Gamma aminobutyric acid (GABA) receptor agonists for acute stroke
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease]
#2 MeSH descriptor: [Brain Ischemia]
#3 MeSH descriptor: [Carotid Artery Diseases]
#4 MeSH descriptor: [Cerebrovascular Trauma]
#5 MeSH descriptor: [Intracranial Arterial Diseases]
#6 MeSH descriptor: [Intracranial Arteriovenous Malformations]
#7 MeSH descriptor: [Intracranial Embolism and Thrombosis]
#8 MeSH descriptor: [Intracranial Hemorrhages]
#9 MeSH descriptor: [Stroke] explode all trees
#10 MeSH descriptor: [Brain Infarction]
#11 MeSH descriptor: [Vasospasm, Intracranial] explode all trees
#12 MeSH descriptor: [Cerebrovascular Disorders] explode all trees
#13 (stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab,kw
#14 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw
#15 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*))
#16 {or #1‐#15}
#17 MeSH descriptor: [GABA Agonists] explode all trees
#18 MeSH descriptor: [GABA‐A Receptor Antagonists]
#19 MeSH descriptor: [GABA‐B Receptor Agonists]
#20 MeSH descriptor: [GABA Modulators] explode all trees
#21 MeSH descriptor: [gamma‐Aminobutyric Acid]
#22 MeSH descriptor: [Receptors, GABA]
#23 (("gamma aminobutyric acid" or "gamma‐aminobutyric acid" or gaba or "gaba‐A" or "gaba‐B") near/5 (agonist* or modulator* or stimulat* or stimulant*)):ti,ab,kw
#24 ((gabaergic or "gaba‐ergic" or gabamimetic) near/5 (agent* or drug* or stimul*)):ti,ab,kw
#25 (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine* or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or "Hopantenate calcium" or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or "Oxybate sodium" or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or "Valproic acid" or Vigabatrin or Zolazepam or XP19986):ti,ab,kw
#26 {or #17‐#25}
#27 #16 and #26
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. ((transi$ adj3 isch?em$ adj3 attack$) or TIA$1).tw.
6. 1 or 2 or 3 or 4 or 5
7. gaba agonists/ or exp gaba‐a receptor agonists/ or exp gaba‐b receptor agonists/ or exp gaba modulators/
8. exp gamma‐Aminobutyric Acid/tu [Therapeutic Use]
9. exp Receptors, GABA/de [Drug Effects]
10. ((gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) adj5 (agonist$ or modulator$ or stimulat$ or stimulant$)).tw.
11. ((gabaergic or gaba‐ergic or gabamimetic) adj5 (agent$ or drug$ or stimul$)).tw.
12. (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986).tw,nm.
13. 7 or 8 or 9 or 10 or 11 or 12
14. Randomized Controlled Trials as Topic/
15. Random Allocation/
16. Controlled Clinical Trials as Topic/
17. control groups/
18. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
19. double‐blind method/
20. single‐blind method/
21. Placebos/
22. placebo effect/
23. Drug Evaluation/
24. Research Design/
25. randomized controlled trial.pt.
26. controlled clinical trial.pt.
27. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
28. (random$ or RCT or RCTs).tw.
29. (controlled adj5 (trial$ or stud$)).tw.
30. (clinical$ adj5 trial$).tw.
31. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
32. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseud or random$).tw.
33. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
34. placebo$.tw.
35. controls.tw.
36. exp animals/ not humans.sh.
37. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35
38. 6 and 13 and 37
39. 38 not 36
40. limit 39 to yr="2013 ‐Current"
Appendix 3. Embase (Ovid) search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or vertebrobasilar insufficiency/ or stroke/ or stroke patient/ or stroke unit/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. 1 or 2 or 3 or 4
6. exp 4 aminobutyric acid receptor stimulating agent/
7. 4 aminobutyric acid/ct, ad, dt or exp 4 aminobutyric acid receptor/ct, dt
8. ((gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) adj5 (agonist$ or modulator$ or stimul$)).tw.
9. ((gabaergic or gaba‐ergic or gabamimetic) adj5 (agent$ or drug$ or stimul$)).tw.
10. (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986).tw.
11. 6 or 7 or 8 or 9 or 10
12. 5 and 11
13. Randomized Controlled Trial/ or Randomization/
14. Controlled Study/
15. control group/
16. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
17. Double Blind Procedure/
18. Single Blind Procedure/ or triple blind procedure/
19. placebo/
20. "types of study"/
21. trial.ti.
22. (random$ or RCT or RCTs).tw.
23. (controlled adj5 (trial$ or stud$)).tw.
24. (clinical$ adj5 trial$).tw.
25. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
26. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
27. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
28. placebo$.tw.
29. controls.tw.
