Antibióticos para las sibilancias o la tos persistente después de la bronquiolitis aguda en niños
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Ir a:
| Methods | Double‐blind, placebo‐controlled, parallel‐group, randomised study | |
| Participants | Inclusion criteria:
Exclusion criteria:
Number screened: not stated Number refused: not stated Number randomised: total n = 30 (intervention group n = 15 and control group n = 15) | |
| Interventions | Oral clarithromycin (15 mg/kg/d) or placebo (15 mg/kg/d) daily for 3 weeks | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Single site Small sample size High attrition Funding: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation provided. Paper describes that participants were "randomised by a single study nurse" |
| Allocation concealment (selection bias) | Unclear risk | No information provided on how the study nurse randomised each participant and how concealment was maintained |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and families remained blinded to randomisation until end of study |
| Blinding of outcome assessment (detection bias) | Low risk | Paper described that investigators remained blinded to randomisation until end of study |
| Incomplete outcome data (attrition bias) | Unclear risk | 30 participants were enrolled and randomised. During the study phase, 9 were excluded (30%) because they had received corticosteroids. Groups and outcomes of these infants were not reported |
| Selective reporting (reporting bias) | Unclear risk | The results table appears to show analysis including only children remaining in the trial (n = 21). ITT was not used |
| Other bias | Unclear risk | It is unclear whether the trial was registered, and if outcomes were selected a priori |
| Methods | Multi‐centre, double‐blinded, placebo‐controlled, parallel‐group, randomised study | |
| Participants | Inclusion criteria:
Exclusion criteria:
Number screened: n = 698 (n = 479 excluded for various reasons) Number refused: n = 89 Number randomised: total n = 219 (intervention group n = 106 and control group n = 113) | |
| Interventions | Oral azithromycin (30 mg/kg once weekly) or placebo (30 mg/kg once weekly) for 3 weeks | |
| Outcomes | Primary outcomes:
Secondary outcomes:
| |
| Notes | International RCT between Australia and New Zealand RCT was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN1261000036099) Funding: National Health and Medical Research Council (605809) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician used a computer‐generated, permuted block design to generate randomisation sequences |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes concealed treatment allocation |
| Blinding of participants and personnel (performance bias) | Low risk | All participants, family, assessor, and hospital staff remained blinded during the study |
| Blinding of outcome assessment (detection bias) | Low risk | Neither the study team (researchers hospital staff) nor parents were aware of assigned treatment groups until data analysis was completed |
| Incomplete outcome data (attrition bias) | Low risk | 219 participants were randomised and intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | All participants were analysed as 'intention‐to‐treat' |
| Other bias | Low risk | The trial was registered. Details of adverse events were reported ‐ none of the participants discontinued the trial |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| In this RCT, treatment occurred during the acute phase of bronchiolitis (e.g. ≤ 14 days) | |
| This open randomised trial included children up to 62 months of age with a diagnosis of pneumonia; treatment provided up to 6 days did not include use of antibiotics beyond the acute period | |
| In this RCT, the treatment period lasted only up to 7 days | |
| In this RCT, the treatment period lasted only up to 3 days | |
| Length of treatment period for this RCT is not clear. Analysis describes up to 5 days, thus not eligible for post‐acute bronchiolitis | |
| In this RCT, treatment was provided during the acute phase of bronchiolitis (i.e. ≤ 14 days) | |
| In this RCT, treatment was provided during the acute phase of bronchiolitis (i.e. ≤ 14 days) |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
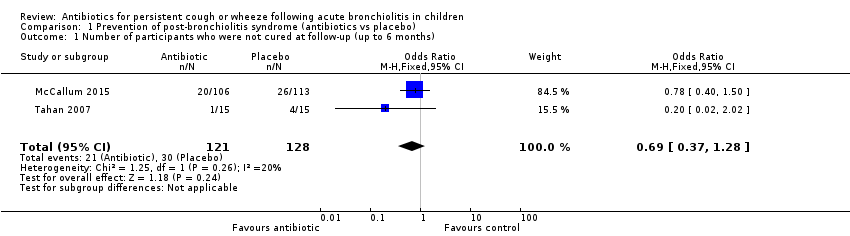
| 1 Number of participants who were not cured at follow‐up (up to 6 months) Show forest plot | 2 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.37, 1.28] |
| Analysis 1.1  Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 1 Number of participants who were not cured at follow‐up (up to 6 months). | ||||
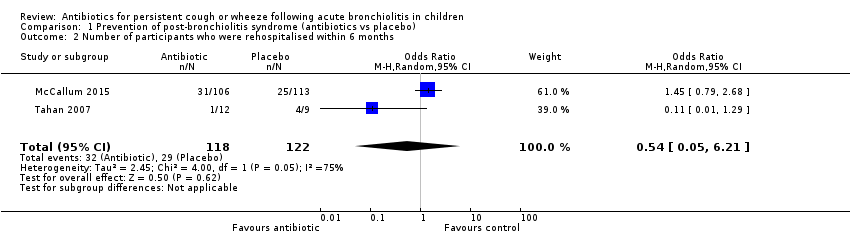
| 2 Number of participants who were rehospitalised within 6 months Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.21] |
| Analysis 1.2 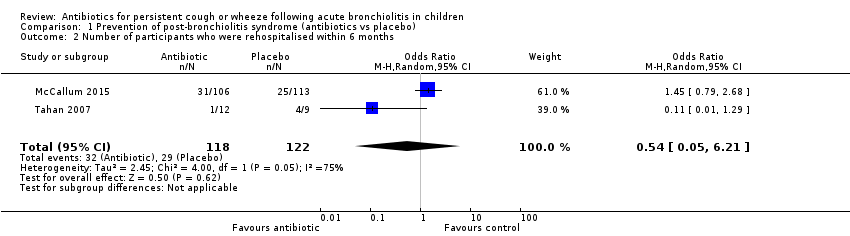 Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 2 Number of participants who were rehospitalised within 6 months. | ||||
| 3 Proportion of participants with wheeze (within 6 months of intervention) Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.95] |
| Analysis 1.3  Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 3 Proportion of participants with wheeze (within 6 months of intervention). | ||||

