Antibióticos para las sibilancias o la tos persistente después de la bronquiolitis aguda en niños
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009834.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
GBM and ABC wrote the protocol. PSM reviewed the protocol. For the 2012 review, GBM and ABC, and for the 2016 search, GBM and EJP, independently reviewed the search, double‐entered data, and wrote the manuscript. ABC amended the manuscript, and PSM reviewed the manuscript.
Sources of support
Internal sources
-
The authors declare that no such funding was received for this systematic review, Other.
External sources
-
National Health Medical Research Council, Australia.
Salary support for GBM, early career fellowship, grant number 1111705
-
National Health Medical Research Council, Australia.
Salary support for ABC, practitioner fellowship, grant number 1058213
-
NHMRC Centre of Research Excellence Grant, Australia.
CRE in respiratory health in Aboriginal and Torres Strait Islander children, grant number 1040830
Declarations of interest
Three review authors (GBM, PSM, and ABC) were authors of the McCallum 2015 paper. Review author EJP, who was not involved in the McCallum 2015 trial, extracted data for this review.
Acknowledgements
We thank Dr. Chris Cates, Dr. Emma Dennett, Jessica Thomas, and Emma Jackson for support for the protocol and the review. We also thank Elizabeth Stovold from the Cochrane Airways Group for performing the searches.
Dr. Chris Cates served as the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 22 | Antibiotics for persistent cough or wheeze following acute bronchiolitis in children | Review | Gabrielle B McCallum, Erin J Plumb, Peter S Morris, Anne B Chang | |
| 2012 Dec 12 | Antibiotics for persistent cough or wheeze following acute bronchiolitis in children | Review | Gabrielle B McCallum, Peter S Morris, Anne B Chang | |
| 2012 Jun 13 | Antibiotics for persistent cough or wheeze following acute bronchiolitis in children | Protocol | Gabrielle B McCallum, Peter S Morris, Anne B Chang | |
Differences between protocol and review
The previous review used a hierarchy of assessment and was difficult to read. For this review update, instead of using a hierarchy of assessment, we modified primary and secondary outcomes for the purpose of providing clarity. We included an additional primary outcome for exacerbation rate and moved adverse events and bacterial resistance to secondary outcomes. We updated the methods to reflect current reporting, which included adding a risk of bias tool and an SOF table. For this review update, we planned but did not perform three sensitivity analyses, which we will likely perform in the future; we updated the inclusion criteria to make them more applicable to the primary question. Using a random‐effects model (when we detected heterogeneity), we performed our analysis by treatment received.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Disease;
- Anti‐Bacterial Agents [*therapeutic use];
- Bronchiolitis [*complications, virology];
- Clarithromycin [*therapeutic use];
- Cough [*drug therapy, etiology];
- Randomized Controlled Trials as Topic;
- Respiratory Sounds [*drug effects, etiology];
- Respiratory Syncytial Virus Infections [complications, *drug therapy];
Medical Subject Headings Check Words
Humans; Infant;
PICO

Study flow diagram.
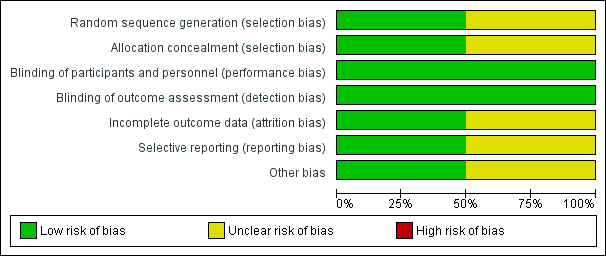
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.1 Number of participants who were not cured at follow‐up (up to 6 months).

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.2 Number of participants who were rehospitalised within 6 months.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.3 Proportion of participants with wheeze (within 6 months of intervention).
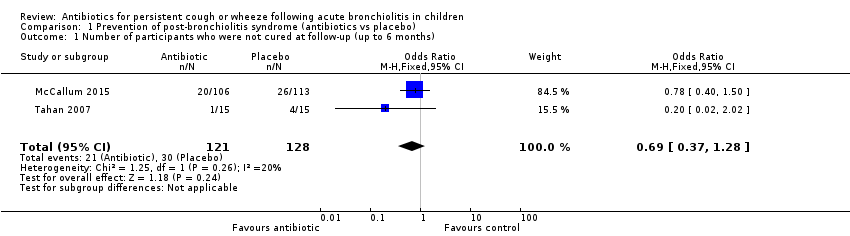
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 1 Number of participants who were not cured at follow‐up (up to 6 months).
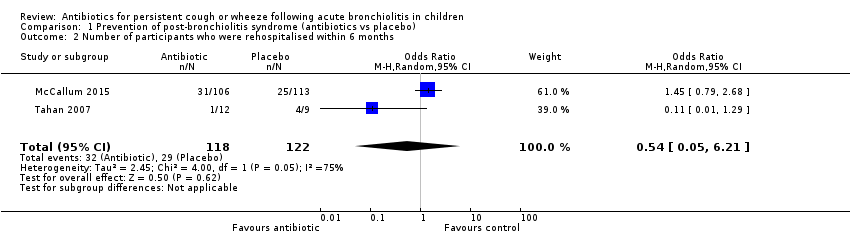
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 2 Number of participants who were rehospitalised within 6 months.

Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 3 Proportion of participants with wheeze (within 6 months of intervention).
| Antibiotics compared with placebo or no treatment for persistent respiratory symptoms following acute bronchiolitis | ||||||
| Patient or population: children < 24 months with persistent respiratory symptoms following acute bronchiolitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with antibiotics | |||||
| Number of participants who were not cured at follow‐up | 234 per 1000 | 174 per 1000 | OR 0.69 | 249 | ⊕⊕⊝⊝ | |
| Number of participants rehospitalised for a respiratory illness | 238 per 1000 | 271 per 1000 | OR 1.19 | 240 | ⊕⊕⊝⊝ | |
| Proportion of participants with recurrent wheeze | 123 per 1000 | 99 per 1000 | OR 0.47 (0.06 to 3.95) | 240 | ⊕⊕⊝⊝ | |
| ^Intervention/Comparison group: treatment initiated during child's hospitalisation for acute bronchiolitis *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| We used GRADEPro software to create this table (GRADEpro GDT 2015) GRADE Working Group grades of evidence | ||||||
| aQuality downgraded because of small numbers of studies and participants and high attrition in the Tahan study. Hence, we cannot be confident of the effect estimate | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who were not cured at follow‐up (up to 6 months) Show forest plot | 2 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.37, 1.28] |
| 2 Number of participants who were rehospitalised within 6 months Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.21] |
| 3 Proportion of participants with wheeze (within 6 months of intervention) Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.95] |

