Prostatectomía radical laparoscópica y asistida por robot versus abierta para el tratamiento del cáncer de próstata localizado
Resumen
Antecedentes
El cáncer de próstata se diagnostica con frecuencia en hombres de todo el mundo. La cirugía, en forma de prostatectomía radical, es uno de los procederes principales de tratamiento para los pacientes con cáncer de próstata localizado. De forma tradicional la prostatectomía se ha realizado como una cirugía abierta, generalmente a través de un abordaje retropúbico. El advenimiento de los abordajes laparoscópicos, que incluyen los asistidos por robot, proporciona una opción mínimamente invasiva con respecto a la prostatectomía radical abierta (PRA).
Objetivos
Evaluar los efectos de la prostatectomía radical laparoscópica o la prostatectomía radical asistida por robot en comparación con la prostatectomía radical abierta en pacientes con cáncer de próstata localizado.
Métodos de búsqueda
Se realizó una búsqueda exhaustiva utilizando múltiples bases de datos (CENTRAL, MEDLINE, EMBASE) y actas de congresos sin restricciones de idioma de publicación o estado de publicación, hasta el 9 junio 2017. También se realizaron búsquedas bibliográficas de estudios incluidos y actas de congresos.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios (ECA) con una comparación directa de la prostatectomía radical laparoscópica (PRL) y la prostatectomía radical asistida por robot (PRAR) con la PRA, incluidos los ensayos controlados cuasialeatorios.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente clasificaron los estudios y resumieron los datos. Los resultados primarios fueron: la supervivencia asociada con el cáncer de próstata, la calidad de vida relacionada con la función urinaria y la calidad de vida relacionada con la función sexual. Los resultados secundarios fueron la supervivencia sin recidiva bioquímica, la supervivencia general, las complicaciones quirúrgicas generales, las complicaciones quirúrgicas posoperatorias graves, el dolor posoperatorio, la estancia hospitalaria y las transfusiones de sangre. Para realizar los análisis estadísticos se utilizó un modelo de efectos aleatorios y la calidad de la evidencia se evaluó según GRADE.
Resultados principales
Se incluyeron dos estudios únicos con 446 participantes asignados al azar que presentaban cáncer de próstata clínicamente localizado. La media de la edad, el volumen de la próstata y el antígeno específico de la próstata (PSA) de los participantes fueron 61,3 años, 49,78 ml y 7,09 ng/ml, respectivamente.
Medidas de resultado principales
No se encontraron estudios que analizaran el resultado supervivencia asociada con el cáncer de próstata. Según los datos de un ensayo, la PRAR probablemente da lugar a poca a ninguna diferencia en la calidad de vida relacionada con la función urinaria (DM ‐1,30; IC del 95%: ‐4,65 a 2,05) y la calidad de vida relacionada con la función sexual (DM 3,90; IC del 95%: ‐1,84 a 9,64). La calidad de la evidencia se consideró moderada para ambos resultados de calidad de vida y se disminuyó por las limitaciones del estudio.
Medidas de resultado secundarias
No se encontraron estudios que analizaran los resultados supervivencia sin recidiva bioquímica y supervivencia general.
Según un ensayo, la PRAR puede dar lugar a poca a ninguna diferencia en las complicaciones quirúrgicas generales (CR 0,41; IC del 95%: 0,16 a 1,04) o en las complicaciones posoperatorias graves (CR 0,16; IC del 95%: 0,02 a 1,32). La calidad de la evidencia se consideró baja para ambas complicaciones quirúrgicas y se disminuyó por las limitaciones del estudio y la imprecisión.
Según dos estudios, la PRL o la PRAR pueden dar lugar a una mejoría pequeña, posiblemente no importante, en el dolor posoperatorio el primer día (DM ‐1,05; IC del 95%: ‐1,42 a ‐0,68) y hasta una semana (DM ‐0,78; IC del 95%: ‐1,40 a ‐0,17). La calidad de la evidencia se consideró baja para ambos puntos temporales y se disminuyó por las limitaciones de los estudios y la imprecisión. Según un estudio, la PRAR probablemente da lugar a poca a ninguna diferencia en el dolor posoperatorio a las 12 semanas (DM 0,01; IC del 95%: ‐0,32 a 0,34). La calidad de la evidencia se consideró moderada y se disminuyó por las limitaciones del estudio.
Según un estudio, es probable que la PRAR reduzca la duración de la estancia hospitalaria (DM ‐1,72; IC del 95%: ‐2,19 a ‐1,25). La calidad de la evidencia se consideró moderada y se disminuyó por las limitaciones del estudio.
Según dos estudios, la PRL o la PRAR pueden reducir la frecuencia de transfusiones de sangre (CR 0,24; IC del 95%: 0,12 a 0,46). Si se supone un riesgo inicial para una transfusión de sangre del 8,9%, la PRL o la PRAR darían lugar a 68 transfusiones de sangre menos por 1000 pacientes (IC del 95%: 78 menos a 48 menos). La calidad de la evidencia se consideró baja y se disminuyó por las limitaciones de los estudios y la falta de direccionalidad.
A partir de la evidencia disponible no fue posible realizar ninguno de los otros análisis secundarios predeterminados. Todos los datos de resultados disponibles fueron a corto plazo y no fue posible considerar la cantidad de cirujanos ni la experiencia.
Conclusiones de los autores
No existe evidencia de alta calidad para informar la efectividad comparativa de la PRL o la PRAR en comparación con la PRA para los resultados oncológicos. Los resultados vinculados con la calidad de vida relacionada con la función urinaria y sexual parecen similares.
Las tasas de complicaciones generales y posoperatorias graves parecen similares. La diferencia en el dolor posoperatorio puede ser mínima. Los pacientes sometidos a PRL o PRAR pueden tener una estancia hospitalaria más corta y recibir menos transfusiones de sangre. Todos los datos de resultados disponibles fueron a corto plazo y en este estudio no fue posible considerar la cantidad de cirujanos ni la experiencia.
PICOs
Resumen en términos sencillos
Prostatectomía radical laparoscópica y asistida por robot versus abierta para el tratamiento del cáncer de próstata localizado
Pregunta de la revisión
¿Cómo se comparan la cirugía laparoscópica y la cirugía laparoscópica asistida por robot en el tratamiento de los pacientes con cáncer de próstata?
