Remisión inmediata a colposcopia versus vigilancia citológica para las anomalías citológicas menores del cuello uterino en ausencia de prueba del VPH
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | ||
| Methods | 3‐arm RCT | |
| Participants | 5060 women (3488 women, ASCUS smear and 1572 women, LSIL smear) Study arms:
Inclusion criteria:
Exclusion criteria:
| |
| Interventions | All
Colposcopy indications: 1. Immediate colposcopy at the date of recruitment or within 3 weeks. Treatment if CIN2 or 3 in biopsy. 2. Referral to colposcopy if the HPV test positive or missing or if enrolment cytology HSIL. Treatment if CIN2 or 3 in biopsy 3. Referral to colposcopy if cytology HSIL. Treatment if CIN2 or 3 in biopsy LSIL only
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone‐based central randomisation service used. Sequence generation not described in detail, but not estimated to induce bias |
| Allocation concealment (selection bias) | Low risk | Allocation done from central point |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible and personnel were not blinded of allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of investigators undertaking follow‐up assessments was not described in detail. "all available clinical information was unmasked and provided to the clinician conducting the exit pelvic examination and colposcopy." |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up were disclosed (ASCUS: 14.2% at 24 months in the surveillance group, LSIL: 16.3% at 24 months in the surveillance group) and the analyses were conducted as specified a priori. Loss to follow‐up might have introduced only small bias to histology results. |
| Selective reporting (reporting bias) | Low risk | Pre‐published trial protocol available, outcome reporting done according to protocol |
| Other bias | High risk | Source of funding reported; competing interests reported. Ethical approval & informed consent from participants obtained. Repeat smear at randomisation visit and exit colposcopy at 24 months, both could potentially introduce detection bias and over inflate the detection rate of pre‐invasive lesions in the cytological surveillance arm at 24 months. |
| Study characteristics | ||
| Methods | 4‐arm RCT | |
| Participants | 902 women, presenting with mildly or moderately dyskaryotic smear for the first time. Study arms:
Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Group 1. Immediate colposcopy with biopsies +/‐ treatment (n = 227) Group 2. 6‐month surveillance (n = 225):
Group 3. 12‐month surveillance (n = 223):
Group 4. 24‐month surveillance (n = 227):
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...all eligible women randomised by serial allocation to one of four groups." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described in sufficient detail |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants was not possible and blinding of personnel was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 109/675 women in the surveillance arms defaulted from study, rates being higher with longer follow‐up (9.8% at 6 months, 15.2% at 12 months, and 23.3% at 24 months). More specific reasons not available. Significant default rates might bias histology results |
| Selective reporting (reporting bias) | Low risk | Protocol not available. Results still reported comprehensively and reporting considered not to introduce bias |
| Other bias | High risk | Conflict of interest not reported; source of funding not reported; details of ethical approval not stated. Missing information still considered not to introduce bias All women in the surveillance arm had colposcopy in addition to smear, which could introduce bias and over inflate the detection rates in the surveillance arm at 6, 12 and 24 months of surveillance |
| Study characteristics | ||
| Methods | 2‐arm RCT with Zelen randomisation | |
| Participants | 712 women pre‐randomised, with mild dyskaryosis for the first time or recurrent borderline change on routine cervical screening in primary care. Aged 20 to 60, not pregnant, no abnormal vaginal bleeding Of 712 pre‐randomised women, 476 decided to participate in the study. Study arms:
Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Group 1. No‐choice arm (n = 243)
Group 2. Choice arm (n = 233) Women were given the opportunity to choose between a) and b):
