Traitements pharmacologiques pour la prévention de l'épilepsie suite à un traumatisme crânien
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT; single center Enrolled: 1978‐1979 Duration of treatment: long‐term; 18‐24 months Follow‐up: 18‐24 months Setting: Neurosurgery Department of University of Wurzburg Type of agent: traditional AED | |
| Participants | 151 participants > 15 years of age, 88.5% male, were admitted due to moderate and severe TBI. Severity determined by GCS and presence of retrospective amnesia Carbamazepine: 75 participants Placebo: 76 participants Pre‐existing epilepsy was excluded | |
| Interventions | Carbamazepine: participants were treated according to serum levels 300‐600 µg. First dose given immediately after accident (no dosage given), other details not specified Placebo: details not provided First dose given before FPS NOTE: 61% of all 139 participants received additional phenobarbital for brain edema (administered in first week). Mean cumulative dosage: 2780 µg in placebo group vs. 1500 µg in intervention group. 59% of all 139 participants received diazepam: 248 µg in placebo group vs. 150 µg in carbamazepine group: acute phase only | |
| Outcomes |
Seizure identification: EEG and clinical findings | |
| Notes | Imbalance at baseline: carbamazepine group had more permanent vegetative states 26% vs. 13% placebo Unable to confirm the data with respect to late seizures; therefore, not included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence predicable ‐ based on birthdays. carbamazepine = even birthdays, placebo = odd birthdays |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up: 6 from carbamazepine group, 5 from placebo group (poor description of reasons) |
| Selective reporting (reporting bias) | High risk | Very detailed description of severity of injury (over 50 baseline descriptive tables) but no adverse events reported |
| Other bias | High risk | Majority of participants received phenobarbital or diazepam, or both (both active AEDs) in the first week for edema treatment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Is not stated if participants or physicians were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study indicated as "blinded"; blinding of assessment not specifically reported |
| Methods | RCT; multicenter; parallel group study Enrolled: 1983‐1985 Duration of treatment: long‐term; 2 years Follow‐up: 5 years Setting: Japan Type of agent: traditional AED | |
| Participants | 244 participants ages 7‐88 years admitted due to TBI Analyzed: Group I with severe TBI (mean age 38 ± 19.9 years):
Group II with mild TBI: 65 participants; mean age 29.3 ± 19.6 years): treatment not described Proportion male: not reported Did not specify if pre‐existing epilepsy was excluded | |
| Interventions | Group IA: received phenobarbital, 10‐25 µg/mL, started 4 weeks after TBI. Full dose for 2 years, tapered in third year Group IB: some participants received nothing, some participants received anticonvulsants Group II: intervention not specified First dose given before an FPS: not reported | |
| Outcomes |
Seizure identification: not specified | |
| Notes | Baseline characteristics not reported Drug not administered until approximately 2 months post‐injury; some early seizures occurred Control group appeared to include participants who were taking other anticonvulsant drugs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No further details provided; therefore, no confirmation method was carried out appropriately |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment process not described: used envelope method; no further details provided. |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up at 5 years was 25%. 52/244 excluded due to drop‐out or "against" protocol. No mention of which groups these participants were in, their characteristics, if they were randomized or if they received treatment prior to drop‐out. Intention‐to‐treat not performed No Table 1 to clearly describe participant characteristics No clear description of drug protocol or control protocol for Group IB and Group II |
| Selective reporting (reporting bias) | High risk | Did not report on adverse events or mortality. Baseline characteristics were not reported; therefore, cannot assess balance of baseline characteristics |
| Other bias | High risk | Co‐intervention in control group. Group IB had some participants who received anticonvulsant medication and other participants who did not receive anticonvulsant medication. Not sure what proportion received medication or what drugs were |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding participants or personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of blinding outcome assessors |
| Methods | RCT; double‐blind, multicenter (2 sites), parallel‐group study Enrolled: Newcastle, UK: November 1977 to October 1979; Edinburgh, UK: 1 January 1977 to October 1979 Duration of treatment: mid‐term; 12 months Follow‐up: 2 years Setting: Neurological Surgery Departments Type of agent: traditional AED | |
| Participants | 164 participants ages 5‐65 years admitted due to TBI Phenytoin: 84 participants; 35% were 5‐15 year olds; 79% male Placebo: 80 participants; 18% were 5‐15 year olds; 80% male 85% of participants had injuries associated with high risk of post‐traumatic epilepsy Pre‐existing epilepsy was excluded | |
| Interventions | Phenytoin: child (5‐15 years 5 mg/kg; adults 300 mg) Placebo: Therapeutic dose: during follow‐up, adjusted to achieve plasma concentration 40‐80 µmol/L Dose administration: capsule of phenytoin 50 or 100 mg and matching placebo capsules Timing of dose: single or divided daily dose; not precisely reported but participants received a full dose every 24 hours First dose given before post‐traumatic seizure; early seizure was an exclusion criteria | |
| Outcomes |
Seizures diagnosed based on clinical findings | |
| Notes | Potentially significant difference in participant characteristics at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was administered by the hospital pharmacist using a prepared list of random treatment allocation ‐ but report did not indicate how the list was made. |
| Allocation concealment (selection bias) | Low risk | Treatment was administered by the hospital pharmacist using a prepared list of random treatment allocation ‐ but report did indicate how the list was made. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up over 2 years was 1%. Authors explained causes of participants lost to follow‐up. These participants were counted in the treatment group to which they were originally assigned |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. Prespecified outcomes reported |