Antecedentes
El cáncer de próstata es un cáncer frecuente en hombres y a menudo se trata mediante la extracción quirúrgica. Tradicionalmente, los cirujanos solían hacer una incisión en el abdomen inferior para extraer la próstata. A este procedimiento se le llama prostatectomía radical abierta (PRA). Más recientemente, los cirujanos han comenzado a utilizar otras formas de realizar la misma operación. La prostatectomía radical laparoscópica (PRL) permite a los cirujanos operar dentro del paciente con instrumentos largos y una cámara diminuta a través de incisiones pequeñas. La cirugía laparoscópica se puede realizar mediante el uso de un dispositivo robotizado que le permite al cirujano tener una visión aumentada, tridimensional y operar desde una consola, lejos del paciente. A este procedimiento se le llama prostatectomía radical asistida por robot (PRAR). No está claro si los abordajes más nuevos mediante la PRL y la PRAR son mejores para los pacientes.
Características de los estudios
Esta revisión identificó dos ensayos controlados aleatorios con 446 pacientes que presentaban cáncer de próstata y tenían una edad promedio de aproximadamente 60 años, que compararon PRL o PRAR con PRA.
Resultados clave
No se encontró evidencia sobre cómo se comparan la PRL o la PRAR con la PRA en cuanto a la reducción del riesgo de muerte por cáncer de próstata, la prevención de que regrese el cáncer o de la muerte por cualquier causa. Con respecto a la función urinaria y sexual, la calidad de vida de los pacientes fue probablemente similar. Parece no haber diferencias en las complicaciones quirúrgicas posoperatorias. La PRL o la PRAR posiblemente pueden tener un efecto pequeño no importante sobre el dolor posoperatorio al primer día y hasta una semana. Sin embargo, no se encontraron diferencias entre la PRAR y la PRA a las 12 semanas posoperatorias. Es probable que los pacientes sometidos a PRL o PRAR tengan una estancia hospitalaria más corta y puedan necesitar menos transfusiones de sangre.
Calidad de la evidencia
No se encontró evidencia a partir de ensayos para ningún resultado del cáncer. La calidad de la evidencia sobre la calidad de vida fue moderada; para las complicaciones quirúrgicas generales y graves fue baja. La calidad de la evidencia para el dolor posoperatorio fue baja (hasta una semana) y moderada (a las 12 semanas). La calidad de la evidencia para la estancia hospitalaria y las transfusiones de sangre fue moderada y baja, respectivamente. En conjunto, para la mayoría de los resultados la calidad de la evidencia fue baja a moderada. Lo anterior significa que es probable que las estimaciones sean cercanas a la verdad, pero existe la posibilidad de que puedan ser diferentes.
Authors' conclusions
Summary of findings
| Laparaoscopic radical prostatectomy and robotic‐assisted laparoscopic radical prostatectomy compared to open radical prostatectomy for the treatment of localised prostate cancer | |||||
| Participants: men with prostate cancer Setting: single surgeon or single centre Intervention: laparoscopic radical prostatectomy/robotic‐assisted laparoscopic radical prostatectomy Control: open radical prostatectomy | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with ORP | Risk difference with LRP/RARP | ||||
| Prostate cancer‐specific survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Urinary quality of life (short‐term) | 248 | ⊕⊕⊕⊝ | ‐ | The mean score was 83.8 | MD 1.3 lower |
| Sexual quality of life (short‐term) | 248 | ⊕⊕⊕⊝ | ‐ | The mean score was 35.0 | MD 3.9 higher |
| Biochemical recurrence‐free survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Overall survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Overall surgical complications (short‐term) | 308 | ⊕⊕⊝⊝ | RR 0.41 | Study population | |
| 40 per 1000 | 23 fewer per 1000 | ||||
| Moderate | |||||
| 238 per 1000d | 140 fewer per 1000 (200 fewer to 10 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Expanded Prostate Cancer Index Composite contains five symptom domains (urinary incontinence, urinary irritative/obstructive, sexual, bowel, hormonal), scored from 0 (worst) to 100 (best). b Downgraded by one level for study limitation: unclear risk or high risk of one or more domains in included study or studies. d Estimates for control event rates for surgical complications come from Gandaglia 2014. | |||||
Background
Description of the condition
Prostate cancer is a leading disease affecting men worldwide and accounting for 15% of cancers diagnosed in men (IARC 2012). In 2012 prostate cancer accounted for 14% of the total new cancers diagnosed worldwide, and 6% of total cancer deaths in men (Jemel 2011; Torre 2015). Incidence rates of prostate cancer vary by more than 25‐fold worldwide, with the highest rates found in North America, Europe and Australasia (Jemel 2011). The age‐standardised rate of prostate cancer incidence is higher in high‐income countries than it is in low‐ and middle‐income countries (56.2 per 100,000 versus 9.4 per 100,000) (Baade 2009). A similar trend is apparent with prostate cancer‐specific mortality between high‐income and low‐ and middle‐income countries, with an age‐standardised mortality rate of 13.5 per 100,000 and 5.2 per 100,000 respectively (Baade 2009).
Description of the intervention
Men diagnosed with localised prostate cancer have a variety of management options available, including radical prostatectomy (RP) (whether by open radical prostatectomy (ORP), laparoscopic approach (LRP), or robotic‐assisted radical prostatectomy approach (RARP)). Other types of management include external beam radiation therapy, brachytherapy (including both high and low dose), active surveillance, watchful waiting, as well as investigational treatments, including whole‐gland ablation therapy and focal‐gland ablation therapy (Heidenreich 2011). Each respective treatment option aims to reduce the risk of prostate cancer‐specific mortality, whilst minimising treatment‐related morbidity and maintaining a good quality of life. Radical prostatectomy is recommended as a front‐line treatment for men diagnosed with localised prostate cancer and with a life expectancy greater than 10 years (AUA 2013; EAU 2015; Heidenreich 2011).