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation into two study arms prior to consent using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation used. In one of the study arms the participants themselves chose the intervention group. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and investigators were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | High risk | 712 pre‐randomised, 476 participated. Reasons for non‐participation reported, no clear differences between randomisation groups 172/476 were lost to follow‐up, no specific reasons stated. Adequate sample size might not have been achieved for all outcomes. |
| Selective reporting (reporting bias) | Low risk | Pre‐trial protocol was not available. Results still reported comprehensively |
| Other bias | High risk | Project funding reported; conflicts of interest not stated. Local ethical approval and informed consent from participants obtained. Missing information still considered not to introduce bias. Used Zelen randomisation, where some participants were able to choose whether to have immediate colposcopy or cytological surveillance, which might well bias the results included here. |
| Study characteristics | ||
| Methods | 2‐arm RCT | |
| Participants | 353 women with borderline or mild dyskaryotic smear Study arms:
Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Group 1. Immediate treatment (n = 182)
Group 2. Deferred treatment (n = 171)
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a sequence of random numbers generated by computer. |
| Allocation concealment (selection bias) | Low risk | Total allocation concealment as randomisation performed individually from a central point. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding participants was not possible and personnel were not blinded to allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Investigators undertaking follow‐up were not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 36/171 lost to follow‐up in deferred treatment group, 1/181 in the immediate colposcopy group. More specific reasons not reported. The 20% lost to follow‐up in other arm might introduce bias to histology results |
| Selective reporting (reporting bias) | Low risk | Pre‐trial protocol was not available. Results still reported comprehensively |
| Other bias | High risk | Individual sources of funding reported, conflicts of interests not declared. Local ethical approval and informed consent from participants obtained. Missing information still considered not to introduce bias. All women in the surveillance arm had deferred treatment at 24 months regardless of cytology, which could introduce bias and over inflate the CIN detection rate in the surveillance‐arm at 24 months. |
| Study characteristics | ||
| Methods | 2‐arm RCT | |
| Participants | 4439 women, aged 20‐59, with cytological result showing: Study arms:
Inclusion criteria:
Exclusion criteria
| |
| Interventions | Group 1. Surveillance arm (n = 2223)
Group 2. Immediate colposcopy arm (n = 2216), second randomisation at colposcopy to a) or b) a) Biopsy and selective recall for LLETZ based on histology on biopsy
b) Immediate treatment (LLETZ)
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Touch‐telephone stratified randomisation used |
| Allocation concealment (selection bias) | Low risk | Allocation done from central point |
| Blinding of participants and personnel (performance bias) | High risk | Participants not possible to blind. Blinding of personnel not described |
| Blinding of outcome assessment (detection bias) | Low risk | At exit examination the colposcopist was blinded to the women's initial cytology status, her randomisation and any clinical outcomes. Blinding of colposcopists or pathologists at other stages was not reported. |
| Incomplete outcome data (attrition bias) | High risk | Before the first examination 107/2223 in the surveillance arm, 155/2216 in the colposcopy arm were lost to follow‐up. 1296 (58.3%) women in the surveillance arm and 1389 (62.7%) in the colposcopy arm attended the exit examination. Power calculations were based on 4500 participants. The significant loss to follow‐up and differences between arms might introduce bias to histology results. |
| Selective reporting (reporting bias) | Low risk | Pre‐published trial protocol available, outcome reporting according to protocol. |
| Other bias | High risk | Source of funding reported; Competing interests reported. Ethical approval & informed consent from participants obtained. All women were invited to an exit examination at 36 months, where colposcopy was performed regardless of smear results. This could introduce bias and over inflate the CIN detection rates in surveillance arm at 36 months. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not RCT (retrospective cohort study) | |
