdominal pain.

Comparison 5 Adverse effects, Outcome 1 Number of participants who experienced any adverse effects related to antibiotics.
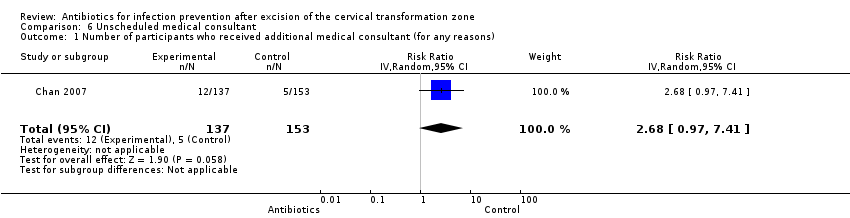
Comparison 6 Unscheduled medical consultant, Outcome 1 Number of participants who received additional medical consultant (for any reasons).

Comparison 7 Additional self‐medication, Outcome 1 Number of participants who had additional self‐medication.
| Antibiotics compared with placebo or no treatment for infection prevention after excision of the cervical transformation zone | ||||||
| Patient or population: Women undergoing excision of the cervical transformation zone for cervical neoplasia Settings: Outpatients setting, the colposcopy clinic Intervention: Prophylactic antibiotics Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| [Placebo or no treatment] | [Antibiotics] | |||||
| Number of participants who experienced prolonged vaginal discharge Follow‐up period: 2 weeks after the procedure | 103 per 1000 | 133 per 1000 | RR 1.29 (0.72 to 2.31) | 348 | ⊕⊕⊝⊝ | |
| Number of participants who had to be admitted for post‐procedure bleeding Follow‐up period: 2‐3 weeks after the procedure | 31 per 1000 | 38 per 1000 | RR 1.21 (0.52 to 2.82) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who developed fever Follow‐up period: 3 weeks after the procedure | 7 per 1000 | 16 per 1000 | RR 2.23 (0.20 to 24.36) | 290 (1 study) | ⊕⊝⊝⊝ | |
| Number of participants who experienced lower abdominal pain Follow‐up period: 3 weeks after the procedure | 163 per 1000 | 168 per 1000 | RR 1.03 (0.61 to 1.72) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who experienced any adverse effects related to antibiotics Follow‐up period: 2‐3 weeks after the procedure | 37 per 1000 | 63 per 1000 | RR 1.69 (0.85 to 3.34) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who received additional medical consultant (for any reasons) Follow‐up period: 3 weeks after the procedure | 33 per 1000 | 88 per 1000 | RR 2.68 (0.97 to 7.41) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who had additional self‐medication Follow‐up period: 3 weeks after the procedure | 72 per 1000 | 88 per 1000 | RR 1.22 (0.56 to 2.67) | 290 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on the high risk of attrition bias (high rate of incomplete participants' record charts) | ||||||
| Study | Antibiotic group | Placebo or no treatment |
| Number of participants who experienced prolonged vaginal discharge | ||
| 23/173 (13.3%) | 18/175 (10.3%) | |
| Number of participants who had to be admitted for post‐procedure bleeding | ||
| 2/173 (1.2%) | 3/175 (1.7%) | |
| 9/137 (6.6%) | 7/153 (4.6%) | |
| Number of participants who experienced lower abdominal pain | ||
| 23/137 (16.8%%) | 25/153 (16.3%) | |
| Number of participants who developed fever | ||
| 2/137 (1.5%) | 1/153 (0.7%) | |
| Number of participants who experienced adverse events | ||
| 20/173 (11.6%) | 12/175 (6.9%) | |
| 0/137 (0%) | 0/153 (0%) | |
| Number of participants who required unscheduled medical consultation | ||
| 12/137 (8.8%) | 5/153 (3.3%) | |
| Number of participants reported to have additional self‐medication | ||
| 12/137 (8.8%) | 11/153 (7.2%) |
| Outcomes reported in Gornall 1999 | Sultrin (sample size = n) | Control (sample size = 77‐n) | 95% confidence interval | P value |
| Mean duration of bleeding (days) | 15.2 | 11.2 | ‐7.7 to ‐0.2 | 0.04 |
| Mean duration of discharge (days) | 16.4 | 13.1 | ‐7.2 to 0.7 | 0.11 |
| Mean duration of pain (days) | 7.7 | 5.7 | ‐5.4 to 1.5 | 0.26 |
| Number of participants who received additional antibiotic therapy | 2 | 7 | Not reported | Not reported |
| Number of participants who had to be admitted for postoperative bleeding | 0 | 2 | Not reported | Not reported |
| Numbers of the participant in each comparison group were not reported. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced prolonged vaginal discharge Show forest plot | 1 | 348 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.72, 2.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had to be admitted for postoperative bleeding Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.52, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who developed fever Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.23 [0.20, 24.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced lower abdominal pain Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.61, 1.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced any adverse effects related to antibiotics Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.85, 3.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who received additional medical consultant (for any reasons) Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.68 [0.97, 7.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had additional self‐medication Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.56, 2.67] |