The effectiveness of radical prostatectomy in treating localised prostate cancer has been evaluated in several recently published randomised controlled trials (RCTs) (Bill‐Axelson 2014; Hamdy 2016; Wilt 2012). A 2014 RCT compared radical prostatectomy to watchful waiting with a median follow‐up period of 23.2 years (Bill‐Axelson 2014). In this study, radical prostatectomy significantly reduced prostate cancer‐specific mortality when compared to watchful waiting (RR 0.56, 95% CI 0.41 to 0.77), with an overall number needed to treat for an additional beneficial outcome of 8 (Bill‐Axelson 2014). The benefit of surgery was largest in men aged below 65 years, and in those with a low‐ and moderate‐risk prostate cancer (Bill‐Axelson 2014). It should also be noted that the participants in this study were men whose cancers were identified through symptoms, rather than widespread prostate‐specific antigen (PSA) testing. The study by Wilt 2012 examined the effectiveness of surgery versus observation for men with localised prostate cancer, detected via PSA testing. A follow‐up period of 10 years identified no significant difference in prostate cancer mortality between the two interventions (HR 0.88, 95% CI 0.71 to 1.08). Radical prostatectomy was associated with a reduction in all‐cause mortality for men with a PSA value greater than 10 ng/mL and those with intermediate and high‐risk tumours. The most recent study by Hamdy 2016 compared active monitoring, radical prostatectomy, and external‐beam radiotherapy for the treatment of clinically localised prostate cancer. Prostate cancer mortality measured at 10‐year median follow‐up identified no significant difference between the treatment arms.
The late 1990s saw the introduction of minimally invasive techniques for performing RP with the aim of reducing postoperative morbidity and allowing faster recovery when compared to traditional ORP (Schuessler 1997). Initially, surgeons adopting the LRP (also known as minimally invasive radical prostatectomy) approach needed to overcome significant technical challenges and a significant learning curve (Ficcara 2007). The introduction of robotic assistance using the da Vinci® surgical system (Intuitive Surgical 2011) led to the development of RARP in the early 2000s (Binder 2001), and offered technical innovations (3D visualisation, articulated instruments, tremor filtration) which addressed some of the technical limitations of LRP.
How the intervention might work
An initial feasibility report into the use of LRP concluded that it was a feasible procedure, but benefits with regard to tumour removal, continence, erectile function, and length of stay were negligible compared to open radical prostatectomy (Schuessler 1997). Other studies have been reported, since this initial feasibility study that standardised the techniques used for LRP (Guillonneau 1999). The robotic systems were introduced to provide surgeons with the ability to reduce the difficulty in performing LRPs (Hemel 2002). The first RARP was performed in 2000, and has since been widely adopted internationally for urological, gynaecological and cardiothoracic surgery (Binder 2001;Brandina 2009;Measo 2010;Uberoi 2010).
Why it is important to do this review
Since the introduction of RARP a number of advantages and limitations have been reported in comparison to ORP and LRP. Such comparisons centre on the amount of blood loss during surgery, hospital length of stay, surgical margin status, urinary continence, erectile function, and other quality‐of‐life indicators (Parsons 2008).
Objectives
To assess the effects of laparoscopic radical prostatectomy or robotic‐assisted radical prostatectomy compared to open radical prostatectomy in men with localised prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
All forms of RCTs were eligible for inclusion in this review, including pseudo‐RCTs.
Types of participants
Adult men, 18 years of age or older, of any ethnicity, diagnosed with clinically localised prostate cancer were eligible for inclusion in this review. Men with a diagnosis of prostate cancer, who had been treated previously for prostate cancer with any intervention (e.g. surgery, brachytherapy, complementary medicines) were not eligible for inclusion.
Types of interventions
We planned to investigate the following comparisons of experimental intervention versus comparator intervention.
Experimental interventions
-
Laparoscopic radical prostatectomy (LRP) or robotic‐assisted radical prostatectomy (RARP)
Comparator interventions
-
Open radical prostatectomy (ORP)
Comparisons
-
LRP or RARP versus ORP
Types of outcome measures
We did not use the measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
-
Prostate cancer‐specific survival
-
Urinary quality of life
-
Sexual quality of life
Secondary outcomes
-
Biochemical recurrence‐free survival
-
Overall survival
-
Overall surgical complications
-
Serious postoperative complications
-
Postoperative pain
-
Hospital stay
-
Blood transfusions
Method and timing of outcome measurement
We used minimal clinically important difference (MCID) for the review outcomes to rate overall quality of the evidence in the 'Summary of findings' table (Jaeschke 1989).
Prostate cancer‐specific survival
-
Measured as the date of randomisation to the date of death due to prostate cancer (Mariotto 2014).
-
We did not find any published information on MCID for prostate cancer‐specific survival.
Urinary quality of life
-
Mean change assessed with a validated questionnaire such as urinary domain of Expanded Prostate Cancer Index Composite (EPIC; Chang 2011; Szymanski 2000; Wei 2000).
-
We considered the MCID in the urinary quality of life domain of EPIC‐short form (EPIC‐26) to be 6 points (Skolarus 2015).
Sexual quality of life
-
Mean change assessed with validated questionnaires such as sexual domain of EPIC (Chang 2011; Szymanski 2000; Wei 2000), erectile function (EF) domain of international index of erectile function (IIEF; Rosen 1997), or total score of IIEF‐5 (Rosen 1997).
-
We used the MCID of sexual domain of EPIC‐26 of 10 points (Skolarus 2015). We considered the MCID in the EF domain score of IIEF of four points (Rosen 2011). We also considered improvement of IIEF‐5 of over five points as MCID (Spaliviero 2010).
Biochemical recurrence‐free survival
Overall survival
-
Measured as the date of randomisation to the date of death due to any cause (Mariotto 2014).
-
We did not find any published information on MCID for overall survival.
Overall surgical complications
-
All postoperative surgical complications such as postoperative haemorrhage, fever, pain, wound infection or dehiscence, hospitalisation, or life‐threatening complications requiring treatment.
-
While no threshold was established for the major and minor adverse events, we considered the MCID as relative risk reduction (RRR) of at least 25% (Guyatt 2011).
Serious surgical complications
-
Such as postoperative haemorrhage requiring admission or intervention, or life‐threatening complications.
-
We used the Clavien‐Dindo classification system (Dindo 2004) to assess the complications and categorized grade III, IV and V complications as serious surgical complications. We considered the MCID as RRR of at least 25% (Guyatt 2011).
Postoperative pain
-
Assessed with validated questionnaires such as visual analogue scale (VAS) score (DeLoach 1998).
-
We considered the MCID to be 10.0 mm on a 100 mm VAS scale to assess efficacy and comparative effectiveness (Kelly 2001).
Hospital stay
-
Measured in days from admission to discharge.