| Other bias | High risk | Potentially significant difference at baseline. In phenytoin group, more 5‐15 year olds than in placebo group. In phenytoin group, more participants admitted in 8‐10 days post injury. In placebo group, more participants admitted > 30 days post‐injury Low compliance in treatment group. 80% were dispensed capsules for up to 6 months, 68% for up to 9 months, 49% for up to 12 months When tested, the level of phenytoin in the plasma of the phenytoin group often below the therapeutic level with only 48% of participants achieving plasma concentrations of > 40 µmol/L on at least 1 occasion, 36% had plasma concentrations of 20‐39 µmol/L, 12% in range 10‐19 µmol/L |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was conducted 'double‐blind' with prescribed treatment known only to the hospital pharmacy and the trial co‐ordinators, who had no responsibility for participant care or follow‐up |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors involved in the prescribed treatment were not involved in follow‐up |
| Methods | RCT Enrolment: January 1982 to March 1985 Duration of treatment: short‐term and mid‐term; 3 months and 1 year Follow‐up: 2 years Setting: France Type of agent: traditional AED | |
| Participants | 86 participants aged 5‐60 years, 80% males admitted due to severe TBI Phenytoin: 34 participants); mean age 26 years; 74% male Placebo: 52 participants; mean age 30.3 years; 85% male Pre‐existing epilepsy was excluded Severity determined by EEG and repeat CT scans | |
| Interventions | Phenytoin: 10 mg/kg by slow intravenous pump 40 mg/minute Placebo: Therapeutic dose: determined by serum results at 48 hours and 7 days ‐ adjusted therapeutic does using formula by Young 1979. Dose administration: capsule of phenytoin 50 or 100 mg and matching placebo capsules Timing of doses: 4 divided doses on first day; on second day, oral phenytoin (gastric tube in some participants), mean dose 8 mg/kg in 2 divided doses. Treated within 24 hours of accident and upon arrival in ICU Not reported if first dose was given before post‐traumatic seizure | |
| Outcomes |
Seizures diagnosed based on clinical findings and EEG | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized by day of arrival even or odd day (predictable sequence) |
| Allocation concealment (selection bias) | Unclear risk | Did not describe allocation concealment. Predictable sequence of randomization |
| Incomplete outcome data (attrition bias) | Unclear risk | 5 participants excluded from analysis due to death in first 5 days ‐ group allocation not indicated. Loss to follow‐up other than mortality was not discussed |
| Selective reporting (reporting bias) | High risk | Indicated that some participants received phenytoin for > 3 months but did not describe outcomes by length of treatment. Adverse events and mortality not reported for included participants |
| Other bias | Unclear risk | No clear description of the control group |
| Blinding of participants and personnel (performance bias) | High risk | Did not mention blinding strategies ‐ but given nature of randomization, it would be easy to determine which participants were in control/treatment groups |
| Blinding of outcome assessment (detection bias) | High risk | Given the allocation by date of enrolment ‐ it is unlikely that treatment was blinded as the assessors could easily determine which participants were in which group by date of admission |
| Methods | RCT; double‐blind, single‐center, parallel group study Enrolled: not reported Duration of treatment: short‐term; 7 days Duration of follow‐up: outcomes assessed at 3 and 6 months Setting: USA; neuroscience ICU Type of agent: traditional AED and newly licensed AEDs | |
| Participants | 52 participants with severe TBI or subarachnoid hemorrhage ages 17‐80 years. Randomization up to 24 hours post admission, at a 2 : 1 ratio levetiracetam : phenytoin Levetiracetam: 34 participants; 30 with TBI; ages 17‐75 years, median 44 years; 77% male Phenytoin: 18 participants; 16 with TBI; ages 18‐80 years, median 25 years; 72% male Inclusion: TBI or subarachnoid hemorrhage, GCS (3‐8 inclusive) or GCS of ≤ 5 and abnormal CT scan showing intracranial pathology, hemodynamically stable, at least 1 reactive pupil, ages ≥ 17 years and informed consent Exclusion: spinal cord injury, previous brain injury, known hypersensitivity to anticonvulsant, hemodynamically unstable, anoxic events Report did not indicate exclusion of pre‐existing seizures prior to study inclusion but author confirmed exclusion by email | |
| Interventions | Levetiracetam: loading dose 20 mg rounded to nearest 250 mg over 60 minutes. Maintenance dose of 1000 mg, IV every 12 hours over 15 minutes. Therapeutic dose: up to 1500 mg (3000 mg/day). Duration of treatment: 1‐7 days Phenytoin: loading dose of 20 mg/kg IV, maximum 2000 mg over 60 minutes and then phenytoin maintenance of 5 mg/kg/day rounded to nearest 100 mg, dose every 12 hours. Therapeutic dose: 10‐20 µg/dL. Duration of treatment range: 1‐7 days Not reported if drug was given before first seizure | |
| Outcomes |
Seizures identified based on clinical findings. Continuous EEG monitoring for first 72 hours | |
| Notes | Baseline characteristics appeared balanced between study groups People with TBI or subarachnoid hemorrhage recruited and it was not possible to obtain data exclusively for the people with TBI, which represented 89% of the participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on method of randomization. Randomized at a 2 : 1 ratio of levetiracetam : phenytoin |
| Allocation concealment (selection bias) | Low risk | Participants randomized and treatment group assigned by the pharmacy |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants were analyzed as survivors and as per their treatment group assignment. No indication of any loss to follow‐up other than death |
| Selective reporting (reporting bias) | Low risk | All expected and pre‐specified outcomes were reported |
| Other bias | Unclear risk | No report of drug levels to assess efficacy |
| Blinding of participants and personnel (performance bias) | Unclear risk | Physicians "partially" blinded. Were not "told" group assignment, but PHT levels could be reviewed. Physicians were also unblinded if a seizure occurred to optimize treatment |
