Traitements pharmacologiques pour la prévention de l'épilepsie suite à un traumatisme crânien
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 MeSH DESCRIPTOR Craniocerebral Trauma Explode All
#2 craniocerebral next injur*
#3 craniocerebral next trauma*
#4 brain next injur*
#5 brain next trauma*
#6 head next injur*
#7 head next trauma*
#8 "post‐trauma" or "post trauma" or posttrauma
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 MeSH DESCRIPTOR Phenytoin Explode All
#11 MeSH DESCRIPTOR Phenobarbital Explode All
#12 MeSH DESCRIPTOR Carbamazepine Explode All
#13 phenytoin or phenobarb* or carbamazepine
#14 levetiracetam or etiracetam or lamotrigine or oxcarbazepine
#15 topiramate or gabapentin or lacosamide or harkeroside
#16 MeSH DESCRIPTOR Magnesium Sulfate Explode All
#17 "magnesium sulphate" or "magnesium sulfate"
#18 MeSH DESCRIPTOR Neuroprotective Agents Explode All
#19 MeSH DESCRIPTOR Nerve Growth Factors Explode All
#20 neurotrophic next factor*
#21 MeSH DESCRIPTOR Hormones Explode All
#22 MeSH DESCRIPTOR Antioxidants Explode All
#23 antioxida*
#24 MeSH DESCRIPTOR Anticonvulsants Explode All
#25 antiepilep* or "anti‐epilep*"
#26 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
#27 #9 AND #26
#28 #27 AND >16/12/2013:CRSCREATED AND INREGISTER
Appendix 2. CENTRAL (via CRSO) search strategy
#1 MESH DESCRIPTOR Craniocerebral Trauma EXPLODE ALL TREES
#2 (craniocerebral next injur*):TI,AB,KY
#3 (craniocerebral next trauma*):TI,AB,KY
#4 (brain next injur*):TI,AB,KY
#5 (brain next trauma*):TI,AB,KY
#6 (head next injur*):TI,AB,KY
#7 (head next trauma*):TI,AB,KY
#8 ((post‐trauma) or (post trauma) or (posttrauma)):TI,AB,KY
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 MESH DESCRIPTOR Phenytoin EXPLODE ALL TREES
#11 MESH DESCRIPTOR Phenobarbital EXPLODE ALL TREES
#12 MESH DESCRIPTOR Carbamazepine EXPLODE ALL TREES
#13 (phenytoin or phenobarb* or carbamazepine):TI,AB,KY
#14 (levetiracetam or etiracetam or lamotrigine or oxcarbazepine):TI,AB,KY
#15 (topiramate or gabapentin or lacosamide or harkeroside):TI,AB,KY
#16 MESH DESCRIPTOR Magnesium Sulfate EXPLODE ALL TREES
#17 ("magnesium sulphate" or "magnesium sulfate"):TI,AB,KY
#18 MESH DESCRIPTOR Neuroprotective Agents EXPLODE ALL TREES
#19 MESH DESCRIPTOR Nerve Growth Factors EXPLODE ALL TREES
#20 (neurotrophic next factor*):TI,AB,KY
#21 MESH DESCRIPTOR Hormones EXPLODE ALL TREES
#22 MESH DESCRIPTOR Antioxidants EXPLODE ALL TREES
#23 antioxida*:TI,AB,KY
#24 MESH DESCRIPTOR Anticonvulsants EXPLODE ALL TREES
#25 (antiepilep* or anti‐epilep*):TI,AB,KY
#26 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
#27 (epilep* or convuls* or seizure*):TI,AB,KY
#28 MESH DESCRIPTOR Epilepsy, Post‐Traumatic EXPLODE ALL TREES
#29 #27 OR #28
#30 #9 AND #26 AND #29
#31 * NOT INMEDLINE AND 30/11/2013 TO 28/02/2015:DL
#32 #30 AND #31
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2011.
1. (randomized controlled trial or controlled clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. exp Craniocerebral Trauma/
8. ((craniocerebral or brain or head) adj (injur* or trauma*)).tw.
9. (post‐trauma or post trauma or posttrauma).tw.
10. 7 or 8 or 9
11. exp Phenytoin/
12. exp Phenobarbital/
13. exp Carbamazepine/
14. (phenytoin or phenobarb* or carbamazepine).tw.