-
No threshold was established for the hospital stay. We used a MCID of one day to assess efficacy and comparative effectiveness.
Blood transfusions
-
Measured as frequencies after surgery.
-
No threshold was established for the blood transfusion. We considered the MCID in the blood transfusions as a RRR of at least a 25% (Guyatt 2011).
We planned to assess outcomes as short‐term (one year or less post‐intervention) and long‐term (more than one year post‐intervention) for prostate cancer‐specific survival, urinary quality of life, sexual quality of life, biochemical recurrence‐free survival, overall survival. We assessed overall surgical complications, serious surgical complications, postoperative pain, hospital stay, and blood transfusions as short‐term only.
Main outcomes for 'Summary of findings' table
Based on our published protocol, we have presented a 'Summary of findings' table reporting the following outcomes, listed according to priority.
-
Prostate cancer‐specific survival
-
Urinary quality of life
-
Sexual quality of life
-
Biochemical recurrence‐free survival
-
Overall survival
-
Overall postoperative complications
Search methods for identification of studies
We performed a comprehensive search with no restrictions on the language of publication or publication status. We initially ran searches on 13 December 2016 followed by an updated search on 9 June 2017.
Electronic searches
We conducted electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, issue 6) in the Cochrane Library (for the search strategy, see Appendix 1), MEDLINE Ovid (1946 to 9 June 2017) (Appendix 2) and EMBASE Ovid (1974 to June 2017) (Appendix 3).
Searching other resources
We searched bibliographies of identified studies for additional studies and contacted authors of identified studies for knowledge of any published or unpublished studies, including new, additional studies, or works in progress. We handsearched relevant conference proceedings for three years, from 2015 to 2017, for unpublished studies from annual meetings of the American Urological Association (EUA) and the European Association of Urology (EAU).
Data collection and analysis
Selection of studies
Two review authors (DI and CA) independently screened the titles and abstracts of all articles identified through the search strategy. Two review authors (DI and CA) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We documented reasons for exclusion of studies that may have reasonably been expected to be included in the review in a Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We developed a dedicated data abstraction form that we pilot tested ahead of time.
For studies that fulfilled inclusion criteria, two review authors (DI and CA) independently abstracted the following information, which we provided in the Characteristics of included studies table:
-
Study design
-
Study dates (if dates were not available then this was reported as such)
-
Study settings and country
-
Participant inclusion and exclusion criteria
-
Participant details, baseline demographics (e.g. age, prostate‐specific antigen, tumour stage, and Gleason score)
-
The number of participants by study and by study arm
-
Details of relevant experimental intervention (e.g. LRP and RARP) and ORP
-
Definitions of relevant outcomes, and method (e.g. type of instrument such as EPIC and IIEF) and timing of outcome measurement (e.g. in months) as well as any relevant subgroups (e.g. based on type of interventions and prostate risk classification)
-
Study funding sources
-
Declarations of interest by primary investigators
We extracted outcomes data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for population of a 2 x 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information.
We planned to provide information, including trial identifier, about potentially relevant ongoing studies in the table Characteristics of ongoing studies, however we did not identify any ongoing studies.
We attempted to contact authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data‐set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (DI and CA) independently conducted a 'Risk of bias' assessment using the Cochrane 'Risk of bias' assessment tool (Higgins 2011b) on all included trials. each review author independently assessed the following domains and graded each criterion as 'low', 'unclear', or 'high' risk of bias.
Assessment of risk of bias in RCTs
We assessed risk of bias using Cochrane's 'Risk of bias' assessment tool (Higgins 2011b). We assessed the following domains:
-
Random sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants and personnel (performance bias)
-
Blinding of outcome assessment (detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective reporting (reporting bias)
-
Other sources of bias
For selection bias (random sequence generation and allocation concealment), we evaluated risk of bias at a trial level.
For performance bias (blinding of participants and personnel), we evaluated the risk of bias separately for each outcome. We considered all outcomes similarly susceptible to performance bias. We considered all outcomes were susceptible and assessed them in one group.
For detection bias (blinding of outcome assessment), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective).
-
Susceptible/subjective: prostate cancer‐specific survival, urinary quality of life, sexual quality of life, biochemical recurrence‐free survival, overall surgical complications, and serious postoperative complications
-
Not susceptible/objective: overall survival, postoperative pain, hospital stay, blood transfusions
We assessed attrition bias (incomplete outcome data) and reporting bias (selective reporting) on a per‐outcome basis but sought to create groups of outcomes based on similar reporting characteristics.
-
Oncological outcomes: prostate cancer‐specific survival, biochemical recurrence‐free survival, overall survival
-
Quality‐of‐life outcomes: urinary quality of life, sexual quality of life
-
Overall surgical complications, serious postoperative complications, postoperative pain, hospital stay, and blood transfusions
Measures of treatment effect
We performed statistical analyses according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We expressed dichotomous outcomes as risk ratio (RR) with 95% confidence intervals (CIs) and reported continuous outcome measures as mean difference (MD) with 95% CIs. For measures recorded at different time points, we used the longest point after randomisation for any comparative analysis between studies. We planned to express time‐to‐event data as hazard ratios (HRs) with 95% CIs. However, we did not find any published time‐to‐event data.
Unit of analysis issues
We included simple, parallel‐group design RCTs in this review, with participants randomised to one of two intervention groups (ORP or LRP/ RARP). We obtained a single unit of measurement for each individual in the study.
Dealing with missing data
We obtained missing data from study authors, if feasible, and performed intention‐to‐treat analyses if data were available; we otherwise performed available case analyses. We investigated attrition rates, (e.g. drop‐outs, losses to follow‐up and withdrawals) and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
We assessed heterogeneity statistically with the I2 statistic (Higgins 2003), and graphically via graphical interpretation of forest plots. An I2 statistic above 75% was considered to be an indicator of considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We attempted to obtain study protocols to assess for selective outcome reporting.
Data synthesis
We summarised data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method. We used Review Manager 5 (RevMan 5) software to perform analyses (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analyses.
-
LRP versus RARP
-
Pathological tumour stage (i.e. T2 versus T3 disease)
These subgroup analyses were based on the following observations.
-
RARP and LRP are different procedures. LRP has been reported to require a high level of surgical skills and has a steeper learning curve (Ficcara 2007; Hemel 2002). RARP requires the use of a robotic‐assisted device that is associated with high acquisition and maintenance costs (Binder 2001).
