자궁 절제 수술 후 감염 예방을 위한 항생제 투여
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | A randomised‐controlled trial conducted in the colposcopy clinic at Queen Mary Hospital, University of Hong Kong. Study duration: May 2003 to August 2006. | |
| Participants | All women who attended the clinic for LLETZ. Exclusion criteria: history of antibiotic hypersensitivity, liver or renal disease, previous psychiatric problems or receiving antibiotics within the 2 weeks prior to the visit. All participants receive a diary to record the daily amount of vaginal bleeding, vaginal discharge, and lower abdominal pain for 3 weeks after the procedure Of 321 participants complying with inclusion/exclusion criteria; 157 were randomly allocated to receive antibiotics and 164 did not receive any medications. Mean age of participants in antibiotic and those in control groups were 38.4 years and 40.5 years, respectively. Approximately 57.7% and 61.4% of participants assigned to antibiotic and control groups, respectively, had positive endocervical swabs. Approximately 27.0% and 34.8% of participants assigned to antibiotic and control groups, respectively, had positive high vaginal swabs. Approximately 4.4% and 2.0% of participants assigned to antibiotic and control groups, respectively, had positive test for Chlamydia. Weight of excised specimens obtained among the participants allocated to antibiotic and control groups, respectively, was 2.2 g and 1.9 g. Approximately 67.2% and 63.4% of participants assigned to antibiotic and control groups, respectively, had high‐grade lesion on conization specimens. | |
| Interventions | Antimicrobial vaginal pessary containing 100 mg tetracycline and 50 mg amphotericin B (Talsutin®, Bristol‐Myers Squibb New York, NY, USA) two times a day for 14 days, starting on the day of LLETZ. The control group did not receive any medications. Tetracycline is mainly an anti‐chlamydial agent. Amphotericin B is an antifungal antibiotic. | |
| Outcomes | The primary outcome was the incidence of post‐LLETZ bleeding that required medical attention. The secondary outcomes were the severity of postoperative vaginal bleeding, vaginal discharge, and lower abdominal pain in the 3 weeks after the procedure recorded in the participants' diary. All participants were assessed about the complications at 3 weeks after LLETZ. | |
| Notes | The authors excluded 23 participants (12 in antibiotic group and 11 in control group) because they did not return the diary. In addition, the authors excluded 8 participants assigned to antibiotic group because of incomplete the course of treatment prescribed, leaving 290 charts for final analyses (approximately 90% of total group). Therefore, the analyses performed in this study did not follow an intention‐to‐treat basis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This trial used block randomisation with a randomised size of four. This study declared that "generation of randomisation schedule was performed by a person independent of the recruitment, and the seed from which the randomisation schedule was generated was kept securely by the randomiser." |
| Allocation concealment (selection bias) | Low risk | A quote from the study: "Sealed opaque envelopes containing the randomised treatment allocation was prepared and kept by the research assistant prior to the start of patient recruitment" |
| Blinding of participants and personnel (performance bias) | High risk | A quote from the study: "The result of the randomisation was blinded to the research assistant but not to the colposcopist who needed to prescribe the medication for the treatment group at the clinic immediately after the procedure". In addition, the authors did not use placebo for the participant assigned to the control group so the participants were not blinded to treatment allocated." |
| Blinding of outcome assessment (detection bias) | High risk | The study outcomes were based on the participants' self‐assessment on their symptoms after the procedure. As participants were aware about the treatment received, there is high risk of detection bias. |
| Incomplete outcome data (attrition bias) | Low risk | Twenty‐three participants did not return the diary (7.2%). |
| Selective reporting (reporting bias) | Low risk | All potential relevant outcomes were reported. |
| Other bias | High risk | This study excluded 23 participants due to not return their diary (12 in antibiotic group and 11 in control group) and 8 participants in the treatment group from the final analyses because of incomplete the course of treatment. So, the analyses performed in this study was not based on intention‐to‐treat. |
| Methods | A randomised double‐blind, placebo‐controlled study conducted in a large teaching hospital colposcopy clinic in UK. Study duration: July 1994 to August 1996 | |