| Blinding of outcome assessment (detection bias) | Low risk | EEG monitoring occurred for 72 hours. Electrophysiologist was blinded to the group assignment and diagnosis The clinical research co‐ordinator remained blinded to participant medication and conducted all assessments |
| Methods | RCT; double‐blind, single center, parallel group study Enrolled: November 1983 to December 1987 Duration of treatment: mid‐term; 12 months Follow‐up: 2 years Setting: USA, Level 1 trauma center Type of Agent: traditional AED | |
| Participants | 404 participants with severe TBI, mean age 34 ± 18 years Phenytoin: 208 participants; mean age 34 ± 18 years; 78% male Placebo: 196 participants; mean age 34 ± 17 years; 75% male Eligibility ‐ meet at least 1 of following criteria: cortical contusion visible on CT scan; a subdural, epidural or intracerebral hematoma; a depressed skull fracture; penetrating head wound; seizure within 24 hours of injury or a GCS ≤ 10 on admission. If any criteria met ‐ estimated 20% chance of seizure Excluded participants with previous documented unprovoked seizures | |
| Interventions | Phenytoin (Dilantin): initial dose 20 mg/kg IV within 24 hours of injury Therapeutic dose: total 40‐80 µmol/L, 10‐20 mg/L Dose administration: daily dose varied based on individual serum level; range 200‐1200 mg to maintain serum levels Placebo: given daily First dose not given before an FPS | |
| Outcomes |
Seizure identification based on clinical findings. Clinicians who were blinded to treatment diagnosed seizures primarily on basis of clinical manifestations especially involuntary movements; alterations in consciousness; or abnormal motor, sensory or psychosensory phenomena. Participants and caregivers were trained to recognize subtle manifestations of seizures | |
| Notes | Baseline characteristics were comparable between groups Additional data regarding the group without prior seizure history was received from Dr. Temkin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization process not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation process not reported |
| Incomplete outcome data (attrition bias) | Low risk | Randomized participants were analyzed in the groups they were allocated to. Withdrawal from treatment well reported. 24% of participants lost to follow‐up; 23% in phenytoin group and 26% in placebo group over 24 months |
| Selective reporting (reporting bias) | Low risk | Expected outcomes of interest appear to be reported |
| Other bias | Low risk | Study groups similar baseline characteristics with respect to demographic characteristics, cause of injury, and severity of injury. 70% of participants had therapeutic levels of phenytoin |
| Blinding of participants and personnel (performance bias) | Low risk | Dose modified by unblinded study staff. Similar "mock" adjustments made to placebo group. Treatment code was not broken unless phenytoin appeared to be responsible for reaction and the participant's condition warranted such action |
| Blinding of outcome assessment (detection bias) | Low risk | Clinicians who were blinded to treatment diagnosed seizures primarily on basis of clinical manifestations |
| Methods | RCT; double‐blind, single‐center, parallel‐group study Enrolled: November February 1991 to December 1995 Duration of treatment: varied 2 groups short‐term; 1 week and 1 month treatments; 1 group mid‐term ‐ 6 month treatment Follow‐up: 2 years Setting: USA, level 1 trauma center Type of agent: traditional AED | |
| Participants | 379 participants with TBI randomized to:
Qualifying injury had 1 of the following characteristics: immediate posttraumatic seizures. Depressed skull fracture, penetrating brain injury, or CT evidence of cortical contusion or subdural, epidural, intracerebral hematoma Excluded people with previous documented unprovoked seizures | |
| Interventions | Phenytoin (1 week): loading dose IV 20 mg/kg ‐ administered within 24 hours. Maintenance dose 5 mg/kg/day in two divided doses. Therapeutic dose: 40‐80 µmol/L (10‐20 µg/mL) Valproate (1 and 6 months): loading dose IV 20 mg/kg. Maintenance dose 15 mg/kg/day in 4 divided doses. Therapeutic dose ‐ 277‐693 µmol/L (40‐100 µg/mL) First dose not given before an FPS | |
| Outcomes |
Seizure identification: based on clinical findings. Early seizures were witnessed by medical personnel. Late seizures recognized by participants and caregivers who reported them to study neurologist. A blinded study neurologist reviewed all suspected seizures; if in doubt, the event was not counted as a seizure | |
| Notes | For early seizures the valproate group was considered as 1 group regardless of length of time to be treated All participants were included in the analysis of late seizures regardless of whether they had experienced early seizures Baseline characteristics were comparable between groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomization list, generated by statistician and kept in locked part of pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation by pharmacist |
| Incomplete outcome data (attrition bias) | Unclear risk | 87% followed for full 2 years. But not all participants followed through with assigned treatment. Of randomized cases: 75% of 1 month and 70% of 6 month valproate group followed up for 2 years. 79% of phenytoin group followed for 2 years Because most participants were unconscious or had cognitive impairments during the first week, early seizures without a prominent motor component were likely to be overlooked 113 participants initially randomized were subsequently found to be ineligible after randomization for issues such as prior history of epilepsy. These participants were only observed for 28 days for incidence of adverse effects and for mortality |
| Selective reporting (reporting bias) | Unclear risk | Although most expected and pre‐specified outcomes appeared to be reported, denominators of counts not reported clearly |
| Other bias | Low risk | Valproate concentrations were at or above the target valproate range in 97% of participants in first week; 90% in first month; 85% in fifth month Phenytoin concentrations were at or above the target phenytoin range in 91% of participants in the first week Compliance: 16% stopped taking blinded medication before 1 month because of participant preference or mild adverse effects. 21% stopped before 6 months compliance reported for each group |
| Blinding of participants and personnel (performance bias) | Low risk | Identical‐appearing IV solutions and call‐backs to check placebo "drug levels" to maintain blind conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Neurologist blinded to the assignment made the final determination on seizure diagnosis for the study |
