Tramadol para el tratamiento del dolor postoperatorio en niños
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT Parallel design | |
| Participants | Adeno‐tonsillectomy, n = 90 (46 males, 44 females), children aged 4 to 10 years Group 1: 16 males, 14 females; mean age 7.46 years (SD 1.85) Group 2: 15 males, 15 females; mean age 7.53 years (SD 1.88) Group 3: 15 males, 15 females; mean age 7.61 years (SD 1.93) | |
| Interventions | Group 1: placebo (saline) po + placebo (saline) iv (n = 30) Group 2: dextromethorphan 1 mg/kg po + placebo (saline) iv (n = 30) Group 3: placebo (saline) plus tramadol 1 mg/kg iv (n = 30) Administration time: 30 minutes before arrival in the operating room and during induction of anaesthesia | |
| Outcomes | ‐ Postoperative pain scales (FPS) (1, 2, 3, 4, 5, 6 h postoperation) ‐ Analgesic and antiemetic requirements during 6‐h observation period ‐ Number of patients with adverse events (vomiting, respiratory parameters) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "Each patient was randomly assigned to one of three groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "A sealed envelope method was used for randomization." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomized, prospective, doubleblind and placebo‐controlled study design" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes are measured and fully reported. |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Tonsillectomy, n = 45 (20 males, 25 females), children aged 5 to 15 years Group 1: 7 males, 8 females; mean age 12.5 years (SD 1.9) Group 2: 6 males, 9 females; mean age 12.5 years (SD 2.3) Group 3: 7 males, 8 females; mean age 11.9 years (SD 2.4) | |
| Interventions | Group 1: placebo iv (n=15) Group 2: 2 mg/kg ketoprofen iv (n = 15) Group 3: 1 mg/kg tramadol iv (n = 15) Administration time: after the induction of anaesthesia and before surgical incision; after surgery the randomised study treatment was continued as a 6‐h iv infusion in which the child received either another dose of saline control, ketoprofen (2 mg/kg) or tramadol (1 mg/kg) | |
| Outcomes | ‐ Postoperative pain scales (VAS) (30 min, 60 min, 90 min, 2 h, 6 h, and 24 h postoperation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to treatment groups was performed by computer in blocks of nine patients." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Children were prospectively randomized to one of the three treatment groups" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The double‐blind nature of the study was confirmed by having a nurse, not otherwise participating in the treatment of the patient, to prepare the coded study treatment syringes and infusions." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | Open trial Parallel design | |
| Participants | Lower abdominal surgery (appendectomy, hernia repair, testicular or urethral surgery), n = 75 (61 males, 14 females), children aged 2 to 12 years Group 1: 22 males, 9 females; mean age 5.4 years (SD 2.6) Group 2: 20 males, 5 females; mean age 6.3 years (SD 1.9) Group 3: 19 males, 6 females; mean age 6.2 years (SD 2.8) | |
| Interventions | Group 1: 0.1 mg/kg nalbuphine im (n = 25) Group 2: 2 mg/kg tramadol im (n = 25) Group 3: 1 mg/kg pethidine im (n = 25) Administration time: after the end of surgery (first expression of pain) an additional injection of the half initial dose was administrated 30 and 60 min, if inadequate analgesia | |
| Outcomes | ‐ Postoperative pain scales (VRS) (0.5, 1, 3, 6, 12, 24 h postoperation) ‐ Number of patients with no or slight pain 1 h postoperation ‐ Total analgesic consumption 24 h postoperation ‐ Frequency of administration 24 h postoperation ‐ Investigators' assessment of overall pain relief (excellent, very good, good, satisfactory, poor) at 24 h postoperation; number, nature, time of onset of adverse events (vomiting, haemodynamic, respiratory parameters); overall assessment of tolerability 24 h postoperation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "The children were randomised into 3 groups of 25,..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The study was intended to be a double‐blind (observer blinded),.." |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "drug dosages were recorded in the patient records,..." |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | High risk | Verbal rating scale (VRS) was also used in the youngest children, which were 2 years old. However, at this age, this scale is not validated |
| Methods | RCT Parallel design | |
| Participants | Inguinal surgery (orchidopexy, herniotomy), n = 88 (87 males, 1 female), children aged 2 to 10 years Group 1: 22 males, mean age 6 years (SD 3) Group 2: 22 males, mean age 5 years (SD 2) Group 3: 21 males, 1 female; mean age 5 years (SD 2) Group 4: 22 males, mean age 5 years (SD 2) | |
| Interventions | Group 1: tramadol 1 mg/kg iv (n = 22) Group 2: tramadol 2 mg/kg iv (n = 22) Group 3: pethidine 1 mg/kg iv (n = 22) Group 4: placebo (n = 22) Administration time: after induction of anaesthesia | |