| Participants | The participants were women aged between 20 and 65 undergoing loop diathermy excision. Exclusion criteria: a history of antibiotic hypersensitivity, renal or hepatic diseases, previous psychiatric problems, and receiving antibiotic within the preceding 14 days. All participants received pictorial chart to record the amount of vaginal loss and adverse events for 2 weeks after the procedure.The participants had to return their chart in a stamped addressed envelopes which were provided by the trial authors. Of 500 participants complying with inclusion/exclusion criteria; 250 were randomly allocated to receive antibiotics and 250 to receive placebo. The mean ages for the participants in antibiotic and placebo groups were 37.0 and 37.1 years, respectively. Approximately 60.4% and 53.6% of participants assigned to antibiotic and control groups, respectively, had underlying high‐grade cervical lesion on excised specimens. Approximately 32.8% and 28.8% of participants enrolled to antibiotic and control groups, respectively, underwent multiple passes of loop excision. 418 participants (70%) returned pictorial chart, but 70 were subsequently excluded due to incomplete data, leaving 348 charts (173 in the antibiotic group and 175 in the placebo group) for final analyses. | |
| Interventions | Participants enrolled in the antibiotic group received ofloxacin 400 mg (2 x 200 mg) once daily for five consecutive days,starting immediately after the procedure. Participants assigned to the control group received identical placebo given in the same fashion. Ofloxacin is antimicrobial antibiotics with special activity against Chlamydial infections. | |
| Outcomes | The amount of postoperative vaginal discharge estimated by participants' self assessment using pictorial chart. | |
| Notes | Of 500 participants recruited, only 348 participants' charts were eligible for study analyses, corresponding to a rate of approximately 70%. The authors stated that the prevalence of Chlamydial infections among their colposcopy population was 10%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A quote from the study: " the study medication had been randomly assigned to the patient numbers in advance by the manufacture on a 1:1 basis" |
| Allocation concealment (selection bias) | Low risk | Following the procedure, participants were allocated their study number and received the study medication carrying her number. |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators and participants involving in this trial were blinded to the treatment received. Patients received either antibiotic or identical placebo, which had been prepared in the same packaging. |
| Blinding of outcome assessment (detection bias) | Low risk | The study outcome was the participants' self‐assessment on postoperative vaginal loss using pictorial charts. As the participants were blinded about the treatment received, there is low risk of detection bias. |
| Incomplete outcome data (attrition bias) | High risk | Of 500 participants enrolled, adequate information for study analyses were obtained from only 348 participants. |
| Selective reporting (reporting bias) | Low risk | All potential relevant outcomes were reported. |
| Other bias | High risk | This study excluded participants who did not return their charts and those with inadequate information on their charts.Thus, the analyses performed did not follow an intention‐to‐treat basis. |
| Methods | A randomised‐controlled trial conducted in the colposcopy clinic at the Princess Anne Hospital, Southamton, UK. Study duration: not reported | |
| Participants | The participants were women undergoing LLETZ. The trial authors did not stated exclusion criteria. All participants received patients' charts contained a daily record of vaginal bleeding, vaginal discharge, and pain for 4 weeks after the procedure. The participants were asked to record the requirement of unscheduled medical visits and additional antibiotic treatment, if any. The participants had to return their chart at 3 months following the procedure. Initially, this study recruited 100 participants but only 77 returned their charts for analyses. | |
| Interventions | Participants allocated to the intervention group received Sultrin which is antimicrobial vaginal pessary containing sulphatiazole 3.42%, sulphacetamide 2.86%, and sulphabenzamide 3.7%, twice daily for 5 days. Participants assigned as the control group received no treatment. Sultrin vaginal pessary is a preparation used intravaginally against Haemophilus (Garnerella)vaginalis. | |
| Outcomes |
| |
| Notes | The authors did not reported the details regarding the processes of randomisation and treatment allocation. In addition, of 100 participants recruited, only 77 participants' charts were available for analyses, corresponding to a rate of 77%.The authors did not reported the baseline characteristics of the participants, treatment‐related events, and actual number of participants assigned in each comparison group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not reported the details of the processes of randomisation. |
| Allocation concealment (selection bias) | Low risk | The authors used sealed envelope during randomisation |
| Blinding of participants and personnel (performance bias) | High risk | The authors did not use placebo for the participant assigned to the control group so the participants were not blinded to treatment received. |