| Exposure not of interest (natural history of CIN 1) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Occurrence of CIN2+ Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1: Occurence of CIN2+ at different lengths of follow‐up: immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN2+ | ||||
| 1.1.1 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 1.50 [1.12, 2.01] |
| 1.1.2 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.66, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Occurrence of CIN3+ Show forest plot | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2: Occurence of CIN3+ at different lengths of follow‐up: immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN3+ | ||||
| 2.1.1 12 months' surveillance | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 2.07 [1.54, 2.79] |
| 2.1.2 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.77, 1.98] |
| 2.1.3 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Presence of any CIN in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.09, 2.70] |
| Analysis 3.1  Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 1: Presence of any CIN in histology at 12 months | ||||
| 3.2 Presence of CIN1/2 in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.83, 3.43] |
| Analysis 3.2  Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 2: Presence of CIN1/2 in histology at 12 months | ||||
| 3.3 Presence of CIN3+ in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.63 [1.25, 2.12] |
| Analysis 3.3  Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 3: Presence of CIN3+ in histology at 12 months | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Histology at 24 months Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4: Histology at 24 months: immediate colposcopy versus cytological surveillance, Outcome 1: Histology at 24 months | ||||
| 4.1.1 HPV/Koilocytic atypia | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.17, 1.90] |
| 4.1.2 Any CIN | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 2.02 [1.33, 3.08] |
| 4.1.3 CIN1 | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 2.58 [1.69, 3.94] |
| 4.1.4 CIN2 | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.80, 1.96] |
| 4.1.5 CIN2+ | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.66, 1.97] |
| 4.1.6 CIN3+ | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Occurrence of CIN2 Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5: Occurence of CIN2 at different lengths of follow‐up: Immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN2 | ||||
| 5.1.1 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 2.04 [1.52, 2.73] |
| 5.1.2 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.45 [0.87, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Default rates Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6: Default rates: immediate colposcopy versus cytological surveillance, Outcome 1: Default rates | ||||
| 6.1.1 Default rates at 6 months | 3 | 5117 | Risk Ratio (IV, Random, 95% CI) | 3.85 [1.27, 11.63] |
| 6.1.2 Default rates at 12 months | 3 | 5115 | Risk Ratio (IV, Random, 95% CI) | 6.60 [1.49, 29.29] |
| 6.1.3 Default rates at 24 months | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 19.10 [9.02, 40.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 CIN incidence at 24 months, after LSIL/mild dyskaryosis at baseline Show forest plot | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7: Histology at 24 months: immediate colposcopy versus cytological surveillance, LSIL/mild dyskaryosis only, Outcome 1: CIN incidence at 24 months, after LSIL/mild dyskaryosis at baseline | ||||
| 7.1.1 CIN2 incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.66, 4.48] |
| 7.1.2 CIN2+ incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.51, 4.01] |
| 7.1.3 CIN3+ incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.39, 3.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Presence of CIN2 in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.41, 5.78] |
| Analysis 8.1  Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 1: Presence of CIN2 in histology at 24 months | ||||
| 8.2 Presence of CIN2+ in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.86, 3.47] |
| Analysis 8.2  Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 2: Presence of CIN2+ in histology at 24 months | ||||
| 8.3 Presence of CIN3+ in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.11, 2.92] |
| Analysis 8.3  Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 3: Presence of CIN3+ in histology at 24 months | ||||
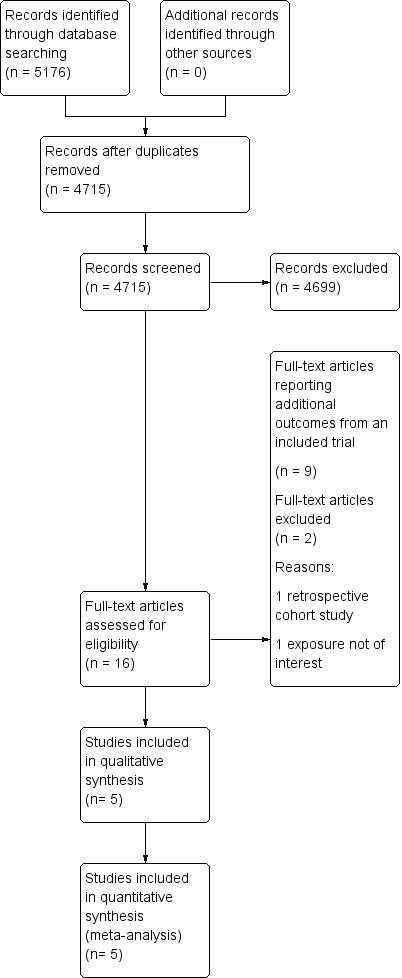
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: occurrence of CIN2+ at different lengths of follow‐up

