Gamma aminobutyric acid (GABA) receptor agonists for acute stroke
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009622.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 04 octubre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Accidentes cerebrovasculares
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Jia Liu and Lu‐Ning Wang formulated the idea for the review and developed the basis for the review. Jia Liu took the primary role in searching, identifying and assessing studies; extracting and analyzing the data; and writing up the full review. Lu‐Ning Wang provided general advice on this review, as well as helping to identify trials, assess studies and extract data. Xin Ma served as the independent third author in selection of studies and assessment of risk of bias. Jia Liu supervised the quality of the methodology and statistics used. The manuscript was written by Jia Liu and Lu‐Ning Wang, and revised by Xunming Ji. Jia Liu will be responsible for updating the review.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
Jia Liu: none known.
Lu‐Ning Wang: none known.
Xin Ma: none known.
Xunming Ji: none known.
Acknowledgements
The authors would like to acknowledge the help provided by the Cochrane Stroke Group.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Oct 30 | Gamma aminobutyric acid (GABA) receptor agonists for acute stroke | Review | Jia Liu, Jing Zhang, Lu‐Ning Wang | |
| 2016 Oct 04 | Gamma aminobutyric acid (GABA) receptor agonists for acute stroke | Review | Jia Liu, Lu‐Ning Wang, Xin Ma, Xunming Ji | |
| 2014 Aug 06 | Gamma aminobutyric acid (GABA) receptor agonists for acute stroke | Review | Jia Liu, Lu‐Ning Wang | |
| 2013 Feb 28 | Gamma aminobutyric acid (GABA) receptor agonists for acute stroke | Review | Jia Liu, Lu‐Ning Wang | |
| 2012 Feb 15 | Gamma aminobutyric acid (GABA) receptor agonists for acute stroke | Protocol | Jia Liu, Luning Wang | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Disease;
- Chlormethiazole [adverse effects, *therapeutic use];
- Diazepam [*therapeutic use];
- Disorders of Excessive Somnolence [chemically induced];
- GABA Agonists [adverse effects, *therapeutic use];
- Neuroprotective Agents [adverse effects, *therapeutic use];
- Randomized Controlled Trials as Topic;
- Rhinitis [chemically induced];
- Stroke [*drug therapy, mortality];
Medical Subject Headings Check Words
Humans;
PICO
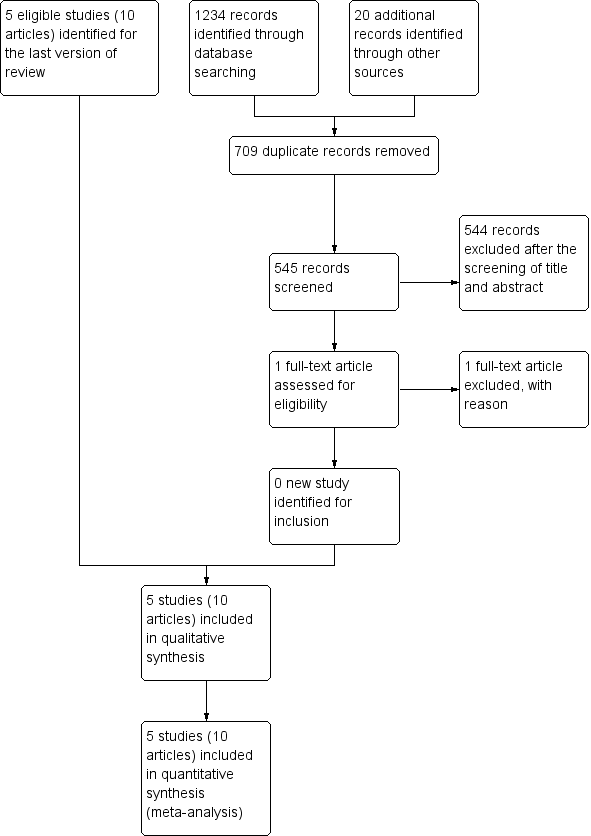
Study flow diagram.
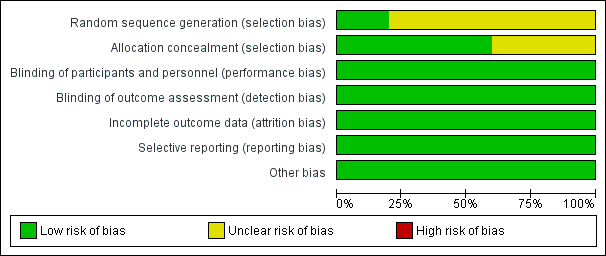
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
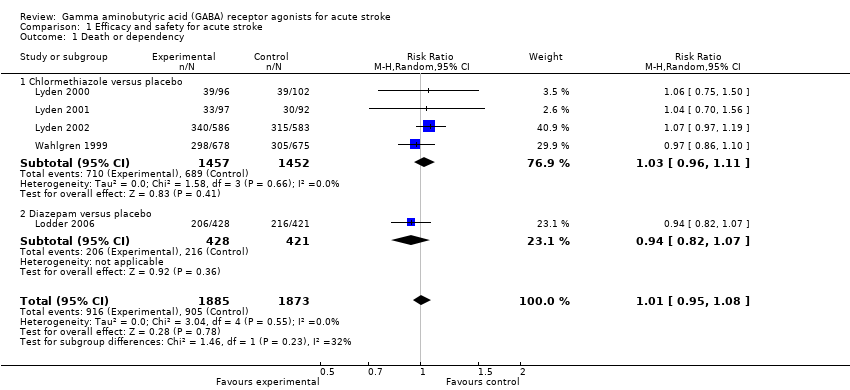
Comparison 1 Efficacy and safety for acute stroke, Outcome 1 Death or dependency.