30. or/13‐29
31. 12 and 30
32. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
33. 31 not 32
34. limit 33 to yr="2013 ‐Current"
Appendix 4. CINAHL (EBSCO) search strategy
S1. (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
S2. (MH "Stroke Patients") OR (MH "Stroke Units")
S3. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S4. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral)
S5. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S6. S4 and S5
S7. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S8. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)
S9. S7 and S8
S10. S1 OR S2 OR S3 OR S6 OR S9
S11. (MH "GABA Agonists+") or (MH "GABA Modulators+")
S12. (MH "GABA/TU")
S13. TI (gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) or AB (gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B)
S14. TI (agonist* or modulator* or stimulat* or stimulant*) or AB (agonist* or modulator* or stimulat* or stimulant*)
S15. S13 and S14
S16. TI (gabaergic or gaba‐ergic or gabamimetic) or AB (gabaergic or gaba‐ergic or gabamimetic)
S17. TI (agent* or drug* or stimul*) or AB (agent* or drug* or stimul*)
S18. S16 and S17
S19. TI (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986) or AB (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986)
S20. S11 OR S12 or S15 or S18 or S19
S21. S10 AND S20
Appendix 5. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. 1 or 2 or 3 or 4
6. ((gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) adj5 (agonist$ or modulator$ or stimulat$ or stimulant$)).tw.
7. ((gabaergic or gaba‐ergic or gabamimetic) adj5 (agent$ or drug$ or stimul$)).tw.
8. (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986).tw.
9. 6 or 7 or 8
10. 5 and 9
11. limit 10 to yr="2013 ‐Current"
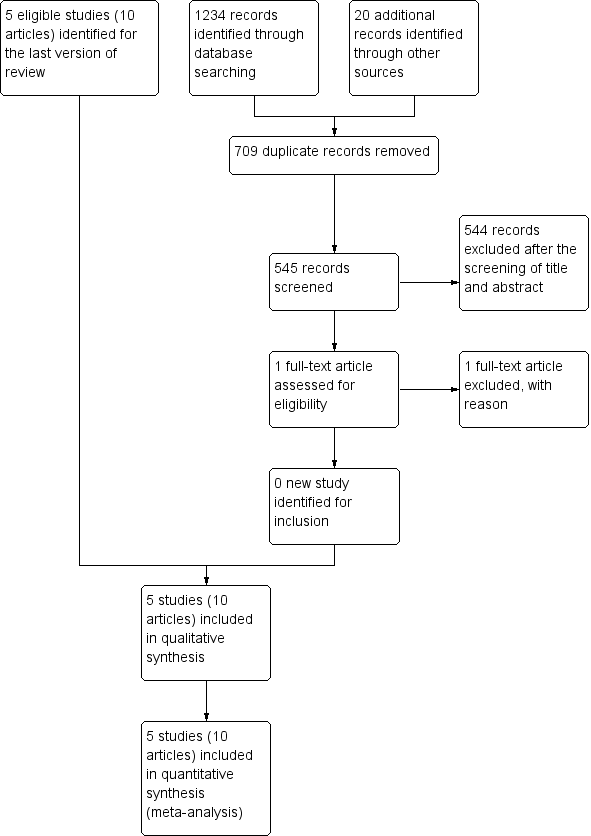
Study flow diagram.
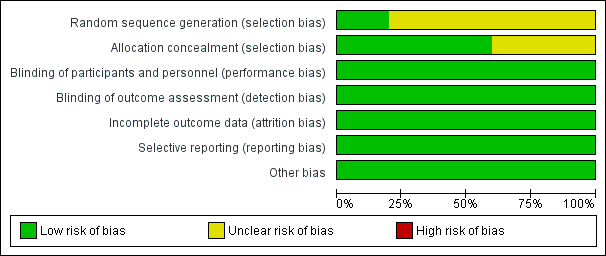
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
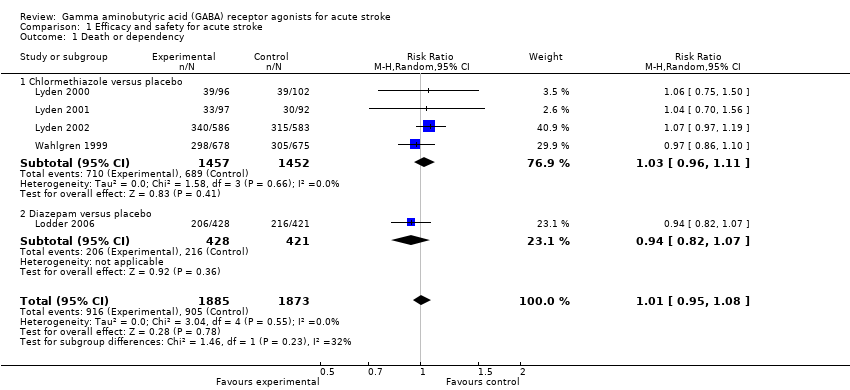
Comparison 1 Efficacy and safety for acute stroke, Outcome 1 Death or dependency.