Forest plot of comparison: occurence of CIN3+ at different lengths of follow‐up

Forest plot of comparison: occurence of CIN2 at different lengths of follow‐up

Forest plot of comparison: default rates at different lengths of follow‐up

Comparison 1: Occurence of CIN2+ at different lengths of follow‐up: immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN2+

Comparison 2: Occurence of CIN3+ at different lengths of follow‐up: immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN3+

Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 1: Presence of any CIN in histology at 12 months

Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 2: Presence of CIN1/2 in histology at 12 months

Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 3: Presence of CIN3+ in histology at 12 months

Comparison 4: Histology at 24 months: immediate colposcopy versus cytological surveillance, Outcome 1: Histology at 24 months

Comparison 5: Occurence of CIN2 at different lengths of follow‐up: Immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN2

Comparison 6: Default rates: immediate colposcopy versus cytological surveillance, Outcome 1: Default rates

Comparison 7: Histology at 24 months: immediate colposcopy versus cytological surveillance, LSIL/mild dyskaryosis only, Outcome 1: CIN incidence at 24 months, after LSIL/mild dyskaryosis at baseline

Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 1: Presence of CIN2 in histology at 24 months

Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 2: Presence of CIN2+ in histology at 24 months

Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 3: Presence of CIN3+ in histology at 24 months
| Immediate colposcopy compared with cytological surveillance for minor cervical cytological abnormalities: occurrence of different grades CIN in histology according to follow‐up time and default rates | ||||||
| Patient or population: women with ASCUS or LSIL Settings: colposcopy clinic Intervention: immediate colposcopy Comparison: cytological surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Risk with cytological surveillance | Risk with immediate colposcopy | |||||
| Occurrence of CIN2+ in histology at 18 months | 101 per 1000 | 151 per 1000 | RR 1.50 (1.12 to 2.01) | 4028 | ⊕⊕⊕⊝ | |
| Occurrence of CIN2+ in histology at 24 months | 183 per 1000 | 209 per 1000 | RR 1.14 (0.66 to 1.97) | 4331 | ⊕⊕⊝⊝ | |
| Occurrence of CIN3+ in histology at 18 months | 69 per 1000 | 86 per 1000 | RR 1.24 (0.77 to 1.98) | 4028 | ⊕⊕⊕⊝ | |
| Occurrence of CIN3+ in histology at 24 months | 119 per 1000 | 121 per 1000 | RR 1.02 (0.53 to 1.97) | 4331 | ⊕⊕⊝⊝ | |
| Occurrence of any CIN in histology at 24 months | 316 per 1000 | 639 per 1000 | RR 2.02 (1.33 to 3.08) | 656 | ⊕⊕⊝⊝ | |
| Default rates at 6 months | 63 per 1000 | 241 per 1000 | RR 3.85 | 5117 | ⊕⊕⊕⊝ moderate6 | |
| Default rates at 12 months | 63 per 1000 | 413 per 1000 | RR 6.60 | 5115 | ⊕⊕⊕⊝ moderate7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). For default rates the relative effect is calculated between cytological surveillance versus immediate colposcopy. For histology the relative effect is calculated between immediate colposcopy versus cytological surveillance. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded to moderate due to substantial inter‐study heterogeneity (P = 0.08, I2= 61%). | ||||||