-
Higher local tumour stage represents a higher risks for positive surgical margins and prompts a different surgical approach, which may impact on urinary quality of life and sexual quality of life (AUA 2013; EAU 2015). It is unclear whether the results differ by LRP/RARP versus ORP.
We planned to perform subgroup analyses limited to the primary outcomes only.
Sensitivity analysis
We planned to perform sensitivity analyses limited to the primary outcomes in order to explore the influence of the following factor (when applicable) on effect sizes.
-
Restricting the analysis by taking into account risk of bias by excluding studies at 'high risk' or 'unclear risk'.
Results
Description of studies
Results of the search
A search of all electronic databases returned 85 citations (Figure 1) with no further records identified through other sources. After removal of duplicates, we found 49 citations eligible for screening against the inclusion criteria for this review. We excluded a total of 46 records based on reading the title and abstract. We screened three full‐text articles, of which one was excluded (Magheli 2014). We assessed two studies as eligible for inclusion in this review (Guazzoni 2006; Yaxley 2016). The flow of literature through the assessment process is shown in the PRISMA flowchart (Figure 1) (Liberati 2009).
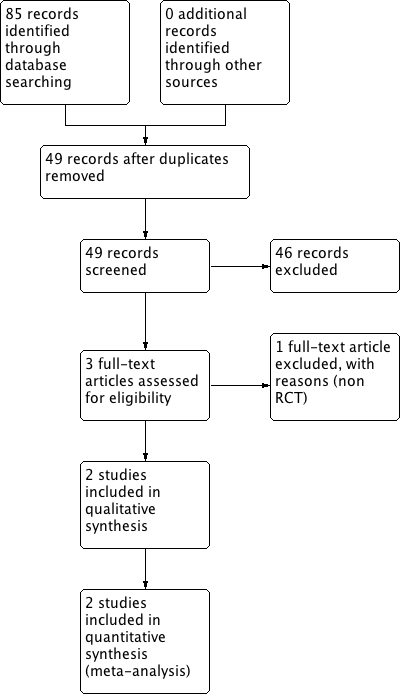
Study flow diagram
Included studies
A detailed description of the baseline characteristics and participants of the included studies is presented elsewhere (see Characteristics of included studies; Table 1; Table 2).
| Study name | Trial | Country | Setting | Description of participants | Intervention(s) | Duration of | Age (yrs) | PSA (ng/mL) | Pathologic stage |
| NR | Italy | Single surgeon | Men aged < 70 years, clinically organ‐confined disease (cT1 ‐ cT2), total serum PSA < 20 ng/dL, Gleason score ≤ 7 | LRP | 6 days | 62.29 ± 8.2 | 6.9 ± 2.9 | T2 (75.0%), T3 (25%), surgical margin positive (26.0%) | |
| ORP | 62.9 ± 7.4 | 6.5 ± 3.0 | T2 (73.3%), T3 (26.6%), surgical margin positive (21.6%) | ||||||
| 2010 to 2014 | Australia | Single centre | Men aged 35‐70 years with newly diagnosed with clinically localised prostate cancer | RARP | 12 weeks | 59.64 ± 6.63 | 7.41 ± 4.10 | Extraprostatic extension (35%), seminal vesicle involvement (3%), surgical margins positive (15%) | |
| ORP | 60.38 ± 5.81 | 7.57 ± 4.07 | Extraprostatic extension (32%), seminal vesicle involvement (6%), surgical margins positive (10%) |
LRP: laparoscopic prostatectomy; NR: not reported; ORP: open radical prostatectomy; PSA: prostate‐specific antigen; RARP: robotic‐assisted radical prostatectomy
| Study name | Intervention(s) and comparator(s) | Sample size (N) | Screened/ eligible (N) | Randomised (N) | Analysed (N) | Finishing trial (N (%)) |
| LRP | NR | NR | 60 | 60 | 60 (100.0) | |
| ORP | NR | 60 | 60 | 60 (100.0) | ||
| Total | 120 | 120 | 120 (100.0) | |||
| RARP | 200 | NR/334 | 163 | QoL: 129 Surgical outcomes: 157a Pain: 130 | 157 (96.3) | |
| ORP | 200 | 163 | QoL: 119 Surgical outcomes: 151a Pain: 120 | 151 (92.6) | ||
| Total | 326 | QoL: 248 Surgical outcomes: 308 Pain: 250 | 308 (94.4) | |||
| Grand total | All interventions | 223 | 217 | |||
| All comparators | 223 | 211 | ||||
| Overall | 446 | 428 | ||||
aSurgical outcomes: surgical complications, hospital stay, and blood transfusions
LRP: laparoscopic prostatectomy; NR: not reported; ORP: open radical prostatectomy; QoL: quality of life; RARP: robotic‐assisted radical prostatectomy
Source of data
The electronic database search identified both of the included trials. We contacted all trial authors of the included trials to identify any further information on study methodology and results, and received reply from Yaxley 2016.
Study design and settings
The two included studies were parallel‐RCTs. Yaxley 2016 used block randomisation with age stratification (40–49 years, 50–59 years, 60‐69 years) and reported to be blinded for study investigators involved in data analysis but participants were telephoned about their designated surgical procedure. Guazzoni 2006 randomised participants using a computer‐generated table. Guazzoni 2006, a single‐surgeon study, was done in Milan, Italy. Yaxley 2016, a single‐centre study, was performed in Queensland, Australia. One study was performed from 2010 to 2014 (Yaxley 2016), but Guazzoni 2006 did not state the study duration.
Participants
This review includes 446 randomised participants in total (LRP or RARP 223, ORP 223), of whom a total of 428 subsequently finished for the intervention and control groups (LRP or RARP 217, ORP 211). The mean age, prostate volume, and PSA of the participants were 61.3 years, 49.78 mL, and 7.09 ng/mL, respectively. Both the studies included participants with clinically localised prostate cancer. However, Yaxley 2016 included participants with a Gleason score of more than 8. In addition, Yaxley 2016 included participants who are able to read and speak English. Men with PSA greater than 20 ng/mL were excluded from both of the included studies. Guazzoni 2006 limited the prostate volume to less than 60 mL.