| Blinding of outcome assessment (detection bias) | High risk | Because the outcome based on the participants' self‐assessment on their symptoms occurring after the procedure but the participants were aware about the treatment received. |
| Incomplete outcome data (attrition bias) | High risk | of 100 participants recruited, only 77 participants' charts were available for analyses, corresponding to a rate of incomplete outcome data of approximately 23%. |
| Selective reporting (reporting bias) | Low risk | All potential relevant outcomes were reported. |
| Other bias | High risk | This study excluded participants who did not return their charts. Therefore, the analyses carried out in this study was not based on an intention‐to‐treat. The authors did not state about the review inclusion and exclusion criteria applied in this study. The authors also did not report the baseline characteristics of participants and number of participants allocated to each comparison group which are mandatory for determining treatment outcomes and assessing the quality of study methodology. |
LLETZ: large loop excision of the transformation zone
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This randomised study was excluded because the intervention used was a Monsel’s solution applied after cervical excision which was not a prophylactic antibiotics, the intervention that this review aims to address. | |
| This randomised study entitled "Antiseptic regimen in the surgical treatment of HPV generated cervical lesions: polyhexamethylene biguanide vs chlorhexidine. A randomised, double blind study" was conducted to determine the effectiveness and safety of polyhexamethylene biguanide‐based vaginal suppositories compared to chlorhexidine‐based preparation. However, the treatment used in this study was an ablation using CO2 laser which did not meet our review inclusion. | |
| This study entitled "Advantages of topical therapy with polydeoxyribonucleotide in reparative processes after cauterization: Experience at a centre for early diagnosis of genital neoplasm" was conducted to evaluate the effectiveness of kanamycin sulphate alternated with placebo versus polydeoxyribonucleotide vaginal suppositories for preventing postoperative infection and promoting tissue healing after cauterisation of the uterine cervix. However, this study was a controlled clinical trail and the treatment used in this study was not an excisional method. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced prolonged vaginal discharge Show forest plot | 1 | 348 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.72, 2.31] |
| Analysis 1.1 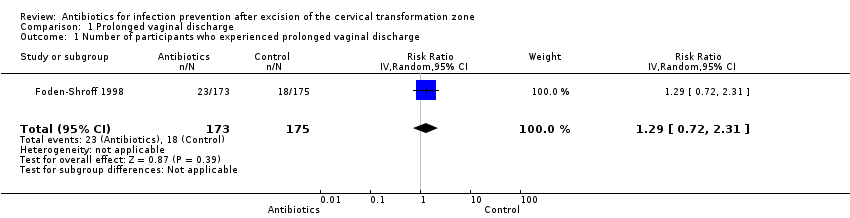 Comparison 1 Prolonged vaginal discharge, Outcome 1 Number of participants who experienced prolonged vaginal discharge. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had to be admitted for postoperative bleeding Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.52, 2.82] |
| Analysis 2.1 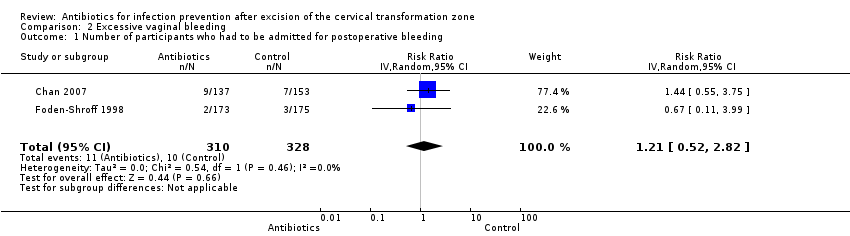 Comparison 2 Excessive vaginal bleeding, Outcome 1 Number of participants who had to be admitted for postoperative bleeding. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who developed fever Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.23 [0.20, 24.36] |
| Analysis 3.1  Comparison 3 Fever, Outcome 1 Number of participants who developed fever. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced lower abdominal pain Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.61, 1.72] |
| Analysis 4.1  Comparison 4 Lower abdominal pain, Outcome 1 Number of participants who experienced lower abdominal pain. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced any adverse effects related to antibiotics Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.85, 3.34] |
| Analysis 5.1  Comparison 5 Adverse effects, Outcome 1 Number of participants who experienced any adverse effects related to antibiotics. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who received additional medical consultant (for any reasons) Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.68 [0.97, 7.41] |
| Analysis 6.1  Comparison 6 Unscheduled medical consultant, Outcome 1 Number of participants who received additional medical consultant (for any reasons). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had additional self‐medication Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.56, 2.67] |
| Analysis 7.1  Comparison 7 Additional self‐medication, Outcome 1 Number of participants who had additional self‐medication. | ||||

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prolonged vaginal discharge, Outcome 1 Number of participants who experienced prolonged vaginal discharge.

Comparison 2 Excessive vaginal bleeding, Outcome 1 Number of participants who had to be admitted for postoperative bleeding.

Comparison 3 Fever, Outcome 1 Number of participants who developed fever.

Comparison 4 Lower abdominal pain, Outcome 1 Number of participants who experienced lower abdominal pain.

Comparison 5 Adverse effects, Outcome 1 Number of participants who experienced any adverse effects related to antibiotics.