| Study | Outcomes | Immediate colposcopy n/N (%) | Cytological surveillance n/N (%) | RR + 95% CI |
| ALTS 2003(ASCUS) | Histology at 18 monthsa | |||
| CIN 2 | 61/1163 (5.2) | 26/1164 (2.2) | 2.35 [1.49, 3.69] | |
| CIN 2+ | 119/1163 (10.2) | 92/1164 (7.9) | 1.29 [1.00, 1.68] | |
| CIN 3+ | 58/1163 (5.0) | 66/1164 (5.7) | 0.88 [0.62, 1.24] | |
| Histology at 24 months | ||||
| CIN 2 | 61/1163 (5.2) | 60/1164 (5.2) | 1.02 [0.72,1.44] | |
| CIN 2+ | 119/1163 (10.2) | 168/1164 (14.4) | 0.71 [0.57, 0.88] | |
| CIN 3+ | 58/1163 (5.0) | 108/1164 (9.3) | 0.54 [0.39, 0.73] | |
| Default rates: | ||||
| Default rate at 24 months | 15/1163 (1.3) | 165/1164 (14.2) | 10.99 [6.52, 18.53] | |
| ALTS 2003(LSIL) | Histology at 18 monthsa | |||
| CIN 2 | 63/673 (9.4) | 36/675 (5.3) | 1.76 [1.18, 2.61] | |
| CIN 2+ | 127/673 (18.9) | 95/675 (14.1) | 1.34 [1.05, 1.71] | |
| CIN 3+ | 64/673 (9.5) | 59/675 (8.7) | 1.09 [0.78, 1.52] | |
| Histology at 24 months | ||||
| CIN 2 | 63/673 (9.4) | 58/675 (8.6) | 1.09 [0.78,1.53] | |
| CIN 2+ | 127/673 (18.9) | 151/675 (22.4) | 0.84 [0.68, 1.04] | |
| CIN 3+ | 64/673 (9.5) | 93/675 (13.8) | 0.69 [0.51, 0.93] | |
| Default rates: | ||||
| Default rate at 24 months | 4/673 (0.6) | 110/675 (16.3) | 27.42 [10.17, 73.93] | |
| Histology at 6 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 28/160 (17.5) | 0.52 [0.28, 0.95] | |
| Any CIN | 121/145 (83.4) | 86/160 (53.8) | 1.55 [1.32, 1.82] | |
| CIN 1 | 23/145 (15.9) | 27/160 (16.9) | 0.94 [0.57, 1.56] | |
| CIN 2 | 32/145 (22.1) | 26/160 (16.3) | 1.36 [0.85, 2.16] | |
| CIN 2+ | 98/145 (67.6) | 59/160 (36.9) | 1.83 [1.45, 2.31] | |
| CIN 3+ | 66/145 (45.5) | 33/160 (20.6) | 2.21 [1.55, 3.14] | |
| Histology at 12 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 18/158 (11.4) | 0.79 [0.40, 1.55] | |
| Any CIN | 121/145 (83.4) | 96/158 (60.8) | 1.37 [1.19, 1.59] | |
| CIN 1 | 23/145 (15.9) | 25/158 (15.8) | 1.00 [0.60, 1.69] | |
| CIN 2 | 32/145 (22.1) | 26/158 (16.5) | 1.34 [0.84, 2.14] | |
| CIN 1 / 2 | 55/145 (37.9) | 51/158 (32.3) | 1.18 [0.86, 1.60] | |
| CIN 2+ | 98/145 (67.6) | 71/158 (44.9) | 1.50 [1.22, 1.85] | |
| CIN 3+ | 66/145 (45.5) | 45/158 (28.5) | 1.60 [1.18, 2.17] | |
| Histology at 24 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 11/158 (7.0) | 1.29 [0.60, 2.78] | |
| Any CIN | 121/145 (83.4) | 53/158 (33.5) | 2.49 [1.97, 3.13] | |
| CIN 1 | 23/145 (15.9) | 9/158 (5.7) | 2.78 [1.33, 5.82] | |
| CIN 2 | 32/145 (22.1) | 12/158 (7.6) | 2.91 [1.56, 5.42] | |
| CIN 2+ | 98/145 (67.6) | 44/158 (27.8) | 2.43 [1.84, 3.20] | |
| CIN 3+ | 66/145 (45.5) | 32/158 (20.3) | 2.25 [1.57, 3.21] | |
| Default rates: | ||||
| Default rate at 6 months | 0/145 (0) | 19/160 (11.9) | 35.37 [2.15, 580.52] | |
| Default rate at 12 months | 0/145 (0) | 23/158 (14.6) | 43.16 [2.65, 704.13] | |
| Default rate at 24 months | 0/145 (0) | 38/158 (24.1) | 70.70 [4.38, 1140.47] | |
| Histology at 12 months | ||||
| Any CIN | 83/130 (63.8) | 71/243 (29.2) | 2.19 [1.73, 2.76] | |
| CIN 1 / 2 | 61/130 (46.9) | 47/243 (19.3) | 2.43 [1.77, 3.32] | |
| CIN 3+ | 22/130 (16.9) | 24/243 (9.9) | 1.71 [1.00, 2.93] | |
| Default rates: | ||||