15. (levetiracetam or etiracetam or lamotrigine or oxcarbazepine).tw.
16. (topiramate or gabapentin or lacosamide or harkeroside).tw.
17. exp Magnesium Sulfate/
18. (magnesium sulphate or magnesium sulfate).tw.
19. exp Neuroprotective Agents/
20. exp Nerve Growth Factors/
21. neurotrophic factor*.tw.
22. exp Hormones/
23. exp Antioxidants/
24. antioxida*.tw.
25. exp Anticonvulsants/
26. (antiepilep* or anti‐epilep*).tw.
27. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28. (epilep* or convuls* or seizure*).tw.
29. exp Epilepsy, Post‐Traumatic/
30. 28 or 29
31. 6 and 10 and 27 and 30
32. limit 31 to ed=20131216‐20150113
Appendix 4. EMBASE search strategy
#1 random*
#2 placebo*
#3doubl* NEAR/3 blind*
#4 assign*
#5 singl* NEAR/3 blind*
#6 allocat*
#7 volunteer*
#8 'double blind procedure'/exp
#9 'randomized controlled trial'/exp
#10 single AND 'blind'/exp
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#12 #10 OR #11
#13 'animal'/exp NOT 'human'/exp
#14 #12 NOT #13
#15 'head injury'/exp
#16 (craniocerebral OR brain OR head) NEAR/3 (injur* OR trauma*)
#17 posttrauma*
#18 post NEAR/3 trauma*
#19 #15 OR #16 OR #17 OR #18
#20 'phenytoin'/exp
#21 'phenobarbital' /exp
#22 'phenobarbital'/exp
#23 'carbamazepine'/exp
#24 'etiracetam' /exp
#25 'gabapentin'/exp
#26 'harkoseride'/exp
#27 'lamotrigine'/exp
#28 'topiramate'exp
#29 'oxcarbazepine'exp
#30 lamotrigine OR topiramate OR oxcarbazepine
#31 levetiracetam OR etiracetam OR lacosamide OR harkoseride
#32 phenytoin OR phenobarb* OR carbamazepine OR gabapentin
#33 'magnesium sulfate'/exp
#34 'magnesium sulphate' OR 'magnesium sulfate'
#35 'neuroprotective agent'/exp
#36 'nerve growth factor'/exp
#37 'hormone'/exp
#38 'anticonvulsive agent'/exp OR 'anticonvulsant activity'/exp
#39 'antioxidant'/exp OR 'antioxidant activity'/exp
#40 neuro* NEAR/3 factor*
#41 antioxida*
#42 anti*epilep*
#43 hormon*
#44 'nerve growth' NEAR/3 factor*
#45 #20 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44
#46 epilep* OR convuls* OR seizure*
#47 'seizure'/exp OR 'epilepsy'/exp OR 'convulsion'/exp
#48 #46 OR #47
#49 #14 AND #19 AND #45 AND #48
#50 #14 AND #19 AND #45 AND #48 AND [15‐8‐2011]/sd NOT [5‐9‐2014]/sd
Appendix 5. SCOPUS search strategy
(TITLE‐ABS‐KEY(randomly OR "clinical trial" OR "controlled trial" OR randomised OR randomized OR placebo*)) AND (TITLE‐ABS‐KEY("craniocerebral trauma*" OR "craniocerebral injur*" OR "brain trauma*" OR "brain injur*" OR "head trauma*" OR "head injur*" OR posttrauma* OR post‐trauma* OR "post trauma*")) AND (TITLE‐ABS‐KEY(phenytoin OR phenobarb* OR carbamazepine OR levetiracetam OR etiracetam OR lamotrigine OR oxcarbazepine OR topiramate OR gabapentin OR lacosamide OR harkeroside OR "magnesium sulphate" OR "magnesium sulfate" OR "neurotrophic factor*" OR antioxida* OR antiepilep* OR anti‐epilep*)) AND (TITLE‐ABS‐KEY(epilep* OR convuls* OR seizure)) AND ( LIMIT‐TO(PUBYEAR,2013) OR LIMIT‐TO(PUBYEAR,2012) ) AND ( LIMIT‐TO(EXACTKEYWORD,"Human" ) OR LIMIT‐TO(EXACTKEYWORD,"Humans" ) )
Appendix 6. Biological Abstracts search strategy
#1 TS=(((craniocerebral or brain or head) NEAR/1 (injur* or trauma*))) Indexes=Biological Abstracts Timespan=All years
#2 TS=("post‐trauma" OR "posttrauma") Indexes=Biological Abstracts Timespan=All years
#3 TS=(phenytoin OR phenobarb* OR carbamazepine OR levetiracetam OR etiracetam OR lamotrigine OR oxcarbazepine OR topiramate OR gabapentin OR lacosamide OR harkeroside OR "magnesium sulphate" OR "magnesium sulfate" OR "neurotrophic factor*" OR antioxida* OR antiepilep* OR anti‐epilep*) Indexes=Biological Abstracts Timespan=All years
#4 TS=(epilep* OR convuls* OR seizure*) Indexes=Biological Abstracts Timespan=All years
#5 #1 OR #2 Indexes=Biological Abstracts Timespan=All years
#6 #3 AND #4 AND #5 Indexes=Biological Abstracts Timespan=All years
#7 TS=(randomly OR "clinical trial" OR "controlled trial" OR randomised OR randomized OR placebo*) Indexes=Biological Abstracts Timespan=All years