| Methods | RCT; double‐blind, single‐center, parallel group study Enrolled: August 1998 to October 2004 Duration of treatment: short‐term; 5 days Follow‐up: 6 months Setting: USA, level 1 trauma center Type of agent: "other" | |
| Participants | 499 participants older than 14 years were admitted due to moderate or severe TBI Treatment 1 (high dose magnesium sulfate; MgSO4):
Treatment 2 (low‐dose magnesium sulfate; MgSO4):
Moderate to severe was defined as: the need for intracranial surgery within 8 hours of injury; a post‐resuscitation GCS score of 3‐12; or intubated, a GCS motor score of 1‐5 without pharmacologic paralysis Pre‐injury seizures were not excluded from the study, but participants with pre‐injury seizures were excluded from seizure outcome analysis | |
| Interventions | Treatment 1 (high dose): magnesium sulfate (MgSO4) high dose 1.2‐2.5 mmol/L. Initial IV load of 0.425 mmol/kg over 15 minutes followed by continuous infusion (0.10 mmol/kg/hour) to maintain target range for 5 days. Therapeutic dose 1.25‐2.5 mmol/L Treatment 2 (low dose): magnesium sulfate (MgSO4) low dose 1.0‐1.85 mmol/L. Initial IV load of 0.30 mmol/kg over 15 minutes followed by continuous infusion (0.05 mmol/kg/hour) to maintain target range for 5 days. Therapeutic dose 1.0‐1.85 mmol/L In both treatments, agent was administered within 8 hours of injury. Infusion rate adjusted daily by pharmacist Placebo: saline, magnesium sulfate given below normal levels Not reported if drug was given before first seizure 96% of participants received phenytoin for the first week as part of clinical care | |
| Outcomes |
Seizure identification not explicitly reported, but, at 1 and 3 months, health status measures were assessed by telephone and as a part of a formal in‐person comprehensive examination at 6 months that included neuropsychologic testing (panel). A family member who knew the person prior to injury also participated in assessment at 6‐month test | |
| Notes | Participants who died before day 8 were excluded from the late seizure analysis Author contacted: contacted for participant details within outcome categories to determine if history of seizure was excluded. Response summary: participants with history of seizure were deleted from early and late seizure outcome. Author provided counts for primary and secondary outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified by sex and age. Randomization was blocked (2‐4 people) to ensure balance. Computer‐generated list kept in a restricted area of the pharmacy |
| Allocation concealment (selection bias) | Low risk | Pharmacist randomly assigned participant sequentially when order received |
| Incomplete outcome data (attrition bias) | Unclear risk | Primary analysis excluded people with fixed dilated pupils and people who were randomized but died before receiving the drug (7 in magnesium sulfate group, 8 in placebo group). In secondary analysis, all participants were analyzed in the groups they were assigned (ITT analysis) 93% were followed for 6 months. 72% had a full neurologic assessment at 6 months Loss to follow‐up similar in both the Mg (18) and placebo (19) groups and for similar reasons |
| Selective reporting (reporting bias) | Unclear risk | Did not report mortality and seizures in a conventional way for these common study outcomes |
| Other bias | Unclear risk | Co‐intervention: phenytoin administered to 96% of participants in the first week Most characteristics were "quite" well balanced at baseline, but the lower magnesium dose had more participants with hematomas and with worse abbreviated‐injury‐scale‐head scores. Noted significant differences in P values between group in age, severity and gender Drug treatment as specified was given in 95% of cases 25 participants stopped taking study drug before the 5 days Pre‐injury seizures were not excluded, but participants with pre‐injury seizures were excluded from seizure outcome analysis Total mean magnesium concentrations were 2.15 mmol/L (SD 0.35) in high‐dose group, 1.45 mmol/L (SD 0.2) in low‐dose group and 0.9 mmol/L (SD 0.1) in placebo group |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, doctors and nurses treating participants were all blinded to assignment. Clinicians were not allowed to order any tests of magnesium concentration during the infusion or for 2 days post drug treatment. Masking was broken in 8% of cases ‐ usually when clinician ordered routine laboratory tests. Research nurse became aware of 4% of cases. Participants remained constantly unaware of assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Research nurses and professionals involved in assessment of outcome were all masked to treatment assignment. There was no formal assessment of the success of the masking. Outcome examiners remained consistently unaware of assignment |
| Methods | RCT; double‐blind, single‐center; parallel‐group study Enrolled: December 1976 to November 1979 Duration of treatment: short‐term 7 days and long‐term 18 months Follow‐up: 1 week to 18 months Setting: USA, neurologic services Type of agent: traditional AED Results of trial reported in 3 reports. Report A: Young 1983. Journal of Neurosurgery 1983;58(2):231‐5; all participants; early seizure only. 1‐week follow‐up. Report B: Journal of Neurosurgery 1983;58(2):236‐41; all participants; late seizures only, 18‐month follow‐up. Report C: Child's Brain 1983;10(3):1985‐92, subanalysis of Report B ‐ all participants aged < 17 years | |
| Participants | Report A: 244 participants of all ages with severe TBI Phenytoin: 136 participants; mean age 24.4 ± 1.29 years; 6 (4.4%) ages 0‐4 years ; 26 (19.1%) ages 5‐15 years; 80.9% male GCS: 14 (10.3%) had GCS 3‐4; 56 (41.2%) had GCS of 5‐7; 56 (48.5%) had GCS ≥ 8. 7 had pre‐randomized seizures (mean age 12 years) Placebo: 108 participants; mean age 25.8 ± 1.47 years; 5 (4.6%)ages 0‐4 years; 17 (15.7%)ages 5‐15 years; 84.3% male GCS: 17 (15.7%) had GCS 3‐4; 46 (42.6%) had GCS 5‐7; 45 (41.7%) had GCS ≥ 8. 3 had pre‐randomized seizures (mean age 12 years) Report B: 214 participants of all ages, mean age of 25.2 years, with severe TBI. 4.7% aged < 5 years, 17.3% ages 5‐16 years, 78.0% > 16 years Phenytoin: 119 participants; mean age 24.4 ± 1.29 years; 6 (4.4%) ages 0‐4 years; 26 (19.1%) ages 5‐15 years; 80.9% male GCS: 9 (8.6%) had GCS 3‐4; 40 (38.1%) had GCS 5‐7; 56 (53.3%) had GCS ≥ 8 Phenobarbital: 20 participants; received phenytoin initially; mean age 21.6 ± 3.01, 75% male. GCS: 0 (0%) had GCS 3‐4; 11 (55.5%) had GCS 5‐7; 9 (45.0%) had GCS of ≥ 8 Placebo: 95 participants; mean age 26.3 ± 2.03 years; 82.4% male GCS: 8 (10.8%) had GCS 3‐4; 33 (44.6%) had GCS 5‐7; 33 (44.6%) had GCS ≥ 8 Report C: 46 participants all age of 17 years with severe TBI. Randomized: 27 to treatment, 19 to placebo. 