| Default rate at 6 months | 5/130 (3.8) | 46/243 (18.9) | 4.92 [2.01, 12.08] | |
| Default rate at 12 months | 5/130 (3.8) | 95/243 (39.1) | 10.16 [4.24, 24.35] | |
| GHQ casenessb | Choicec | No choicec | ||
| Baseline | 134/233 (58) | 119/241 (49) | 1.16 [0.98, 1.38] | |
| 6 months (pre visit) | 71/183 (39) | 77/190 (41) | 0.96 [0.75, 1.23] | |
| 6 months (post visit) | 59/175 (34) | 66/177 (37) | 0.90 [0.68, 1.20] | |
| 12 months | 40/135 (29) | 35/127 (28) | 1.08 [0.73, 1.58] | |
| Histology at 18 monthsa | ||||
| CIN 2 | 8/182(4.4) | 2/171 (1.1) | 3.76 [0.81, 17.45] | |
| CIN 2+ | 43/182 (23.6) | 16/171 (9.4) | 2.53 [1.48, 4.31] | |
| CIN 3+ | 35/182 (19.2) | 14/171(8.2) | 2.35 [1.31, 2.45] | |
| Histology at 24 months | ||||
| HPV / Koilocytic atypia | 92/182 (50.5) | 57/171 (33.3) | 1.52 [1.17, 1.96] | |
| Any CIN | 88/182 (48.4) | 51/171 (29.8) | 1.62 [1.23, 2.13] | |
| CIN 1 | 45/182 (24.7) | 17/171 (9.9) | 2.49 [1.48, 4.17] | |
| CIN 2 | 8/182 (4.4) | 10/171 (5.8) | 0.75 [0.30, 1.86] | |
| CIN 2+ | 43/182 (23.6) | 34/171 (19.9) | 1.19 [0.80, 1.77] | |
| CIN 3+ | 35/182 (19.2) | 24/171 (14.0) | 1.37 [0.85, 2.20] | |
| Default rates: | ||||
| Default rate at 24 months | 1/182 (0.5) | 36/171 (21.1) | 38.32 [5.31, 276.40] | |
| Tombola 2009 | Histology at 30 monthsa | |||
| CIN 2 | 181/2216 (8.2) | 101/2223 (4.5) | 1.80 [1.42, 2.28] | |
| CIN 2+ | 369/2216 (16.7) | 269/2223 (12.1) | 1.38 [1.19, 1.59] | |
| CIN 3+ | 188/2216 (8.5) | 168/2223 (7.6) | 1.12 [0.92, 1.37] | |
| Histology at 36 months | ||||
| CIN 2 | 181/2216 (8.2) | 157/2223 (7.1) | 1.16 [0.94, 1.42] | |
| CIN 2+ | 369/2216 (16.7) | 350/2223 (15.7) | 1.06 [0.93, 1.21] | |
| CIN 3+ | 188/2216 (8.5) | 193/2223 (8.7) | 0.98 [0.81, 1.18] | |
| Default rates: | ||||
| Default rate at 6 months | 151/2216 (6.8) | 285/2223 (12.8) | 1.88 [1.56, 2.27] | |
| Default rate at 12 months | 151/2216 (6.8) | 327/2223 (14.7) | 2.16 [1.80, 2.59] | |
| Paind | ||||
| Any pain | 304/782 (38.9) | 145/968 (15.0) | 2.60 [2.18, 3.09] | |
| Moderate or more severe | 144/774 (18.6) | 56/965 (5.8) | 3.21 [2.39, 4.30] | |
| Bleedingd | ||||
| Any bleeding | 366/781 (46.9) | 166/967 (17.2) | 2.73 [2.33, 3.19] | |
| Moderate or more severe | 144/772 (18.6) | 16/961 (1.7) | 11.20 [6.74, 18.61] | |
| Discharged | ||||
| Any discharge | 267/780 (34.2) | 83/964 (8.6) | 3.98 [3.17, 4.99] | |
| Moderate or more severe | 133/777 (17.1) | 36/962 (3.7) | 4.57 [3.20, 6.53] | |
| Anxietye | ||||
| 6 weeks | 59/751 (7.9) | 121/900 (13.4) | 0.58 [0.43, 0.79] | |
| 12 months | 190/1161 (16.4) | 218/1130 (19.3) | 0.85 [0.71, 1.01] | |
| 18 months | 162/1050 (15.4) | 177/1008 (17.6) | 0.88 [0.72, 1.07] | |
| 24 months | 179/1001 (17.9) | 177/962 (18.4) | 0.97 [0.81, 1.17] | |
| 30 months | 146/949 (15.4) | 143/887 (16.1) | 0.95 [0.77, 1.18] | |
| Depressionf | ||||
| 6 weeks | 50/757 (6.6) | 68/902 (7.5) | 0.88 [0.62, 1.25] | |
| 12 months | 110/1162 (9.5) | 132/1136 (11.6) | 0.81 [0.64, 1.04] | |
| 18 months | 106/1052 (10.1) | 114/1016 (11.2) | 0.90 [0.70, 1.15] | |
| 24 months | 111/1001 (11.1) | 104/964 (10.8) | 1.03 [0.80, 1.32] | |
| 30 months | 101/948 (10.7) | 108/887 (12.2) | 0.88 [0.68, 1.13] | |