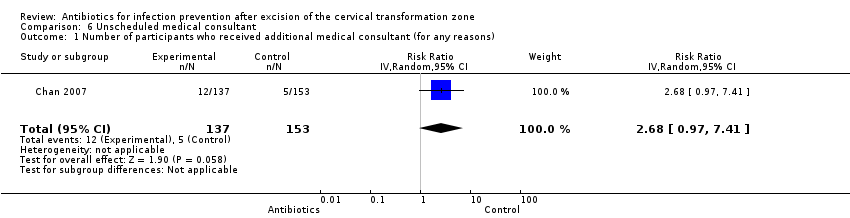
Comparison 6 Unscheduled medical consultant, Outcome 1 Number of participants who received additional medical consultant (for any reasons).

Comparison 7 Additional self‐medication, Outcome 1 Number of participants who had additional self‐medication.
| Antibiotics compared with placebo or no treatment for infection prevention after excision of the cervical transformation zone | ||||||
| Patient or population: Women undergoing excision of the cervical transformation zone for cervical neoplasia Settings: Outpatients setting, the colposcopy clinic Intervention: Prophylactic antibiotics Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| [Placebo or no treatment] | [Antibiotics] | |||||
| Number of participants who experienced prolonged vaginal discharge Follow‐up period: 2 weeks after the procedure | 103 per 1000 | 133 per 1000 | RR 1.29 (0.72 to 2.31) | 348 | ⊕⊕⊝⊝ | |
| Number of participants who had to be admitted for post‐procedure bleeding Follow‐up period: 2‐3 weeks after the procedure | 31 per 1000 | 38 per 1000 | RR 1.21 (0.52 to 2.82) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who developed fever Follow‐up period: 3 weeks after the procedure | 7 per 1000 | 16 per 1000 | RR 2.23 (0.20 to 24.36) | 290 (1 study) | ⊕⊝⊝⊝ | |
| Number of participants who experienced lower abdominal pain Follow‐up period: 3 weeks after the procedure | 163 per 1000 | 168 per 1000 | RR 1.03 (0.61 to 1.72) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who experienced any adverse effects related to antibiotics Follow‐up period: 2‐3 weeks after the procedure | 37 per 1000 | 63 per 1000 | RR 1.69 (0.85 to 3.34) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who received additional medical consultant (for any reasons) Follow‐up period: 3 weeks after the procedure | 33 per 1000 | 88 per 1000 | RR 2.68 (0.97 to 7.41) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who had additional self‐medication Follow‐up period: 3 weeks after the procedure | 72 per 1000 | 88 per 1000 | RR 1.22 (0.56 to 2.67) | 290 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on the high risk of attrition bias (high rate of incomplete participants' record charts) | ||||||
| Study | Antibiotic group | Placebo or no treatment |
| Number of participants who experienced prolonged vaginal discharge | ||
| 23/173 (13.3%) | 18/175 (10.3%) | |
| Number of participants who had to be admitted for post‐procedure bleeding | ||
| 2/173 (1.2%) | 3/175 (1.7%) | |
| 9/137 (6.6%) | 7/153 (4.6%) | |
| Number of participants who experienced lower abdominal pain | ||
| 23/137 (16.8%%) | 25/153 (16.3%) | |
| Number of participants who developed fever | ||
| 2/137 (1.5%) | 1/153 (0.7%) | |
| Number of participants who experienced adverse events | ||
| 20/173 (11.6%) | 12/175 (6.9%) | |
| 0/137 (0%) | 0/153 (0%) | |
| Number of participants who required unscheduled medical consultation | ||
| 12/137 (8.8%) | 5/153 (3.3%) | |
| Number of participants reported to have additional self‐medication | ||
| 12/137 (8.8%) | 11/153 (7.2%) |
| Outcomes reported in Gornall 1999 | Sultrin (sample size = n) | Control (sample size = 77‐n) | 95% confidence interval | P value |
| Mean duration of bleeding (days) | 15.2 | 11.2 | ‐7.7 to ‐0.2 | 0.04 |
| Mean duration of discharge (days) | 16.4 | 13.1 | ‐7.2 to 0.7 | 0.11 |
| Mean duration of pain (days) | 7.7 | 5.7 | ‐5.4 to 1.5 | 0.26 |
| Number of participants who received additional antibiotic therapy | 2 | 7 | Not reported | Not reported |
| Number of participants who had to be admitted for postoperative bleeding | 0 | 2 | Not reported | Not reported |
| Numbers of the participant in each comparison group were not reported. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced prolonged vaginal discharge Show forest plot | 1 | 348 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.72, 2.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had to be admitted for postoperative bleeding Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.52, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who developed fever Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.23 [0.20, 24.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced lower abdominal pain Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.61, 1.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced any adverse effects related to antibiotics Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.85, 3.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who received additional medical consultant (for any reasons) Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.68 [0.97, 7.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had additional self‐medication Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.56, 2.67] |