4 died and 1 early seizure excluded from analysis Follow‐up: Phenytoin: 20 participants; mean age 9.3 ± 0.81 years; 72% male. GCS: 1 (5.0%) had GCS 3‐4; 5 (25.0%) had GCS 5‐7; 14 (70.0%) had GCS ≥ 8. (5 switched to phenobarbital) Phenobarbital: 5 participants; received phenytoin initially. Mean age 9.0 ± 1.92 years, % male unknown GCS: 0 (0%) had GCS 3‐4; 4 (80.0%) had GCS 5‐7; 1 (20.0%) had GCS ≥ 8 Placebo: 16 participants; mean age 9.2 ± 1.15 years; 93.8% male GCS: 2 (12.5%) had GCS 3‐4; 4 (25.0%) had GCS 5‐7; 10 (62.5%) had GCS ≥ 8 Included people with penetrating head wound or blunt head injury providing > 10% chance of developing seizures. Participants had: intracranial hematomas; frontal, temporal, or parietal depressed skull fracture; and other blunt head injuries causing unconsciousness for at least 6 hours or major focal neurologic deficits. Some seizures occurred prior to first dose | |
| Interventions | Phenytoin: initial dose 11 mg/kg at 25 mg/minute plus 13 mg/kg intramuscularly If levels were adequate 8.8 mg/kg administered daily or adjusted as needed. Therapeutic dose: plasma concentrations 10‐20 µg/ml. Timing of dose: administered with 24 hours of admission Placebo: identical IV of phenytoin diluent (10% ethanol, propylene glycol 40% and water 50%) or placebo capsule | |
| Outcomes |
| |
| Notes | Method of identification of early seizure was not reported. Identification of late seizure by interview, exam, written and telephone follow‐ups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported; "randomized" but did not say how |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | A number of participants were lost to follow‐up or excluded from the analysis. Report A: 1 participant who had a drug reaction was excluded from the results. Report B: 179/214 followed for 18 months. 4 participants had early seizures in placebo group and were eliminated as they were administered phenytoin (3 people) or phenobarbital (1 person). 11 participants lost to follow‐up: 3 in drug group, 8 in placebo. 20 participants died in first week: 11 in drug group and 9 in placebo group. Report C: participants were analyzed in the group they were assigned regardless of outcome |
| Selective reporting (reporting bias) | High risk | Results indicated that deaths occurred beyond those reported in first 7 days. Median time to death was reported with ranges from 8 to 450 days but number of deaths between week 2 and 18 months not reported. Types of adverse reactions not reported despite 20 participants switched to phenobarbital. Did not include lost to follow‐up in time to event analysis |
| Other bias | Unclear risk |
|
| Blinding of participants and personnel (performance bias) | Low risk | State: "only the clinical pharmacist or the clinical nurse on the team was aware of which participant was receiving active drug or the placebo and made dosing adjustments" |
| Blinding of outcome assessment (detection bias) | Unclear risk | State: "In all cases the physician evaluators were blinded as to the drug received"; however, unclear if blinding was broken when participants were switched from phenytoin to phenobarbital |
| Methods | RCT; double‐blind, multicenter, parallel‐group study Enrolled: December 1992 to November 1997 Duration of treatment: short‐term; 5 days Follow‐up: 30 days; median time 34.5 days (interquartile range 30‐50 days) Setting: USA, urban pediatric trauma centers Type of agent: traditional AED | |
| Participants | 103 participants aged < 10 years, range of 3.3‐9.4 years, median 6.1 years with moderate and severe TBI. 68% male Phenytoin: 47 participants*; age range 3.7‐9.6 years, median 6.4 years; 67% male Placebo: 56 participants; age range 2.6‐8.8 years, median 5.9 years; 68% male Severity of TBI determined by: acute blunt head injury, with marked alteration in level of consciousness as defined by GCS (≤ 10 in children aged ≥ 4 years; ≥ 9 in children aged < 4 years, and pulse rate > 60 beats/minute | |
| Interventions | Phenytoin: initial IV dose 18 mg/kg over 20 minutes, maintenance 2 mg/kg every 8 hours for 48 hours (5 doses). Drug administered within 60 minute of arrival in emergency room Placebo: dilutent alone. First dose was administered prior to first traumatic seizure | |
| Outcomes |
Seizures were identified with EEG and clinical finding | |
| Notes | Excluded participants who had post‐trauma seizures before randomization * 1 participant in phenytoin group was removed from the study at the request of the family | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolment was intended to be consecutive. Participants stratified into 6 groups according to age and initial GCS. Within each of the 6 stratified groups and study site, participants were randomly allocated to phenytoin or placebo by using randomized permuted blocks to ensure baseline similarity of treatment groups |
| Allocation concealment (selection bias) | Low risk | A code kept locked in an office off site was available only to the principal investigator linking each vial to the contents of the vial (phenytoin or placebo). Sealed envelopes with the identity of the study medication were kept with the vials and in the participant's medical record. The envelopes were to be opened at the end of the 48‐hour observation period, if a participant experienced a seizure or if the attending neurosurgeon wished to withdraw the participant from the study |
| Incomplete outcome data (attrition bias) | Low risk | 68% completed entire observation period; 6 seizures, 9 deaths, 1 surgery, 12 discharged home, 5 protocol violations or neurosurgeons request Of 82/102 remaining participants, 62 (76%) returned for 30‐day follow‐up. Telephone follow‐up obtained from 4 others. Total follow‐up including deaths = 86/102 (84%). Randomized participants were analyzed in the groups they were allocated to. 10 lost to follow‐up from phenytoin group and 6 lost to follow‐up from placebo group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes analyzed and reported |