#8 #6 and #7 Indexes=Biological Abstracts Timespan=All years
#9 #7 AND #6 Indexes=Biological Abstracts Timespan=2011‐2015
Appendix 7. Data extraction form
| Reviewer: | Date of review: |
1. Study Description
| Study ID number: | RefWorksID number: |
| Corresponding author’s name and institution:
| Corresponding author’s email:
|
| Full citation, including all author names:
Only abstract was published. | Author contacted: Yes No If Yes: Indicate reason: _____________________________ ___________________________________________ Date message sent: _____________ _____ Response summary: _________________________ ___________________________________________ Date of response: __________________ |
| Setting of study:
| Language: English Other ___________________________________ |
| Check off inclusion criteria: Study is an RCT or a quasi‐randomized trial. Patients were diagnosed with TBI. Study involves administration of pharmacologic agents for the prevention of post‐traumatic epilepsy. Study excluded patients that had a previous documented unprovoked seizure. Study reports outcomes of interest. Additional notes: | |
Part I: Data extraction form
2. General study design questions
| Was this a multicenter study? | Not reported Yes No |
| Duration of enrolment: |
|
| Duration of follow‐up: |
|
| Study excluded patients six years and under? | Not reported Yes No (indicate details in part 3: Participants) |
3. Participants
|
| Overall | Control | Treatment 1 | Treatment 2 |
| N number randomized |
|
|
|
|
| N number followed up |
|
|
|
|
| Age classification:
| All patients are < 17 yrs All patients are >= 17 Neither of the above. | All patients are < 17 yrs All patients are >= 17 Neither of the above. | All patients are < 17 yrs All patients are >= 17 Neither of the above. | All patients are < 17 yrs All patients are >= 17 Neither of the above. |
| Indicate age: range: or Mean/SD: or Median:
Additional details: |
|
|
|
|
| Gender (Proportion male) |
|
|
|
|
| Trauma severity
| Undefined Minor Moderate Severe | Undefined Minor Moderate Severe | Undefined Minor Moderate Severe | Undefined Minor Moderate Severe |
| Indicate methods of measurement for severity: e.g.: EEG,MRI, or CT scan findings, GCS range, etc. |
|
|
|
|
| How were seizures identified? | Not reported EEG Clinical findings
| Not reported EEG Clinical findings
| Not reported EEG Clinical findings
| Not reported EEG Clinical findings
|
4. Treatment/Control/Comparison
|
| Control | Treatment 1 | Treatment 2 | ||
| Type of agent | Placebo Usual care | Traditional AED Newly licensed AED Other agent | Traditional AED Newly licensed AED Other agent | ||
| Examples for types of agent: | Traditional antiepileptic: antiepileptic Drug that has been on the market for many years (E.g. Carbamazepine, phenytoin, valproate) Newly licensed AED: antiepileptic Drug that has been licensed more recently (E.g. Levetiracetam, topiramate, lamotrigine, oxcarbazepine) Other agent: Any agent or drug that is not marketed as an AED (E.g.Magnesium sulphate | ||||
| Name of agent |
|
|
| ||
| Dose amount& administration method
|
|
|
| ||
| Therapeutic dose |
|
|
| ||
| Timing of doses |
|
|
| ||
| Duration of treatment
| Short‐term Mid‐term Long‐term Additional comment:
| Short‐term Mid‐term Long‐term Additional comment:
| Short‐term Mid‐term Long‐term Additional comment:
| ||
| Definitions for duration of treatment: | Short‐term: Treatment less than or equal to 3 months post injury Mid‐term: Treatment less than or equal to 12 months post injury Long‐term: Treatment more than 12 months post injury | ||||
| First dose given before a first posttraumatic seizure? | Not reported Yes No | Not reported Yes No | Not reported Yes No | ||
5. Primary outcomes
For the purpose of this review, seizure outcomes are classified according to the following definitions:
Early seizures are those that occur within one week of trauma.