| Outcomes | ‐ Documentation of respiratory and haemodynamic parameters during anaesthesia ‐ Postoperative pain scales (five‐point verbal rating scale) (1, 2, 3, 4, 5,6 h postoperation) ‐ Recovery from anaesthesia ‐ Number of patients with the need for rescue analgesia ‐ Number of patients with moderate to severe pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated randomisation." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "preserve blinding, the study medication was prepared in identical syringes by a designated third party" "Randomisation codes were masked to the patient, the investigator and nursing staff." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | Postoperative pain was measured, but was only selectively reported. An overall pain intensity score was described, but no values at time points were specified |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Major neurosurgical surgery, n = 42 (23 males, 20 female) Group 1: 10 males, 8 females; mean age 67.8 months (SD 66.5) Group 2: 6 males, 5 females; mean age 62 months (SD 50) Group 3: 7 males, 7 female; mean age 74 months (SD 47.5) | |
| Interventions | Group 1: tramadol 1 mg/kg iv (n = 14) Group 2: tramadol 0.5 mg/kg iv followed by continuous infusion at the rate of 150 μg/kg/h (n = 14) Group 3: fentanyl by continuous infusion at the rate of 2 µg/kg/h (n = 14) Administration time: at the induction of general anaesthesia | |
| Outcomes | ‐ Postoperative pain scales (AFS scale and CHEOPS score) (every 4 h for 12 to 36 h postoperation) ‐ Documentation of respiratory and haemodynamic parameters postoperation ‐ Number of patients with adverse events (vomiting, nausea, apnoea, and bradycardia) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "Patients were allocated randomly to three intraoperative treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Unclear risk | No detailed information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Tonsillectomy, n = 90 (42 males, 48 female), children aged 2 to 9 years Group 1: 14 males, 16 females; mean age 6.3 years (SD 0.002) Group 2: 14 males, 16 females; mean age 6.17 years (SD 1.599) Group 3: 14 males, 16 female; mean age 6.1 years (SD 1.517) | |
| Interventions | Group 1: placebo (saline) iv (n = 30) Group 2: 1.5 ml 0.75% ropivacaine to the tonsil lodge (n = 30) Group 3: tramadol 1 mg/kg iv (n = 30) Administration time: after induction of anaesthesia | |
| Outcomes | ‐ Documentation of haemodynamic parameters for 24 h postoperation ‐ Postoperative pain scales at resting and swallowing (Maunuksela pain scores) (1, 2, 3, 6, 9, 12, 15, 18, 21, 24 h postoperation) ‐ Number of patients with adverse events (hypotension, breathing difficulties and bleeding, vomiting, nausea) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... paediatric tonsillectomies: A randomized placebo controlled study", "Randomization was provided by the use of a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Unclear risk | No detailed information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Lower abdominal surgery (e.g. hernia repair, appendicectomy, testicular or ureteral surgery), n = 110, children aged 2 to 12 years Group 1: mean age 5.7 years (SD 0.39) Group 2: mean age 5.65 years (SD 0.56) | |
| Interventions | Group 1: pethidine 1 mg/kg iv (n = 60) Group 2: tramadol 2 mg/kg iv (n = 50) Administration time: at the induction of anaesthesia | |
| Outcomes | ‐ Documentation of haemodynamic parameters and sedation score for 24 h postoperation ‐ Postoperative pain scales (4‐point restlessness–pain protocol) (5, 10, 20, 30 min, 6 and 24 h postoperation) ‐ Number of patients with adverse events (episodes of vomiting, nausea, apnoea and bradycardia) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: " ... we designed prospective, randomized, controlled study." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information Quote: "Patients were allocated randomly to receive either.." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: " ...were recorded by an assistant investigating anaesthetist, who was blind to test drug ..." "Physicians in recovery room, blind to the test drug...assessed residual analgesia..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, but pain scores values were not specified |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Tonsillectomy or adeno‐tonsillectomy, n = 60, children aged 2 to 14 years Group 1: mean age 8.1 years (SD 4.2) Group 2: mean age 7.1 years (SD 3.6) Group 3: mean age 7 years (SD 4.2) | |
| Interventions | Group 1: morphine 0.1 mg/kg iv (n = 20) Group 2: tramadol 1 mg/kg iv (n = 20) Group 3: tramadol 2 mg/kg iv (n = 20) Administration time: after induction of anaesthesia All patients received diclofenac (1 mg/kg) rectally prior to commencing surgery | |