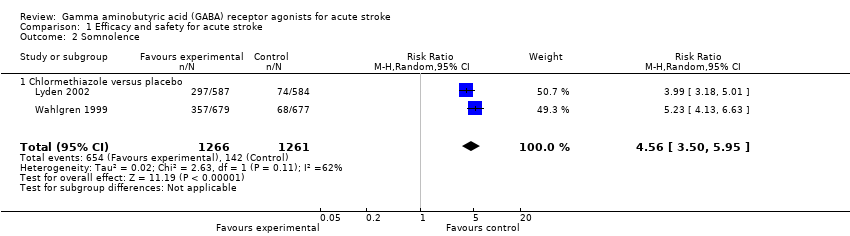
Comparison 1 Efficacy and safety for acute stroke, Outcome 2 Somnolence.
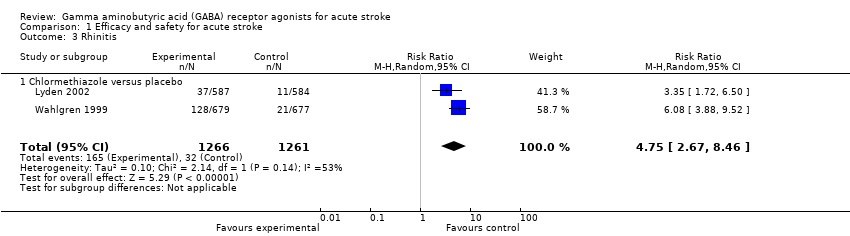
Comparison 1 Efficacy and safety for acute stroke, Outcome 3 Rhinitis.
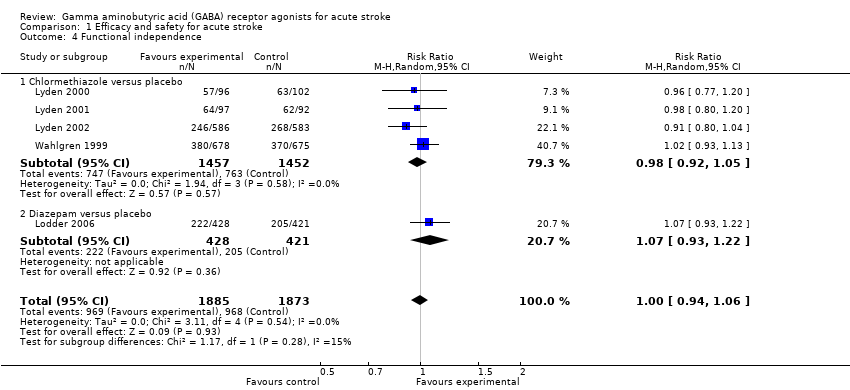
Comparison 1 Efficacy and safety for acute stroke, Outcome 4 Functional independence.
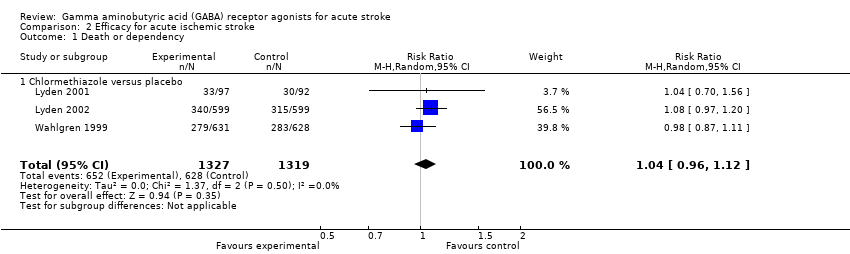
Comparison 2 Efficacy for acute ischemic stroke, Outcome 1 Death or dependency.

Comparison 2 Efficacy for acute ischemic stroke, Outcome 2 Functional independence.
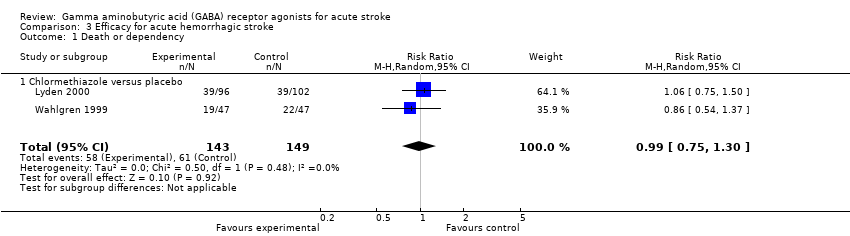
Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 1 Death or dependency.

Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 2 Functional independence.

Comparison 4 Efficacy for TACS, Outcome 1 Functional independence.
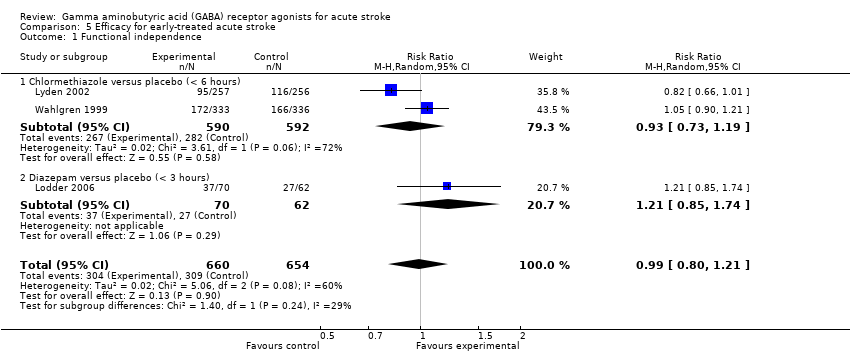
Comparison 5 Efficacy for early‐treated acute stroke, Outcome 1 Functional independence.
| Chlormethiazole compared with placebo for acute stroke | ||||||
| Patient or population: people with acute stroke Settings: inpatients Intervention: chlormethiazole Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Chlormethiazole | |||||
| Death or dependency | 475 per 1000 | 487 per 1000 | RR 1.03 (0.96 to 1.11) | 2909 (4) | ⊕⊕⊕⊝ | — |
| Adverse events | Somnolence 113 per 1000 Rhinitis 25 per 1000 | Somnolence 517 per 1000 Rhinitis 130 per 1000 | RR 4.56 (3.50 to 5.95) RR 4.75 (2.67 to 8.46) | 2527 (2) | ⊕⊕⊕⊝ | — |
| Functional independence | 525 per 1000 | 513 per 1000 | RR 0.98 (0.92 to 1.05) | 2909 (4) | ⊕⊕⊕⊝ | — |
| Other stroke scales | — | — | — | NIHSS 1367 (2) SSS 2727 (3) | ⊕⊕⊕⊝ | In Lyden 2000, the mean change of the NIHSS score was ‐4.5 in the chlormethiazole group (N = 96) and ‐4.0 in the placebo group (N = 102; P = 0.36). In Lyden 2002, the change of NIHSS score (median (quartiles)) was ‐5.5 (‐11, 17) in the chlormethiazole group (N = 586) and ‐6.0 (‐10, 16) in the placebo group (N = 583; P = 0.68). In Wahlgren 1999, no significant difference was found between the placebo and chlormethiazole groups for the change in score in the SSS 48‐point (P = 0.56) and SSS motor power score (P = 0.96). In Lyden 2000 and Lyden 2002, the change in score in the SSS was not significant in the two groups (P = 0.06 and P = 0.23, respectively). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to unclear risk of selection bias Functional independence, defined as a BI score higher than 60 or a mRS score less than 3 | ||||||
| Diazepam compared with placebo for acute stroke | ||||||
| Patient or population: people with acute stroke Settings: inpatients Intervention: diazepam Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Diazepam | |||||
| Death or dependency | 513 per 1000 | 481 per 1000 | RR 0.94 (0.82 to 1.07) | 849 (1) | ⊕⊕⊕⊝ | — |
| Adverse events | 357 per 1000 | 355 per 1000 | RR 0.99 (0.75 to 1.31) | 865 (1) | ⊕⊕⊕⊝ | — |
| Functional independence | 487 per 1000 | 519 per 1000 | RR 1.07 (0.93 to 1.22) | 849 (1) | ⊕⊕⊕⊝ | — |
| Other stroke scales | Not reported | Not reported | — | — | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level: one study with small sample size Functional independence, defined as a BI score higher than 60 or a mRS score less than 3 | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 1.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 1.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 2 Somnolence Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 2.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 3 Rhinitis Show forest plot | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 3.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 4 Functional independence Show forest plot | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 4.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 1.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 2 Functional independence Show forest plot | 4 | 3394 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.08] |
| 2.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.05] |
| 2.2 Diazepam versus placebo | 1 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency Show forest plot | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 1.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 2 Functional independence Show forest plot | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 2.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2.2 Diazepam versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| 1.1 Chlormethiazole versus placebo | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional independence Show forest plot | 3 | 1314 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.21] |
| 1.1 Chlormethiazole versus placebo (< 6 hours) | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 1.2 Diazepam versus placebo (< 3 hours) | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.74] |