| Other bias | Unclear risk | Median serum phenytoin levels: 16.2 mg/L (range 3.3‐61) Serum levels in the participants who had post‐traumatic seizures was 2.3, 34, 13 mg/L Ideal study therapeutic dose of phenytoin not stated Emergency room administration of benzodiazepines and barbiturates: differences approached significance between the groups (see report, Table 3) Administration of paralytic agents in the pediatric ICU and potential seizures were not monitored by EEG. Unlikely to introduce bias due to blinding, but number of seizures reported may underestimate the true early seizure rate 18% of participants had been receiving anticonvulsant medications at some point since hospital discharge and prior to 30 day follow‐up |
| Blinding of participants and personnel (performance bias) | Low risk | Study medication and identical‐appearing placebo were prepared by the pharmacy. A code kept locked in an office off site was available only to the principal investigator linking each vial to the contents of the vial (phenytoin or placebo). Group assignment concealed until end of 48‐hour observation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Group assignment concealed until end of 48‐hour observation (low risk) Unclear for secondary outcomes as the groups would have been unblinded for 30‐day assessment (unclear risk) |
AED: antiepileptic drug; CT: computed tomography; EEG: electroencephalography; FPS: first post‐traumatic seizure; GCS: Glasgow Coma Scale; ICU: intensive care unit; ITT: intention to treat; IV: intravenous; RCT: randomized controlled trial; SD: standard deviation; TBI: traumatic brain injury.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not RCT or quasi‐randomized trial | |
| Secondary publication of Temkin 1999, no further relevant information reported | |
| Not treatment of interest | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not population of interest | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Secondary publication of Temkin 1990, no further relevant information reported | |
| Not treatment of interest | |
| Secondary publication of Temkin 1999, no further relevant information reported | |
| Not treatment of interest; not population of interest | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Outcome Information unavailable (no response from author) | |
| Not RCT or quasi‐randomized: single‐ arm trial | |
| Contacted author: not population of interest (all postoperati on ) | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not treatment of interest | |
| Outcomes of interest not recorded, author unable to provide | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not treatment of interest. Compare d different doses of same drug | |
| Not RCT or quasi‐randomized | |
| Not population of interest | |
| Study terminated due to lack of enrolment; n o outcome data available | |
| Status of trial unknown, further information unavailable (no response from author) | |
| Study terminated due to lack of enrolment; n o outcome data available | |
| Not population of interest. Postoperative participants | |
| Not population of interest. Postoperative participants | |
| Not population of interest. Included some participants with pre‐existing seizures (excluded following confirmation by author) | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Outcome data unavailable from author | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized. No control participants | |
| Secondary publication of Szaflarski 2010, no further relevant information reported | |
| Not RCT or quasi‐randomized. No control participants | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Author did not respond with outcome data | |
| Not population of interest. Participa nt population not traumatic brain injury | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized | |
| Not RCT or quasi‐randomized. No control group |
RCT: randomized controlled trial .
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Use of Biperiden for the Prevention of Post‐Traumatic Epilepsy |
| Methods | Placebo‐controlled, randomized, double‐blind study |
| Participants | 132 |
| Interventions | Biperiden lactate and placebo |
| Outcomes | Onset of post‐traumatic epilepsy, quality of life; cognitive level |
| Starting date | 2013 |
| Contact information | Luiz Eugenio Mello, Federal University of São Paulo, [email protected] |
| Notes |
| Trial name or title | Allopregnanolone for the Treatment of Traumatic Brain Injury |
| Methods | Double‐blind, placebo‐controlled, randomized, dose‐finding, 2‐stage adaptive, clinical trial comparing allopregnanolone to placebo when administered intravenously for 5 days beginning within 8 hours after injury |
| Participants | 136 |
| Interventions | Allopregnanolone and placebo |
| Outcomes | Extended Glasgow Outcome Scale (GOS‐E) Score; mortality; depression; late post‐traumatic epilepsy; Neurobehavioral Rating Scale Revised (NRS‐R); Test of Adult Reading; Tests of Executive Function; Tests of Learning, Delayed Recall, and Recognition; Test of Working Memory; Tests of Psychomotor and Processing Speed; depression; quality of life; anxiety |
| Starting date | 2013 |
| Contact information | University of California, Davis Medical Center. Nancy Rudisill [email protected] / Steffany Lim [email protected] |
| Notes |
| Trial name or title | Traumatic neuroprotection and epilepsy prevention of Valproate acid |
| Methods | 160 participants who were in a vegetative or minimally conscious state 4 to 16 weeks after TBI and who were receiving inpatient rehabilitation. Participants were randomly assigned to receive VPA or placebo for 4 weeks and were followed for 2 weeks after the treatment was discontinued. The rate of functional recovery on the Disability Rating Scale (DRS; range, 0 to 29, with higher scores indicating greater disability) was compared over the 4 weeks of treatment (primary outcome) and during the 2‐week washout period with the use of mixed‐effects regression models. |
| Participants | 160 |
| Interventions | Valproate acid and placebo |
| Outcomes | DRS scores; time of break out and state of epilepsy; brain magnetic resonance imaging scan; the blood concentration of valproate acid |
| Starting date | 2013 |
| Contact information | Hu S Jie, Xijing Hospital [email protected][email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 5 | 987 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.23, 0.73] |
| Analysis 1.1 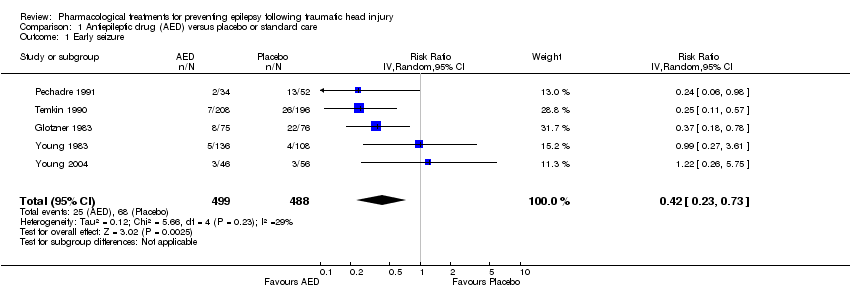 Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 1 Early seizure. | ||||
| 2 Late seizure Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| Analysis 1.2  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 2 Late seizure. | ||||
| 3 All‐cause mortality Show forest plot | 5 | 1065 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.79, 1.46] |