| For Immediate colposcopy, n = n at immediate colposcopy visit, possible follow‐up excluded. a Cumulative incidence during follow‐up, excluding the exit examination or deferred treatment. b GHQ caseness = GHQ (General Health Questionnaire) score ≥ 4. c Analysis for this outcome between the original randomization groups. d Based on Questionnaire 6 weeks after immediate colposcopy or first cytological surveillance visit. e ≥ 11 on hospital anxiety and depression anxiety subscale f ≥ 8 on hospital anxiety and depression subscale | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Occurrence of CIN2+ Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 1.50 [1.12, 2.01] |
| 1.1.2 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.66, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Occurrence of CIN3+ Show forest plot | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 12 months' surveillance | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 2.07 [1.54, 2.79] |
| 2.1.2 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.77, 1.98] |
| 2.1.3 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Presence of any CIN in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.09, 2.70] |
| 3.2 Presence of CIN1/2 in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.83, 3.43] |
| 3.3 Presence of CIN3+ in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.63 [1.25, 2.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Histology at 24 months Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 HPV/Koilocytic atypia | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.17, 1.90] |
| 4.1.2 Any CIN | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 2.02 [1.33, 3.08] |
| 4.1.3 CIN1 | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 2.58 [1.69, 3.94] |
| 4.1.4 CIN2 | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.80, 1.96] |
| 4.1.5 CIN2+ | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.66, 1.97] |
| 4.1.6 CIN3+ | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Occurrence of CIN2 Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 2.04 [1.52, 2.73] |
| 5.1.2 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.45 [0.87, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Default rates Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 Default rates at 6 months | 3 | 5117 | Risk Ratio (IV, Random, 95% CI) | 3.85 [1.27, 11.63] |
| 6.1.2 Default rates at 12 months | 3 | 5115 | Risk Ratio (IV, Random, 95% CI) | 6.60 [1.49, 29.29] |
| 6.1.3 Default rates at 24 months | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 19.10 [9.02, 40.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 CIN incidence at 24 months, after LSIL/mild dyskaryosis at baseline Show forest plot | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.1.1 CIN2 incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.66, 4.48] |
| 7.1.2 CIN2+ incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.51, 4.01] |
| 7.1.3 CIN3+ incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.39, 3.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Presence of CIN2 in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.41, 5.78] |
| 8.2 Presence of CIN2+ in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.86, 3.47] |
| 8.3 Presence of CIN3+ in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.11, 2.92] |