Late seizures are those that occur later than one week post‐trauma.
Note that some studies only report seizure incidence in general and do not indicate when they occurred. Report this in the row labelled “Patients experiencing seizures (general)”.
| Control (n/N) | Treatment 1 (n/N) | Treatment 2 (n/N) | |
| Patients experiencing early seizures * |
|
|
|
| Patients experiencing late seizures |
|
|
|
| Patients experiencing seizures (general) |
|
|
|
* If the study reports actuarial percentages, please note that here.
6. Secondary outcomes
| Control | Treatment 1 | Treatment 2 | |
| Mortality from any cause during follow‐up period |
|
|
|
| Mean number of early seizures (per patient) |
|
|
|
| Mean number of late seizures (per patient) |
|
|
|
| Mean number of seizures (general) (per patient) |
|
|
|
| Time to first seizure (may report hazard ratio, CI) |
|
|
|
| Time to second seizure (hazard ratio, CI) |
|
|
|
| Other adverse effects (Eg. Skin rashes) (Define adverse effect and indicate incidence) (n/N) |
|
|
|
| Number of patients for which treatment was discontinued (include reasons) (n/N) |
|
|
|
| Other outcomes reported by study (e.g. Neurological findings, Status Epilepticus)
|
|
|
|
Part II:Questions for assessing Risk of Bias (ROB)
A summary of the ROB domains, as described by the Cochrane Collaboration in chapter 8of the handbook, are included here for reference.
| Domain | Support for judgement |
| Selection bias. | |
| Random sequence generation. | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. |
| Allocation concealment. | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. |
| Performance bias. | |
| Blinding of participants and personnelAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. |
| Detection bias. | |
| Blinding of outcome assessmentAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. |
| Attrition bias. | |
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. |
| Reporting bias. | |
| Selective reporting. | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. |
| Other bias. | |
| Other sources of bias. | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. |
| Domain | Assessment | Support for judgement |
| Selection bias. |
| |
| Random sequence generation. | Low Unclear High |
|
| Allocation concealment. | Low Unclear High |
|
For performance bias, detection bias and attrition bias, assessments should be made for each main outcome (or class of outcomes). Print off more copies of the following page, if more space is required.
Define outcome: _________________________________________
| Domain | Assessment | Support for judgement |
| Performance bias. |
| |
| Blinding of participants and personnel | Low Unclear High |
|
| Detection bias. |
| |
| Blinding of outcome assessment | Low Unclear High |
|
| Attrition bias. |
| |
| Incomplete outcome data | Low Unclear High |
|
| Domain | Assessment | Support for judgement |
| Reporting bias. |
| |
| Selective reporting. | Low Unclear High |
|
| Other bias. |
| |
| Other sources of bias. | Low Unclear High |
|
Part III. Additional details
Fill in any study shortcomings and other relevant details.