| Analysis 1.3  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 3 All‐cause mortality. | ||||
| 4 Any serious event Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.73, 3.66] |
| Analysis 1.4  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 4 Any serious event. | ||||
| 5 Skin rash Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.54, 5.04] |
| Analysis 1.5  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 5 Skin rash. | ||||
| 6 Sensitivity analysis ‐ early seizure: age of population Show forest plot | 4 | 885 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.21, 0.60] |
| Analysis 1.6 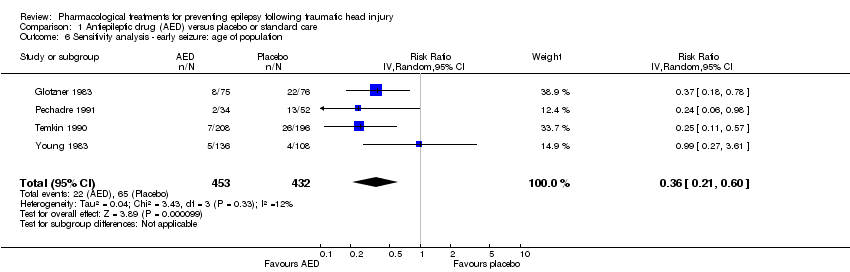 Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 6 Sensitivity analysis ‐ early seizure: age of population. | ||||
| 7 Sensitivity analysis ‐ early seizure: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.11, 2.18] |
| Analysis 1.7 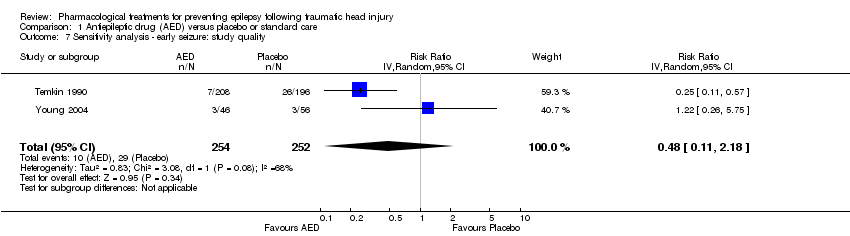 Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 7 Sensitivity analysis ‐ early seizure: study quality. | ||||
| 8 Subgroup: late seizure: type of AED Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| Analysis 1.8  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 8 Subgroup: late seizure: type of AED. | ||||
| 8.1 Late seizure ‐ phenytoin | 4 | 752 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.40, 1.70] |
| 8.2 Late seizure ‐ other AED | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.46, 1.99] |
| 9 Subgroup ‐ late seizure: treatment duration Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| Analysis 1.9  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 9 Subgroup ‐ late seizure: treatment duration. | ||||
| 9.1 Long treatment duration | 5 | 943 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.81, 1.46] |
| 9.2 Short treatment duration | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.03, 0.55] |
| 10 Sensitivity analysis ‐ late seizure: age of population Show forest plot | 5 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.48] |
| Analysis 1.10 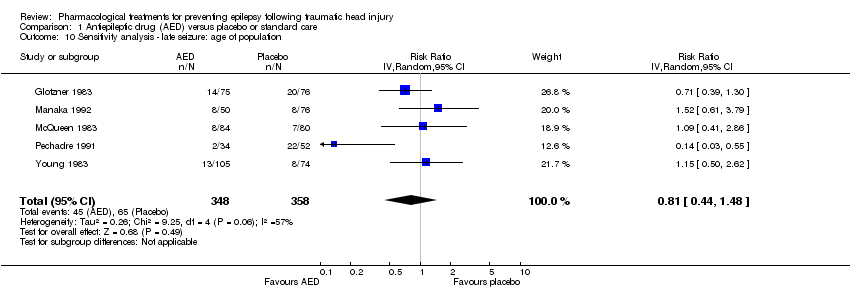 Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 10 Sensitivity analysis ‐ late seizure: age of population. | ||||
| 11 Sensitivity analysis ‐ late seizure: comparison group Show forest plot | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.41] |
| Analysis 1.11 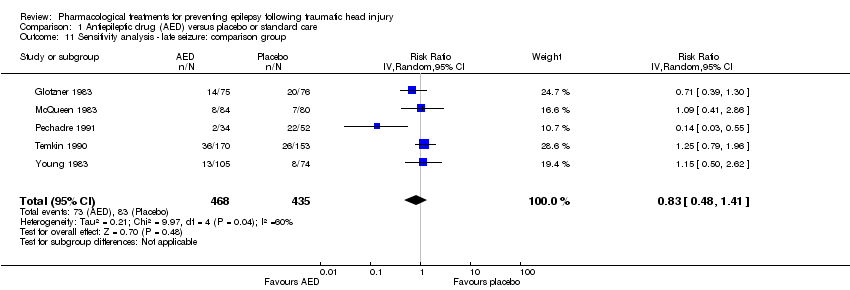 Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 11 Sensitivity analysis ‐ late seizure: comparison group. | ||||
| 12 Sensitivity analysis ‐ late seizure: study quality Show forest plot | 1 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.79, 1.96] |
| Analysis 1.12 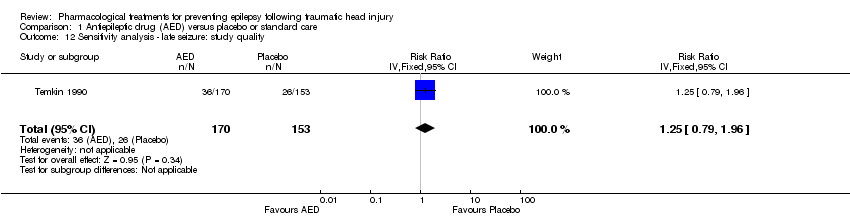 Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 12 Sensitivity analysis ‐ late seizure: study quality. | ||||
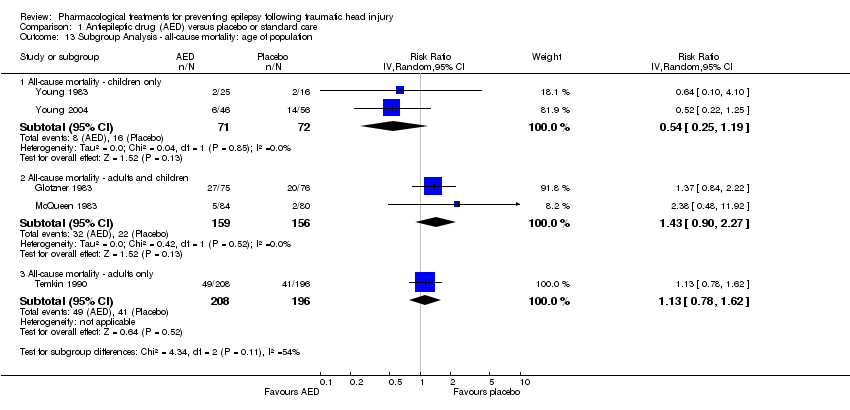
| 13 Subgroup Analysis ‐ all‐cause mortality: age of population Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 13 Subgroup Analysis ‐ all‐cause mortality: age of population. | ||||
| 13.1 All‐cause mortality ‐ children only | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.25, 1.19] |
| 13.2 All‐cause mortality ‐ adults and children | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.90, 2.27] |
| 13.3 All‐cause mortality ‐ adults only | 1 | 404 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.78, 1.62] |
| 14 Subgroup analysis ‐ all‐cause mortality: treatment duration Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 14 Subgroup analysis ‐ all‐cause mortality: treatment duration. | ||||
| 14.1 All‐cause mortality ‐ short‐term treatment duration | 2 | 346 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.39, 1.24] |
| 14.2 All‐cause mortality ‐ long‐term treatment duration | 3 | 719 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.93, 1.65] |
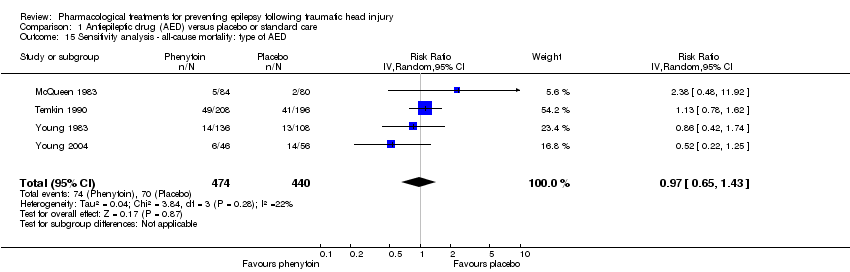
| 15 Sensitivity analysis ‐ all‐cause mortality: type of AED Show forest plot | 4 | 914 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.65, 1.43] |
| Analysis 1.15  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 15 Sensitivity analysis ‐ all‐cause mortality: type of AED. | ||||
| 16 Sensitivity analysis ‐ all‐cause mortality: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.72, 1.41] |
| Analysis 1.16  Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 16 Sensitivity analysis ‐ all‐cause mortality: study quality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
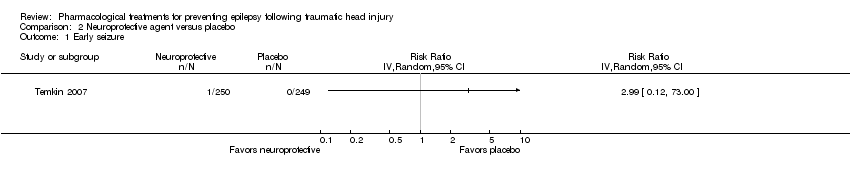
| 1 Early seizure Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Neuroprotective agent versus placebo, Outcome 1 Early seizure. | ||||
| 2 Late seizure Show forest plot | 1 | 498 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.53, 2.17] |
| Analysis 2.2 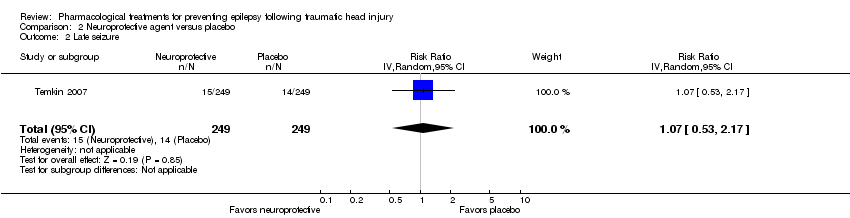 Comparison 2 Neuroprotective agent versus placebo, Outcome 2 Late seizure. | ||||
| 3 All‐cause mortality Show forest plot | 1 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.2 [0.80, 1.81] |
| Analysis 2.3 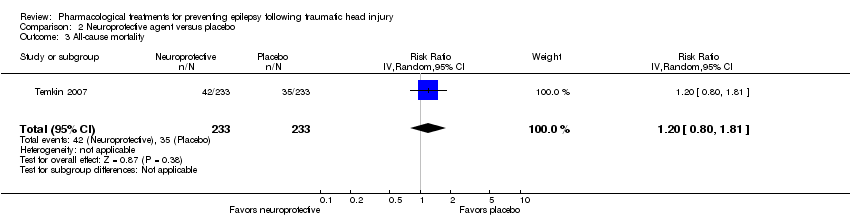 Comparison 2 Neuroprotective agent versus placebo, Outcome 3 All‐cause mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
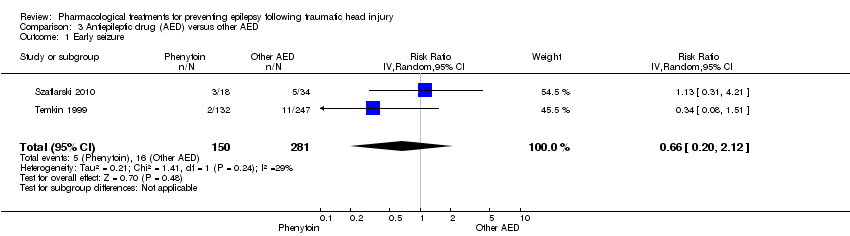
| 1 Early seizure Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.12] |
| Analysis 3.1  Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 1 Early seizure. | ||||
| 2 Late seizure Show forest plot | 2 | 378 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.30] |
| Analysis 3.2  Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 2 Late seizure. | ||||
| 3 All‐cause mortality Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |
| Analysis 3.3  Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 3 All‐cause mortality. | ||||
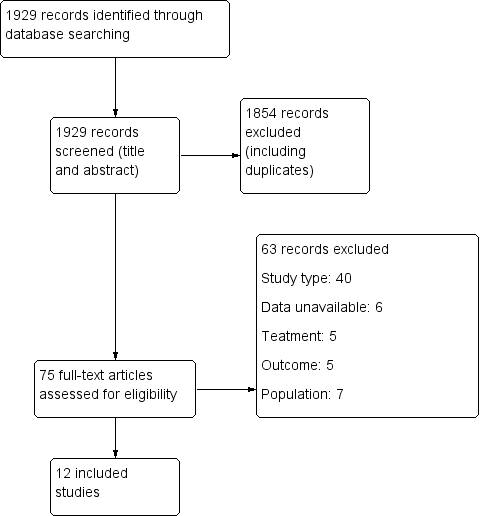
Study flow diagram.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
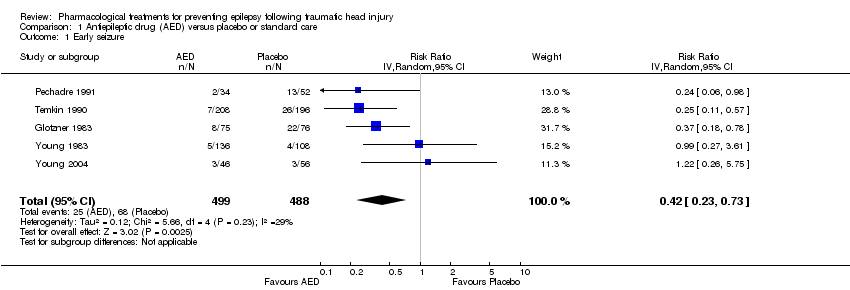
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 1 Early seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 2 Late seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 3 All‐cause mortality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 4 Any serious event.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 5 Skin rash.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 6 Sensitivity analysis ‐ early seizure: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 7 Sensitivity analysis ‐ early seizure: study quality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 8 Subgroup: late seizure: type of AED.