Interventions and comparators
Guazzoni 2006 compared LRP to ORP. LRP was performed via the trans‐peritoneal route according to the Montsouris technique (Guillonneau 2002), and ORP was performed using the anatomic technique described by Walsh 2000. Single surgeons performed all procedures. Yaxley 2016 compared RARP to ORP. To reduce heterogeneity, each surgical procedure was done by the same surgeon. Pelvic lymph node dissection was performed in participants with a serum PSA greater than 10 ng/mL, or Gleason score more than 7 in both studies. Guazzoni 2006 and Yaxley 2016, used limited and standardised templates respectively. Nerve‐sparing procedures were undertaken based on preoperative parameters including clinical staging in both included studies. Pain control was done based on analgesic protocol in Guazzoni 2006, but epidural or spinal anaesthesia was not used routinely in Yaxley 2016.
Outcomes
Guazzoni 2006 compared LRP to ORP and followed up for six days postoperatively, with reported outcomes including length of stay, success rate in nerve sparing, blood loss and quality of life (as measured by pain). Yaxley 2016 reported non‐oncological participant outcomes including, postoperative complications, pain, physical and mental functioning, fatigue, preference‐based utility scores, bowel function, cancer‐specific distress, psychological distress, and time to return to work comparing RARP to ORP. Outcomes were measured preoperatively and at six and 12 weeks postoperatively. They also planned to report oncological outcomes including positive surgical margins and biochemical recurrence at 24 months postoperatively.
Excluded studies
We excluded one study due to not being a RCT after evaluation of the full publication (Magheli 2014) see Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2, Figure 3, and the Characteristics of included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
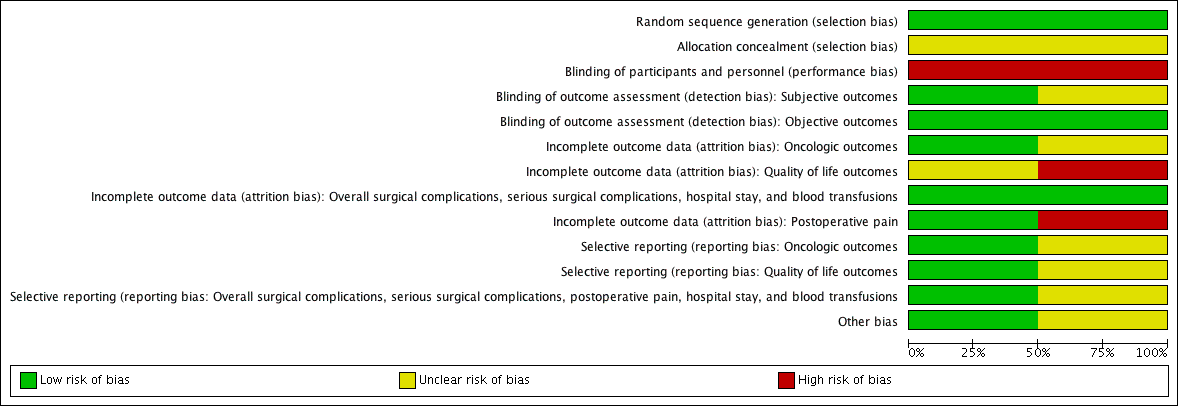
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation
Both studies reported an adequate method of sequence generation and we rated them at low risk of bias.
Allocation concealment
We rated both included studies as unclear risk of bias due to lack of information.
Blinding
Blinding of participants and personnel
We judged both included studies as high risk of bias.
Blinding of outcome assessor
-
Susceptible/subjective outcomes (prostate cancer‐specific survival, urinary quality of life, sexual quality of life, biochemical recurrence‐free survival, overall surgical complications, serious postoperative complications): we rated one study as low risk of bias (Yaxley 2016), but the other as unclear risk of bias (Guazzoni 2006).
-
Not susceptible/objective outcomes (overall survival, postoperative pain, hospital stay, blood transfusions): we judged both included studies as low risk of bias.
Incomplete outcome data
-
Oncological outcomes (prostate cancer specific survival, biochemical recurrence‐free survival, overall survival): we rated one study as low risk of bias (Yaxley 2016), but the other as unclear risk of bias (Guazzoni 2006).
-
Quality‐of‐life outcomes (urinary quality of life, sexual quality of life): we rated one study as high risk of bias (Yaxley 2016), but the other as unclear risk of bias (Guazzoni 2006).
-
Overall surgical complications, serious postoperative complications, hospital stay, and blood transfusions: we judged both included studies as low risk of bias.
-
Postoperative pain: we rated one study as low risk of bias (Guazzoni 2006), but the other as high risk of bias (Yaxley 2016).
Selective reporting
For all outcomes, we rated one study, which had a published protocol, as low risk of bias (Yaxley 2016), but the other as unclear risk of bias (Guazzoni 2006).
Other potential sources of bias
We rated one study as low risk of bias (Yaxley 2016), but the other as unclear risk of bias due to statistically significant differences in the nerve‐sparing procedures between the interventions, which may have affected the prognosis (Guazzoni 2006).
Effects of interventions
Laparascopic or robotic‐assisted versus open radical prostatectomy
Primary Outcomes
Prostate cancer‐specific survival
Neither of the two trials reported this outcome.
Urinary quality of life
One included study (Yaxley 2016) reported data for this outcome for 248 participants (RARP 129, ORP 119). RARP likely results in little to no difference in urinary quality of life (MD ‐1.30, 95% CI ‐4.65 to 2.05). We rated the quality of the evidence as moderate according to GRADE, downgrading for study limitations.
Sexual quality of life
One included study (Yaxley 2016) reported data for this outcome for 248 participants (RARP 129, ORP 119). RARP likely results in little to no difference in sexual quality of life (MD 3.90, 95% CI ‐1.84 to 9.64). We rated the quality of the evidence as moderate according to GRADE, downgrading for study limitation.
Secondary Outcomes
Biochemical recurrence‐free survival
Neither of the two trials included in this review reported this outcome.
Overall survival
Neither of the two trials included in this review reported this outcome.
Overall surgical complications
One included study (Yaxley 2016) reported data for this outcome for 308 participants (RARP 157, ORP 151). RARP likely results in little to no difference in postoperative surgical complications (RR 0.41, 95% CI 0.16 to 1.04). We rated the quality of the evidence as low according to GRADE, downgrading for study limitations and imprecision.