Study flow diagram.
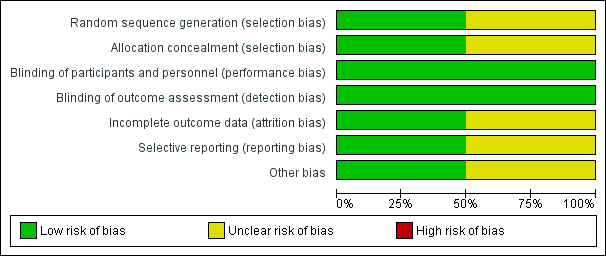
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.1 Number of participants who were not cured at follow‐up (up to 6 months).

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.2 Number of participants who were rehospitalised within 6 months.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.3 Proportion of participants with wheeze (within 6 months of intervention).
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 1 Number of participants who were not cured at follow‐up (up to 6 months).
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 2 Number of participants who were rehospitalised within 6 months.

Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 3 Proportion of participants with wheeze (within 6 months of intervention).
| Antibiotics compared with placebo or no treatment for persistent respiratory symptoms following acute bronchiolitis | ||||||
| Patient or population: children < 24 months with persistent respiratory symptoms following acute bronchiolitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with antibiotics | |||||
| Number of participants who were not cured at follow‐up | 234 per 1000 | 174 per 1000 | OR 0.69 | 249 | ⊕⊕⊝⊝ | |
| Number of participants rehospitalised for a respiratory illness | 238 per 1000 | 271 per 1000 | OR 1.19 | 240 | ⊕⊕⊝⊝ | |
| Proportion of participants with recurrent wheeze | 123 per 1000 | 99 per 1000 | OR 0.47 (0.06 to 3.95) | 240 | ⊕⊕⊝⊝ | |
| ^Intervention/Comparison group: treatment initiated during child's hospitalisation for acute bronchiolitis *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| We used GRADEPro software to create this table (GRADEpro GDT 2015) GRADE Working Group grades of evidence | ||||||
| aQuality downgraded because of small numbers of studies and participants and high attrition in the Tahan study. Hence, we cannot be confident of the effect estimate | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who were not cured at follow‐up (up to 6 months) Show forest plot | 2 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.37, 1.28] |
| 2 Number of participants who were rehospitalised within 6 months Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.21] |
| 3 Proportion of participants with wheeze (within 6 months of intervention) Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.95] |