Appendix 8. Severity of trauma
| Mild | Moderate | Severe | Very severe |
| PTA < 1 hour | PTA 1‐24 hours | PTA 1‐7 days | PTA > 7 days |
| GCS 13‐15 | GCS 9‐12 | GCS 3‐8 | LOC > 48 hours |
| LOC < 15 minutes | LOC < 6 hours | LOC 6‐48 hours | |
| GCS: Glasgow Coma Score; LOC: loss of consciousness; PTA: post‐traumatic amnesia. | |||
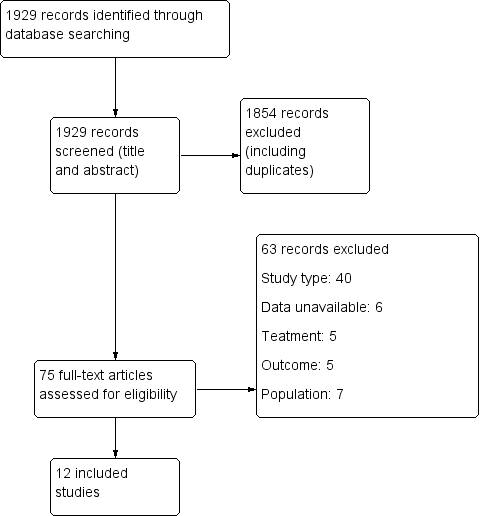
Study flow diagram.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
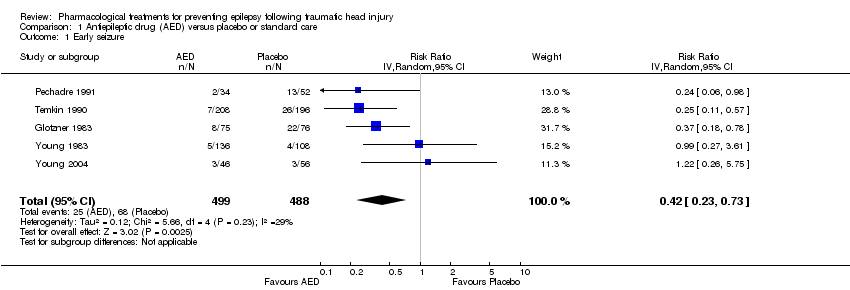
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 1 Early seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 2 Late seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 3 All‐cause mortality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 4 Any serious event.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 5 Skin rash.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 6 Sensitivity analysis ‐ early seizure: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 7 Sensitivity analysis ‐ early seizure: study quality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 8 Subgroup: late seizure: type of AED.
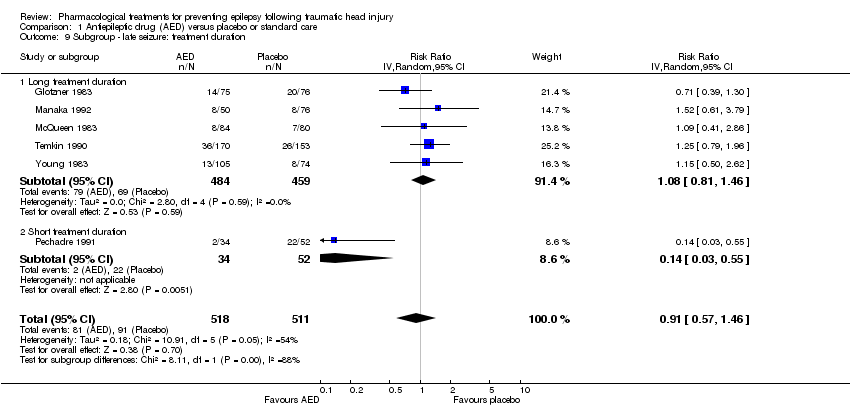
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 9 Subgroup ‐ late seizure: treatment duration.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 10 Sensitivity analysis ‐ late seizure: age of population.
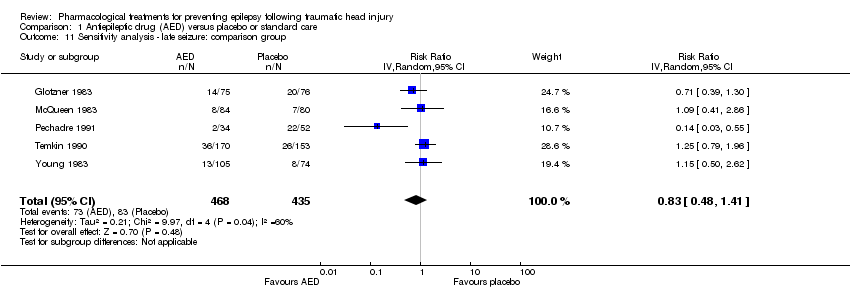
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 11 Sensitivity analysis ‐ late seizure: comparison group.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 12 Sensitivity analysis ‐ late seizure: study quality.