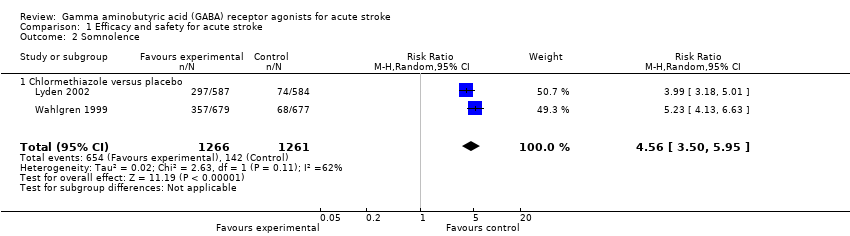
Comparison 1 Efficacy and safety for acute stroke, Outcome 2 Somnolence.
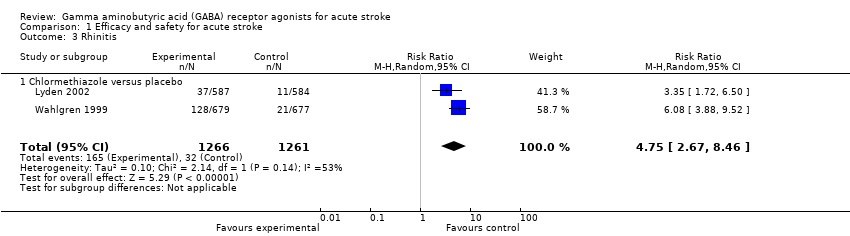
Comparison 1 Efficacy and safety for acute stroke, Outcome 3 Rhinitis.
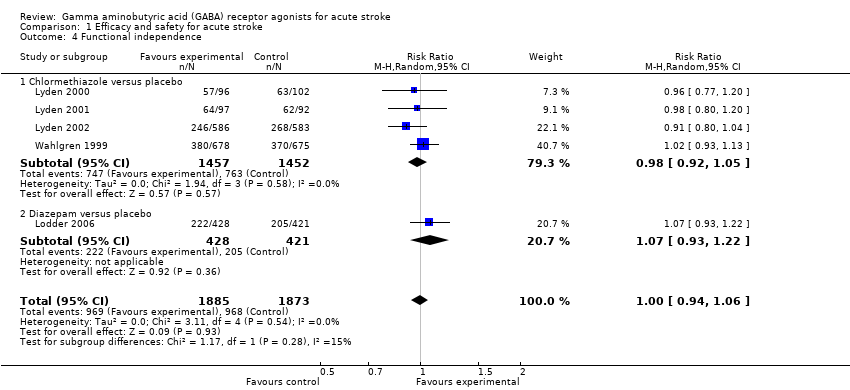
Comparison 1 Efficacy and safety for acute stroke, Outcome 4 Functional independence.
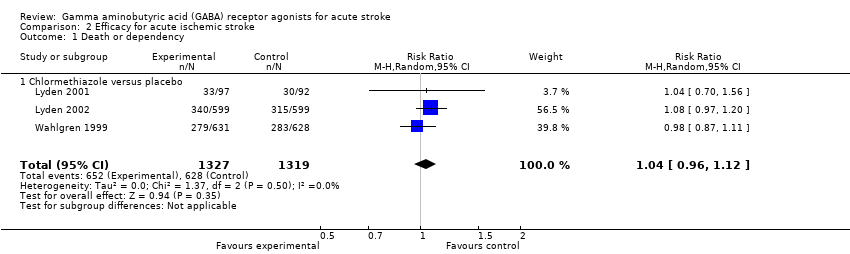
Comparison 2 Efficacy for acute ischemic stroke, Outcome 1 Death or dependency.

Comparison 2 Efficacy for acute ischemic stroke, Outcome 2 Functional independence.