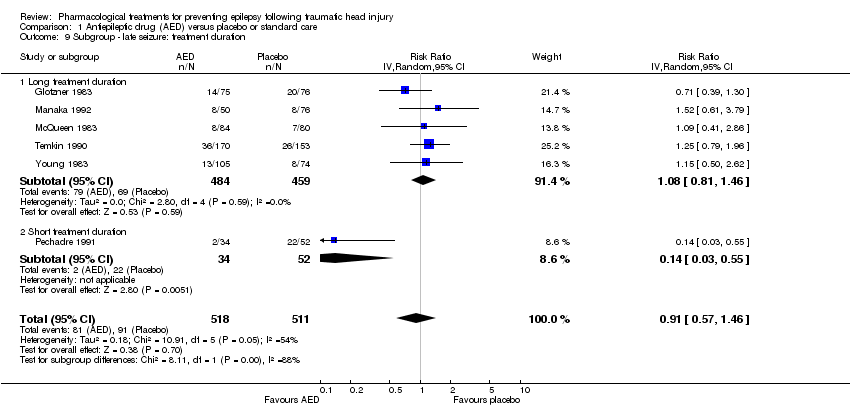
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 9 Subgroup ‐ late seizure: treatment duration.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 10 Sensitivity analysis ‐ late seizure: age of population.
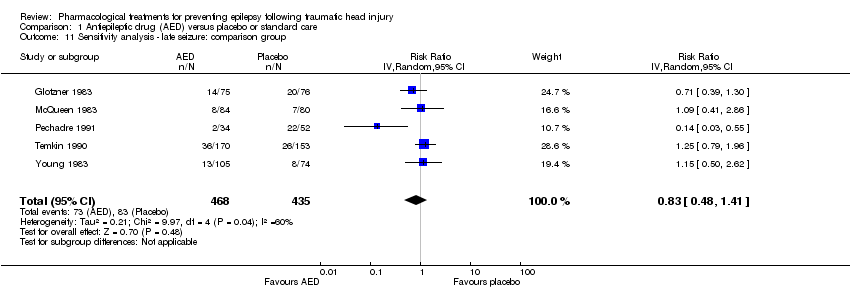
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 11 Sensitivity analysis ‐ late seizure: comparison group.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 12 Sensitivity analysis ‐ late seizure: study quality.
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 13 Subgroup Analysis ‐ all‐cause mortality: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 14 Subgroup analysis ‐ all‐cause mortality: treatment duration.
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 15 Sensitivity analysis ‐ all‐cause mortality: type of AED.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 16 Sensitivity analysis ‐ all‐cause mortality: study quality.
Comparison 2 Neuroprotective agent versus placebo, Outcome 1 Early seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 2 Late seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 3 All‐cause mortality.
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 1 Early seizure.
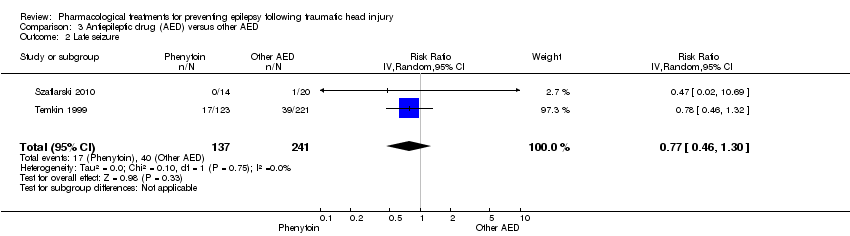
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 2 Late seizure.

Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 3 All‐cause mortality.
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures | 139 per 1000 | 59 per 1000 | RR 0.42 | 987 | ⊕⊕⊝⊝ | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures | 178 per 1000 | 162 per 1000 | RR 0.91 | 1029 | ⊕⊝⊝⊝ | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality | 174 per 1000 | 188 per 1000 | RR 1.08 | 1065 | ⊕⊝⊝⊝ | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
| Any serious adverse event of treatment count of events Follow up: 12 months | 94 per 1000 | 154 per 1000 (69 to 345) | RR 1.63 (0.73 to 3.66) | 568 (2 studies) | ⊕⊕⊝⊝ | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. 6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials | ||||||
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
| Early seizure Count of events Follow‐up: 7 days | 0 per 1000 | 0 per 1000 | RR 2.99 (0.12 to 73.00) | 499 | ⊕⊕⊝⊝ | No events occurred in the control group therefore corresponding risk is also zero |
| Late seizure Count of events Follow‐up: 6 months | 56 per 1000 | 60 per 1000 | RR 1.07 (0.53 to 2.17) | 498 | ⊕⊕⊕⊕ | |
| All‐cause mortality Follow‐up: 6 months | 150 per 1000 | 180 per 1000 | RR 1.20 (0.80 to 1.81) | 466 | ⊕⊕⊕⊕ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
| Early seizure Counts of events Follow up: 7 days | 57 per 1000 | 38 per 1000 | RR 0.66 (0.20 to 2.12) | 431 | ⊕⊕⊝⊝ | |
| Late seizure Counts of events Follow up: 6 months to 2 years | 166 per 1000 | 128 per 1000 | RR 0.77 (0.46 to 1.30) | 378 | ⊕⊕⊕⊝ | |
| All‐cause mortality Follow up: 6 months to 2 years | 164 per 1000 | 87 per 1000 | RR 0.53 (0.30 to 94) | 431 | ⊕⊕⊕⊝ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 5 | 987 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.23, 0.73] |
| 2 Late seizure Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 3 All‐cause mortality Show forest plot | 5 | 1065 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.79, 1.46] |
| 4 Any serious event Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.73, 3.66] |
| 5 Skin rash Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.54, 5.04] |
| 6 Sensitivity analysis ‐ early seizure: age of population Show forest plot | 4 | 885 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.21, 0.60] |
| 7 Sensitivity analysis ‐ early seizure: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.11, 2.18] |
| 8 Subgroup: late seizure: type of AED Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 8.1 Late seizure ‐ phenytoin | 4 | 752 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.40, 1.70] |
| 8.2 Late seizure ‐ other AED | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.46, 1.99] |
| 9 Subgroup ‐ late seizure: treatment duration Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 9.1 Long treatment duration | 5 | 943 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.81, 1.46] |
| 9.2 Short treatment duration | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.03, 0.55] |
| 10 Sensitivity analysis ‐ late seizure: age of population Show forest plot | 5 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.48] |
| 11 Sensitivity analysis ‐ late seizure: comparison group Show forest plot | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.41] |
| 12 Sensitivity analysis ‐ late seizure: study quality Show forest plot | 1 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.79, 1.96] |
| 13 Subgroup Analysis ‐ all‐cause mortality: age of population Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All‐cause mortality ‐ children only | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.25, 1.19] |
| 13.2 All‐cause mortality ‐ adults and children | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.90, 2.27] |
| 13.3 All‐cause mortality ‐ adults only | 1 | 404 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.78, 1.62] |
| 14 Subgroup analysis ‐ all‐cause mortality: treatment duration Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All‐cause mortality ‐ short‐term treatment duration | 2 | 346 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.39, 1.24] |
| 14.2 All‐cause mortality ‐ long‐term treatment duration | 3 | 719 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.93, 1.65] |
| 15 Sensitivity analysis ‐ all‐cause mortality: type of AED Show forest plot | 4 | 914 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.65, 1.43] |
| 16 Sensitivity analysis ‐ all‐cause mortality: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.72, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Late seizure Show forest plot | 1 | 498 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.53, 2.17] |
| 3 All‐cause mortality Show forest plot | 1 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.2 [0.80, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.12] |
| 2 Late seizure Show forest plot | 2 | 378 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.30] |
| 3 All‐cause mortality Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |

