Prostatectomía radical laparoscópica y asistida por robot versus abierta para el tratamiento del cáncer de próstata localizado
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009625.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 12 septiembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Urología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Dragan Ilic initiated the review and wrote the initial protocol. He conducted the literature search, reviewed abstracts and full‐text studies for inclusion, performed quality assessment, data extraction, analysis, and wrote the review. Guarantor of the review.
Sue Evans wrote the initial protocol. She conducted the literature search, reviewed abstracts and full‐text studies for inclusion, performed analysis, and wrote the review.
Christie Allan reviewed abstracts and full‐text studies for inclusion, performed quality assessment, and contributed to the writing of the review.
Jae Hung Jung performed quality assessment, data extraction, analysis and contributed to the writing of the review.
Declan Murphy wrote the initial protocol. He contributed to the data analysis and writing of the review.
Mark Frydenberg wrote the initial protocol. He contributed to the data analysis and writing of the review.
Sources of support
Internal sources
-
Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Australia.
-
Centre of Research Excellence in Patient Safety, School of Public Health and Preventive Medicine, Monash University, Australia.
-
Department of Urology, Yonsei University Wonju College of Medicine, Korea, South.
-
Cancer Surgery, Peter MacCallum Cancer Centre, Australia.
-
Department of Surgery, Monash University, Australia.
External sources
-
None, Other.
Declarations of interest
Dragan Ilic: none declared
Sue Evans: none declared
Christie Allan: none declared
Jae Hung Jung: none declared
Declan Murphy: none declared
Mark Frydenberg: none declared
Acknowledgements
We would like to thank the peer reviewers and Cochrane Urology for their comments and suggestions in writing this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Sep 12 | Laparoscopic and robotic‐assisted versus open radical prostatectomy for the treatment of localised prostate cancer | Review | Dragan Ilic, Sue M Evans, Christie Ann Allan, Jae Hung Jung, Declan Murphy, Mark Frydenberg | |
| 2012 Feb 15 | Laparoscopic versus open prostatectomy for the treatment of localised prostate cancer | Protocol | Dragan Ilic, Sue Evans, Declan Murphy, Mark Frydenberg | |
Differences between protocol and review
-
In the original protocol (Ilic 2012) we had stated that non‐RCTs would be eligible for inclusion in the review to examine secondary objectives. It was also stated that only articles in English would be considered eligible for inclusion. Disease‐specific and general quality of life was originally listed as a secondary outcome but have been moved up to be primary outcomes. The search strategy for non‐RCTs has been removed from the review. The title has been amended from the original title in the protocol. All these changes were made after discussion and with the formal agreement of Cochrane Urology.
-
We have described experimental interventions and control in greater detail than before.
-
We further specified the methods of measurement of primary and secondary outcomes in addition to the description of main outcomes for 'Summary of findings' tab
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Laparoscopy [adverse effects, *methods];
- Organ Size;
- Prostate [pathology];
- Prostate‐Specific Antigen [blood];
- Prostatectomy [adverse effects, *methods];
- Prostatic Neoplasms [blood, mortality, pathology, *surgery];
- Quality of Life;
- Randomized Controlled Trials as Topic;
- Robotic Surgical Procedures [adverse effects, *methods];
- Sexual Behavior;
- Urination;
Medical Subject Headings Check Words
Humans; Male; Middle Aged;
PICO

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 1 Urinary QoL (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 2 Sexual QoL (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 3 Surgical complications (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 4 Serious postoperative complications (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 5 Postoperative pain (at 1 day).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 6 Postoperative pain (at 1 week).
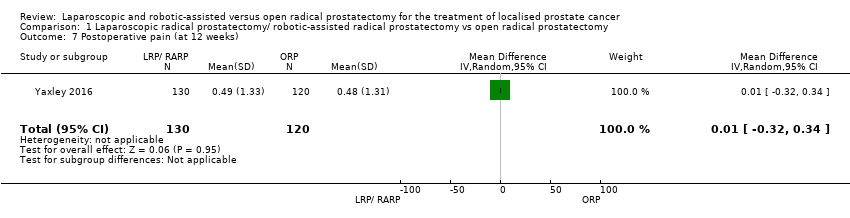
Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 7 Postoperative pain (at 12 weeks).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 8 Hospital stay (short term ).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 9 Blood transfusion (short term).
| Laparaoscopic radical prostatectomy and robotic‐assisted laparoscopic radical prostatectomy compared to open radical prostatectomy for the treatment of localised prostate cancer | |||||
| Participants: men with prostate cancer Setting: single surgeon or single centre Intervention: laparoscopic radical prostatectomy/robotic‐assisted laparoscopic radical prostatectomy Control: open radical prostatectomy | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with ORP | Risk difference with LRP/RARP | ||||
| Prostate cancer‐specific survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Urinary quality of life (short‐term) | 248 | ⊕⊕⊕⊝ | ‐ | The mean score was 83.8 | MD 1.3 lower |
| Sexual quality of life (short‐term) | 248 | ⊕⊕⊕⊝ | ‐ | The mean score was 35.0 | MD 3.9 higher |
| Biochemical recurrence‐free survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Overall survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Overall surgical complications (short‐term) | 308 | ⊕⊕⊝⊝ | RR 0.41 | Study population | |
| 40 per 1000 | 23 fewer per 1000 | ||||
| Moderate | |||||
| 238 per 1000d | 140 fewer per 1000 (200 fewer to 10 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Expanded Prostate Cancer Index Composite contains five symptom domains (urinary incontinence, urinary irritative/obstructive, sexual, bowel, hormonal), scored from 0 (worst) to 100 (best). b Downgraded by one level for study limitation: unclear risk or high risk of one or more domains in included study or studies. d Estimates for control event rates for surgical complications come from Gandaglia 2014. | |||||
| Study name | Trial | Country | Setting | Description of participants | Intervention(s) | Duration of | Age (yrs) | PSA (ng/mL) | Pathologic stage |
| NR | Italy | Single surgeon | Men aged < 70 years, clinically organ‐confined disease (cT1 ‐ cT2), total serum PSA < 20 ng/dL, Gleason score ≤ 7 | LRP | 6 days | 62.29 ± 8.2 | 6.9 ± 2.9 | T2 (75.0%), T3 (25%), surgical margin positive (26.0%) | |
| ORP | 62.9 ± 7.4 | 6.5 ± 3.0 | T2 (73.3%), T3 (26.6%), surgical margin positive (21.6%) | ||||||
| 2010 to 2014 | Australia | Single centre | Men aged 35‐70 years with newly diagnosed with clinically localised prostate cancer | RARP | 12 weeks | 59.64 ± 6.63 | 7.41 ± 4.10 | Extraprostatic extension (35%), seminal vesicle involvement (3%), surgical margins positive (15%) | |
| ORP | 60.38 ± 5.81 | 7.57 ± 4.07 | Extraprostatic extension (32%), seminal vesicle involvement (6%), surgical margins positive (10%) | ||||||
| LRP: laparoscopic prostatectomy; NR: not reported; ORP: open radical prostatectomy; PSA: prostate‐specific antigen; RARP: robotic‐assisted radical prostatectomy | |||||||||
| Study name | Intervention(s) and comparator(s) | Sample size (N) | Screened/ eligible (N) | Randomised (N) | Analysed (N) | Finishing trial (N (%)) |
| LRP | NR | NR | 60 | 60 | 60 (100.0) | |
| ORP | NR | 60 | 60 | 60 (100.0) | ||
| Total | 120 | 120 | 120 (100.0) | |||
| RARP | 200 | NR/334 | 163 | QoL: 129 Surgical outcomes: 157a Pain: 130 | 157 (96.3) | |
| ORP | 200 | 163 | QoL: 119 Surgical outcomes: 151a Pain: 120 | 151 (92.6) | ||
| Total | 326 | QoL: 248 Surgical outcomes: 308 Pain: 250 | 308 (94.4) | |||
| Grand total | All interventions | 223 | 217 | |||
| All comparators | 223 | 211 | ||||
| Overall | 446 | 428 | ||||
| aSurgical outcomes: surgical complications, hospital stay, and blood transfusions LRP: laparoscopic prostatectomy; NR: not reported; ORP: open radical prostatectomy; QoL: quality of life; RARP: robotic‐assisted radical prostatectomy | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urinary QoL (short term) Show forest plot | 1 | 248 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐4.65, 2.05] |
| 2 Sexual QoL (short term) Show forest plot | 1 | 248 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐1.84, 9.64] |
| 3 Surgical complications (short term) Show forest plot | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.04] |
| 4 Serious postoperative complications (short term) Show forest plot | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.32] |
| 5 Postoperative pain (at 1 day) Show forest plot | 2 | 423 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.42, ‐0.68] |
| 6 Postoperative pain (at 1 week) Show forest plot | 2 | 416 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.40, ‐0.17] |
| 7 Postoperative pain (at 12 weeks) Show forest plot | 1 | 250 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.32, 0.34] |
| 8 Hospital stay (short term ) Show forest plot | 1 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐2.19, ‐1.25] |
| 9 Blood transfusion (short term) Show forest plot | 2 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.12, 0.46] |