Serious postoperative complications
One included study (Yaxley 2016) reported data for this outcome for 308 participants (RARP 157, ORP 151). RARP likely results in little to no difference in serious surgical complications (RR 0.16, 95% CI 0.02 to 1.32). We rated the quality of the evidence as low according to GRADE, downgrading for study limitations and imprecision.
Postoperative pain
We included two studies with 428 participants (LRP or RARP 217, ORP 211) and 370 participants (LRP or RARP 190, ORP 180) for postoperative follow‐up at one day and up to one week (Guazzoni 2006; Yaxley 2016). LRP or RARP may result in a small effect that may not be an important improvement in postoperative pain (at one day: MD ‐1.05, 95% CI ‐1.42 to ‐0.68), (up to one week: MD ‐0.78, 95% CI ‐1.40 to ‐0.17). We rated the quality of the evidence as low for both according to GRADE, downgrading for study limitations and imprecision. We included only one study with 250 participants (RARP 130, ORP 120) with postoperative 12‐week follow‐up (Yaxley 2016). RARP likely results in little to no difference in postoperative pain at 12 weeks (MD 0.01, 95% CI ‐0.32 to 0.34). We rated the quality of the evidence as moderate according to GRADE, downgrading for study limitations.
Hospital stay
Only one included study (Yaxley 2016) reported data for this outcome for 308 participants (RARP 157, ORP 151). RARP likely reduces the length of hospital stay (MD ‐1.72, 95% CI ‐2.19 to ‐1.25). We rated the quality of the evidence as moderate according to GRADE, downgrading for study limitations.
Blood transfusions
Both the included studies (Guazzoni 2006; Yaxley 2016) reported data for this outcome for 428 participants (LRP or RARP 217, ORP 211). LRP or RARP may reduce the frequency of blood transfusions postoperatively (RR 0.24, 95% CI 0.12 to 0.46). Assuming a baseline risk of blood transfusion to be 8.9% (Gandaglia 2014), LRP or RARP would result in 68 fewer blood transfusions per 1000 men (95% CI 78 fewer to 48 fewer). We rated the quality of the evidence as low according to GRADE, downgrading for study limitations and indirectness. We downgraded for indirectness since all participants in the Guazzoni 2006 study banked two units of autologous blood, which may have increased transfusion requirements. This study contributed 90% of the weight of the analysis.
Subgroup analysis
We did not perform a subgroup analysis because there were no relevant data.
Sensitivity analysis
We did not perform a sensitivity and subgroup analysis due to a paucity of included studies.
Discussion
Summary of main results
A central finding of this review was the lack of high quality evidence to support equivalence or superiority of oncological outcomes, in particular prostate cancer‐specific survival, but also biochemical and recurrence‐free survival.
RARP likely results in little to no difference in urinary and sexual quality of life. RARP appears similar with regards to overall surgical complications and serious postoperative complications.
LRP or RARP may have a small unimportant effect on postoperative pain up to one week but RARP appears similar at 12 weeks. RARP likely reduces the length of hospital stay and LRP or RARP may reduce the frequency of blood transfusions.
Overall completeness and applicability of evidence
-
This review is based on only two randomised controlled trials with relatively small sample sizes and event rates conducted at tertiary care centres with expert surgeons (Guazzoni 2006; Yaxley 2016). This narrow evidence base stands in marked contrast to the widespread use of RARP in many countries, in particular in the USA (Stitzenberg 2012).
-
A major limitation of the evidence drawn from this review is the lack of high‐quality evidence to inform the comparison of any oncological outcomes, resulting in major uncertainty. An understanding of these outcomes therefore has to come from observational studies that were outside the scope of this review and are likely to only yield low‐quality evidence (Schunemann 2013).
-
All outcome data, including that on quality of life, was short‐term. Given that prostate cancer survivorship, which includes dealing with the potential adverse events of radical surgery, such as urinary incontinence and erectile dysfunction commonly extends over decades, the information provided appears insufficient to guide clinical practice. Longer term, well‐controlled studies are needed.
-
One of the central challenges of assessing surgical innovation lies in the need to account for ongoing evolution of the procedure or device, or both, being used, as well as accounting for the surgical learning curve (Dahm 2014; Dahm 2016). It is well recognised that surgical outcomes are dependent on surgeons' and centres' volume and experience. This review is unable to account for these differences, which may be more important factors than the surgical approach.
-
While RARP (and less so today, LRP) has tremendous appeal to surgeons, due, among other things, to magnification of the operative field and 3‐D imaging, device acquisition and maintenance/service are costly. However, an assessment of the cost‐effectiveness of LRP/RARP was outside the scope of this review.
Quality of the evidence
We consistently downgraded the quality of the evidence for all outcomes to moderate, except for postoperative surgical complications, postoperative pain, and blood transfusions. The main issues that lowered our confidence in the estimates of effect were study limitations, specifically unclear allocation concealment (selection bias) and the lack of blinding (performance and detection bias). For postoperative surgical complications and postoperative pain at one day and up to one week, we rated the quality of the evidence as low due to study limitations and imprecision (confidence interval that crosses predefined MCIDs). We downgraded the quality of the evidence for blood transfusions as low due to study limitations and indirectness (different standard of care; autologous transfusion).
Potential biases in the review process
This review employed a comprehensive search strategy of multiple data sources to search for RCTs irrespective of publication status and language. Despite this, there is a possibility that we may have missed published studies in a language other than English, published in non‐indexed journals or not published at all.
Agreements and disagreements with other studies or reviews
A systematic search of the literature identified no published systematic reviews of RCTs, clinical practice guidelines reporting findings, or recommendations, with respect to the use of ORP versus LRP in the treatment of localised prostate cancer. Several systematic reviews of observational studies have been completed, and generally support the findings reported in this systematic review (Bolenz 2014; De Carlo 2014; Ficcara 2007; Heer 2011; Moran 2013; Novara 2012). Previous systematic review evidence from observational studies has demonstrated lower rates of blood loss, blood transfusion, postoperative complications, pain and length of hospital stay for the LRP/RARP (De Carlo 2014; Heer 2011; Novara 2012; Tooher 2006). Conversely, urinary and sexual quality of life have been reported in observational studies to be significantly better in men receiving RARP compared to ORP (Moran 2013). However, none of these studies used GRADE to assess the quality of evidence. Findings from our systematic review support previous findings that have reported lower rates of blood transfusion, postoperative pain, and length of hospital stay in LRP/RARP groups (De Carlo 2014; Moran 2013; Tooher 2006).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 1 Urinary QoL (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 2 Sexual QoL (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 3 Surgical complications (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 4 Serious postoperative complications (short term).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 5 Postoperative pain (at 1 day).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 6 Postoperative pain (at 1 week).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 7 Postoperative pain (at 12 weeks).

Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 8 Hospital stay (short term ).
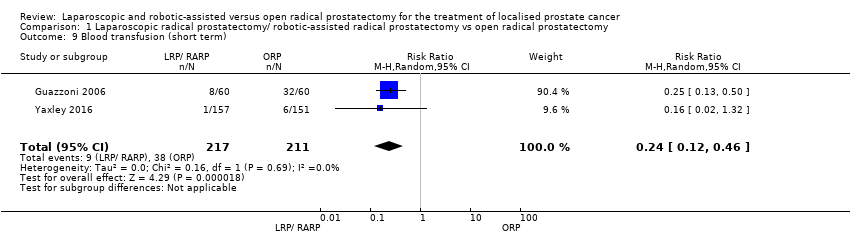
Comparison 1 Laparoscopic radical prostatectomy/ robotic‐assisted radical prostatectomy vs open radical prostatectomy, Outcome 9 Blood transfusion (short term).
| Laparaoscopic radical prostatectomy and robotic‐assisted laparoscopic radical prostatectomy compared to open radical prostatectomy for the treatment of localised prostate cancer | |||||
| Participants: men with prostate cancer Setting: single surgeon or single centre Intervention: laparoscopic radical prostatectomy/robotic‐assisted laparoscopic radical prostatectomy Control: open radical prostatectomy | |||||
| Outcomes | № of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with ORP | Risk difference with LRP/RARP | ||||
| Prostate cancer‐specific survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Urinary quality of life (short‐term) | 248 | ⊕⊕⊕⊝ | ‐ | The mean score was 83.8 | MD 1.3 lower |
| Sexual quality of life (short‐term) | 248 | ⊕⊕⊕⊝ | ‐ | The mean score was 35.0 | MD 3.9 higher |
| Biochemical recurrence‐free survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Overall survival ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Overall surgical complications (short‐term) | 308 | ⊕⊕⊝⊝ | RR 0.41 | Study population | |
| 40 per 1000 | 23 fewer per 1000 | ||||
| Moderate | |||||
| 238 per 1000d | 140 fewer per 1000 (200 fewer to 10 more) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Expanded Prostate Cancer Index Composite contains five symptom domains (urinary incontinence, urinary irritative/obstructive, sexual, bowel, hormonal), scored from 0 (worst) to 100 (best). b Downgraded by one level for study limitation: unclear risk or high risk of one or more domains in included study or studies. d Estimates for control event rates for surgical complications come from Gandaglia 2014. | |||||
| Study name | Trial | Country | Setting | Description of participants | Intervention(s) | Duration of | Age (yrs) | PSA (ng/mL) | Pathologic stage |
| NR | Italy | Single surgeon | Men aged < 70 years, clinically organ‐confined disease (cT1 ‐ cT2), total serum PSA < 20 ng/dL, Gleason score ≤ 7 | LRP | 6 days | 62.29 ± 8.2 | 6.9 ± 2.9 | T2 (75.0%), T3 (25%), surgical margin positive (26.0%) | |
| ORP | 62.9 ± 7.4 | 6.5 ± 3.0 | T2 (73.3%), T3 (26.6%), surgical margin positive (21.6%) | ||||||
| 2010 to 2014 | Australia | Single centre | Men aged 35‐70 years with newly diagnosed with clinically localised prostate cancer | RARP | 12 weeks | 59.64 ± 6.63 | 7.41 ± 4.10 | Extraprostatic extension (35%), seminal vesicle involvement (3%), surgical margins positive (15%) | |
| ORP | 60.38 ± 5.81 | 7.57 ± 4.07 | Extraprostatic extension (32%), seminal vesicle involvement (6%), surgical margins positive (10%) | ||||||
| LRP: laparoscopic prostatectomy; NR: not reported; ORP: open radical prostatectomy; PSA: prostate‐specific antigen; RARP: robotic‐assisted radical prostatectomy | |||||||||
| Study name | Intervention(s) and comparator(s) | Sample size (N) | Screened/ eligible (N) | Randomised (N) | Analysed (N) | Finishing trial (N (%)) |
| LRP | NR | NR | 60 | 60 | 60 (100.0) | |
| ORP | NR | 60 | 60 | 60 (100.0) | ||
| Total | 120 | 120 | 120 (100.0) | |||
| RARP | 200 | NR/334 | 163 | QoL: 129 Surgical outcomes: 157a Pain: 130 | 157 (96.3) | |
| ORP | 200 | 163 | QoL: 119 Surgical outcomes: 151a Pain: 120 | 151 (92.6) | ||
| Total | 326 | QoL: 248 Surgical outcomes: 308 Pain: 250 | 308 (94.4) | |||
| Grand total | All interventions | 223 | 217 | |||
| All comparators | 223 | 211 | ||||
| Overall | 446 | 428 | ||||
| aSurgical outcomes: surgical complications, hospital stay, and blood transfusions LRP: laparoscopic prostatectomy; NR: not reported; ORP: open radical prostatectomy; QoL: quality of life; RARP: robotic‐assisted radical prostatectomy | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urinary QoL (short term) Show forest plot | 1 | 248 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐4.65, 2.05] |
| 2 Sexual QoL (short term) Show forest plot | 1 | 248 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐1.84, 9.64] |
| 3 Surgical complications (short term) Show forest plot | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.04] |
| 4 Serious postoperative complications (short term) Show forest plot | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.32] |
| 5 Postoperative pain (at 1 day) Show forest plot | 2 | 423 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.42, ‐0.68] |
| 6 Postoperative pain (at 1 week) Show forest plot | 2 | 416 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.40, ‐0.17] |
| 7 Postoperative pain (at 12 weeks) Show forest plot | 1 | 250 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.32, 0.34] |
| 8 Hospital stay (short term ) Show forest plot | 1 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐2.19, ‐1.25] |
| 9 Blood transfusion (short term) Show forest plot | 2 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.12, 0.46] |