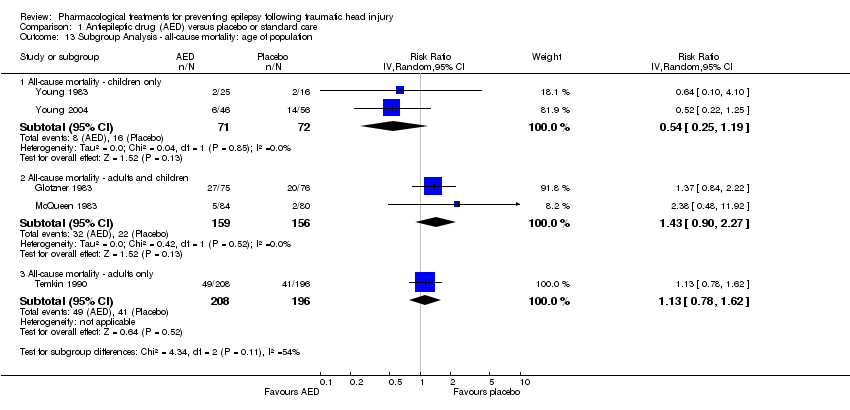
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 13 Subgroup Analysis ‐ all‐cause mortality: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 14 Subgroup analysis ‐ all‐cause mortality: treatment duration.
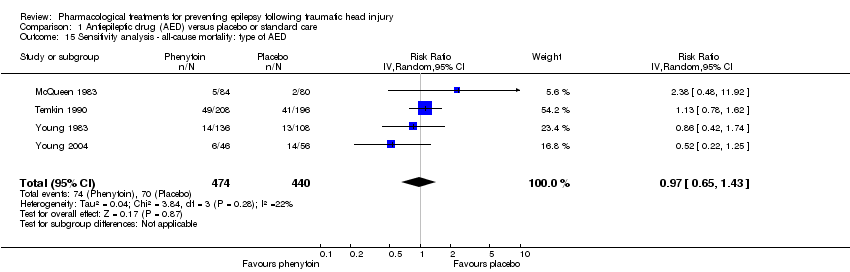
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 15 Sensitivity analysis ‐ all‐cause mortality: type of AED.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 16 Sensitivity analysis ‐ all‐cause mortality: study quality.
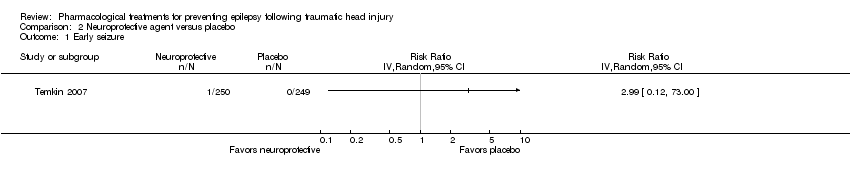
Comparison 2 Neuroprotective agent versus placebo, Outcome 1 Early seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 2 Late seizure.

Comparison 2 Neuroprotective agent versus placebo, Outcome 3 All‐cause mortality.
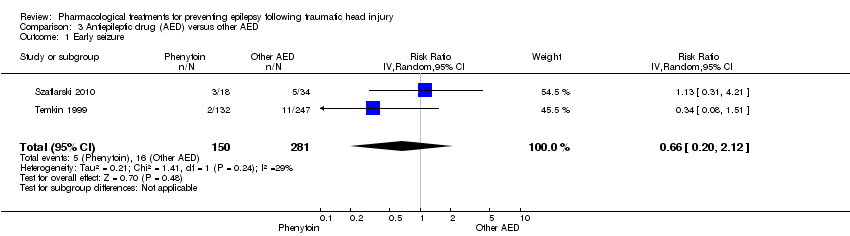
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 1 Early seizure.
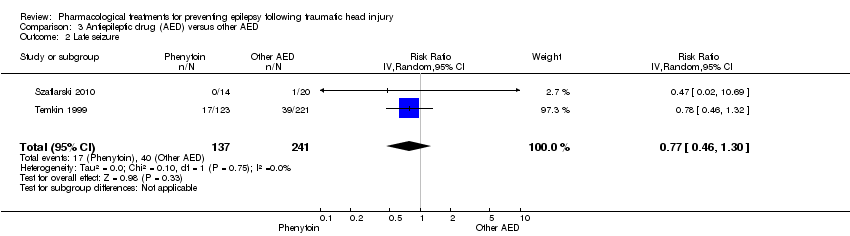
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 2 Late seizure.

Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 3 All‐cause mortality.
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures | 139 per 1000 | 59 per 1000 | RR 0.42 | 987 | ⊕⊕⊝⊝ | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures | 178 per 1000 | 162 per 1000 | RR 0.91 | 1029 | ⊕⊝⊝⊝ | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality | 174 per 1000 | 188 per 1000 | RR 1.08 | 1065 | ⊕⊝⊝⊝ | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
| Any serious adverse event of treatment count of events Follow up: 12 months | 94 per 1000 | 154 per 1000 (69 to 345) | RR 1.63 (0.73 to 3.66) | 568 (2 studies) | ⊕⊕⊝⊝ | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. 6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials | ||||||
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
| Early seizure Count of events Follow‐up: 7 days | 0 per 1000 | 0 per 1000 | RR 2.99 (0.12 to 73.00) | 499 | ⊕⊕⊝⊝ | No events occurred in the control group therefore corresponding risk is also zero |
| Late seizure Count of events Follow‐up: 6 months | 56 per 1000 | 60 per 1000 | RR 1.07 (0.53 to 2.17) | 498 | ⊕⊕⊕⊕ | |
| All‐cause mortality Follow‐up: 6 months | 150 per 1000 | 180 per 1000 | RR 1.20 (0.80 to 1.81) | 466 | ⊕⊕⊕⊕ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
| Early seizure Counts of events Follow up: 7 days | 57 per 1000 | 38 per 1000 | RR 0.66 (0.20 to 2.12) | 431 | ⊕⊕⊝⊝ | |
| Late seizure Counts of events Follow up: 6 months to 2 years | 166 per 1000 | 128 per 1000 | RR 0.77 (0.46 to 1.30) | 378 | ⊕⊕⊕⊝ | |
| All‐cause mortality Follow up: 6 months to 2 years | 164 per 1000 | 87 per 1000 | RR 0.53 (0.30 to 94) | 431 | ⊕⊕⊕⊝ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 5 | 987 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.23, 0.73] |
| 2 Late seizure Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 3 All‐cause mortality Show forest plot | 5 | 1065 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.79, 1.46] |
| 4 Any serious event Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.73, 3.66] |
| 5 Skin rash Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.54, 5.04] |
| 6 Sensitivity analysis ‐ early seizure: age of population Show forest plot | 4 | 885 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.21, 0.60] |
| 7 Sensitivity analysis ‐ early seizure: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.11, 2.18] |
| 8 Subgroup: late seizure: type of AED Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 8.1 Late seizure ‐ phenytoin | 4 | 752 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.40, 1.70] |
| 8.2 Late seizure ‐ other AED | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.46, 1.99] |
| 9 Subgroup ‐ late seizure: treatment duration Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 9.1 Long treatment duration | 5 | 943 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.81, 1.46] |
| 9.2 Short treatment duration | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.03, 0.55] |
| 10 Sensitivity analysis ‐ late seizure: age of population Show forest plot | 5 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.48] |
| 11 Sensitivity analysis ‐ late seizure: comparison group Show forest plot | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.41] |
| 12 Sensitivity analysis ‐ late seizure: study quality Show forest plot | 1 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.79, 1.96] |
| 13 Subgroup Analysis ‐ all‐cause mortality: age of population Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All‐cause mortality ‐ children only | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.25, 1.19] |
| 13.2 All‐cause mortality ‐ adults and children | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.90, 2.27] |
| 13.3 All‐cause mortality ‐ adults only | 1 | 404 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.78, 1.62] |
| 14 Subgroup analysis ‐ all‐cause mortality: treatment duration Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All‐cause mortality ‐ short‐term treatment duration | 2 | 346 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.39, 1.24] |
| 14.2 All‐cause mortality ‐ long‐term treatment duration | 3 | 719 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.93, 1.65] |
| 15 Sensitivity analysis ‐ all‐cause mortality: type of AED Show forest plot | 4 | 914 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.65, 1.43] |
| 16 Sensitivity analysis ‐ all‐cause mortality: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.72, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Late seizure Show forest plot | 1 | 498 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.53, 2.17] |
| 3 All‐cause mortality Show forest plot | 1 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.2 [0.80, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.12] |
| 2 Late seizure Show forest plot | 2 | 378 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.30] |
| 3 All‐cause mortality Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |