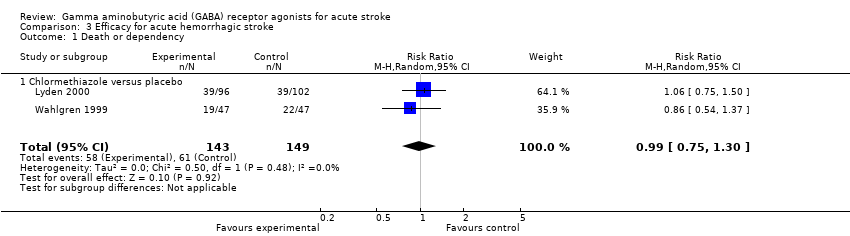
Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 1 Death or dependency.

Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 2 Functional independence.

Comparison 4 Efficacy for TACS, Outcome 1 Functional independence.
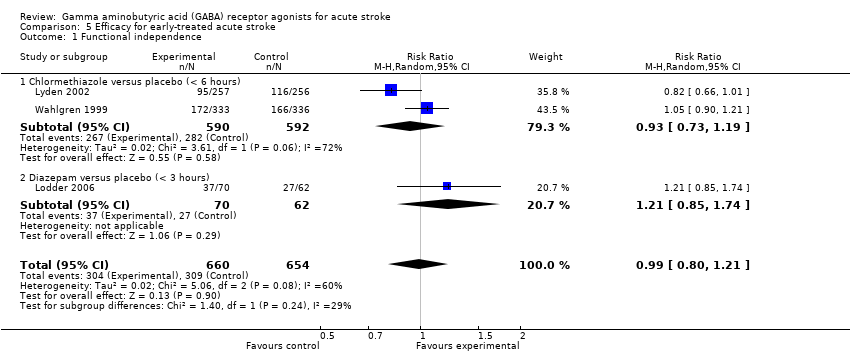
Comparison 5 Efficacy for early‐treated acute stroke, Outcome 1 Functional independence.
| Chlormethiazole compared with placebo for acute stroke | ||||||
| Patient or population: people with acute stroke Settings: inpatients Intervention: chlormethiazole Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Chlormethiazole | |||||
| Death or dependency | 475 per 1000 | 487 per 1000 | RR 1.03 (0.96 to 1.11) | 2909 (4) | ⊕⊕⊕⊝ | — |
| Adverse events | Somnolence 113 per 1000 Rhinitis 25 per 1000 | Somnolence 517 per 1000 Rhinitis 130 per 1000 | RR 4.56 (3.50 to 5.95) RR 4.75 (2.67 to 8.46) | 2527 (2) | ⊕⊕⊕⊝ | — |
| Functional independence | 525 per 1000 | 513 per 1000 | RR 0.98 (0.92 to 1.05) | 2909 (4) | ⊕⊕⊕⊝ | — |
| Other stroke scales | — | — | — | NIHSS 1367 (2) SSS 2727 (3) | ⊕⊕⊕⊝ | In Lyden 2000, the mean change of the NIHSS score was ‐4.5 in the chlormethiazole group (N = 96) and ‐4.0 in the placebo group (N = 102; P = 0.36). In Lyden 2002, the change of NIHSS score (median (quartiles)) was ‐5.5 (‐11, 17) in the chlormethiazole group (N = 586) and ‐6.0 (‐10, 16) in the placebo group (N = 583; P = 0.68). In Wahlgren 1999, no significant difference was found between the placebo and chlormethiazole groups for the change in score in the SSS 48‐point (P = 0.56) and SSS motor power score (P = 0.96). In Lyden 2000 and Lyden 2002, the change in score in the SSS was not significant in the two groups (P = 0.06 and P = 0.23, respectively). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to unclear risk of selection bias Functional independence, defined as a BI score higher than 60 or a mRS score less than 3 | ||||||
| Diazepam compared with placebo for acute stroke | ||||||
| Patient or population: people with acute stroke Settings: inpatients Intervention: diazepam Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Diazepam | |||||
| Death or dependency | 513 per 1000 | 481 per 1000 | RR 0.94 (0.82 to 1.07) | 849 (1) | ⊕⊕⊕⊝ | — |
| Adverse events | 357 per 1000 | 355 per 1000 | RR 0.99 (0.75 to 1.31) | 865 (1) | ⊕⊕⊕⊝ | — |
| Functional independence | 487 per 1000 | 519 per 1000 | RR 1.07 (0.93 to 1.22) | 849 (1) | ⊕⊕⊕⊝ | — |
| Other stroke scales | Not reported | Not reported | — | — | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level: one study with small sample size Functional independence, defined as a BI score higher than 60 or a mRS score less than 3 | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 1.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 1.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 2 Somnolence Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 2.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 3 Rhinitis Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 3.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 4 Functional independence Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 4.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 1.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 2 Functional independence Show forest plot | 4 | 3394 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.08] |
| 2.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.05] |
| 2.2 Diazepam versus placebo | 1 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 1.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 2 Functional independence Show forest plot | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 2.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2.2 Diazepam versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| 1.1 Chlormethiazole versus placebo | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 3 | 1314 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.21] |
| 1.1 Chlormethiazole versus placebo (< 6 hours) | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 1.2 Diazepam versus placebo (< 3 hours) | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.74] |