| Outcomes | ‐ Postoperative pain scales (1 = pain free, 5 = worst possible pain) (every 4 hours for 24 h postoperation) ‐ Sedation scores, analgesic requirements, respiratory rate, nausea and episodes of vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We conducted a prospective, double‐blind, randomized controlled trial in children ..." "A computer‐generated randomization table ..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Each patient volunteer was given a subsequent study number, which determined the drug and dose to be given. The code was not broken during the study." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All study drugs were diluted in water for injection to the total volume of 10 ml and given to the attending anaesthetist without disclosing the drug." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Adeno‐tonsillectomy, n = 45 (18 male, 27 female) children aged 1 to 7 years Group 1: 7 male, 8 female; median age 4.26 years (SD 1.57) Group 2: 6 male, 9 female; mean age 4.07 years (SD 1.54) Group 3: 5 male, 10 female; mean age 3.93 years (SD 1.66) | |
| Interventions | Group 1: ketamine 0.5 mg/kg po (n = 15) Group 2: tramadol 1 mg/kg po (n = 15) Group 3: meperidine 1 mg/kg po (n = 15) Administration time: after induction of anaesthesia | |
| Outcomes | ‐ Postoperative pain scales (Toddler–Preschooler Postoperative Pain Scale) (30, 60, 120 and 240 min after extubation) ‐ Postoperative agitation scores ‐ Time to opening eyes upon command (min) ‐ Number of patients with vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "After induction of anaesthesia and before tracheal intubation, children were randomly allocated to receive.." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Post‐operative pain was scored blind ..."; "Post‐operative pain was assessed in the recovery room 30, 60, 120 and 240 min after tracheal extubation by a blinded observer ..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Adeno‐tonsillectomy, n = 60, children aged 1 to 8 years Group 1: median age 5.0 years (P25 to P75 4.5 to 7.0) Group 2: mean age 5.0 years (P25 to P75 3.0 to 7.0) | |
| Interventions | Group 1: morphine 0.1 mg/kg iv (n = 28) Group 2: tramadol 1 mg/kg iv (n = 32) Administration time: at induction of anaesthesia | |
| Outcomes | ‐ Postoperative pain scales (PMH Pain Assessment Tool) (0, 15, 30 min; 1, 2, 3, 4, 5, 6 h postoperation) ‐ Sedation score ‐ Time to first analgesic requirement and total numbers of doses ‐ Number of patients with respiratory depression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "... in this double blinded, prospective, controlled trial." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Unclear risk | No detailed information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Six other patients were excluded because of protocol violations or inadequate data collection" |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, but pain scores values were only incompletely specified: ‐ only description of number of patient with pain score > 6 at the first three study time points ‐ no pain score values |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Surgical procedures with expected moderate to severe postoperative pain (e.g. perineal fistula and bladder neck closure, bilateral oblique pelvic osteotomy, exploratory laparotomy, bilateral elongation of Achilles tendon, etc), n = 24 (14 males, 10 females; children aged 1 to 10 years) Group 1: 7 males, 5 females; mean age 6.2 years (2.5 to 10) Group 2: 7 males, 5 females; mean age 4.4 years (1.6 to 10) | |
| Interventions | Group 1: nalbuphine iv (bolus 100 μg/kg, 0.2 μg/kg/min for 72 h) (n = 12) Group 2: tramadol iv (bolus 1 mg/kg, 2.0 μg/kg/min for 72 h) (n = 12) Administration time: before the end of surgery | |
| Outcomes | ‐ Postoperative pain scales (FPS, VAS) (every 1 h for the first 24 h, then every 4 h until the end of the 72 h study period) ‐ Documentation of haemodynamic and respiratory parameters during 72 h study period ‐ Sedation scores, episodes of vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By means of a predesigned table of random numbers, children were allocated to receive..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "In order to preserve the double‐blind design of the study, drugs were prepared on a case‐by‐case basis, according to the predesigned table of random numbers, by one of the investigators of the study (JCR‐M) who did not participate in the evaluation of patients’ eligibility or study outcomes. Evaluation of patients’ eligibility, pain score in younger children, application of the visual analogue scale (VAS) in older children, and evaluation of adverse events during the study period were performed by an investigator (JCH‐P) who was unaware of the patients’ allocation group. Adherence to the protocol was also monitored by another investigator (DM‐G)." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, pain intensities were not mentioned within the text |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Adeno‐tonsillectomy, n = 60 (37 males, 23 females), children aged 6 to 12 years Group 1: 18 males, 12 females; mean age 7 years (6 to 11) Group 2: 19 males, 11 females; mean age 6 years (6 to 12) | |
| Interventions | Group 1: tramadol iv (loading dose 1 mg/kg, bolus 0.2 mg/kg/10min) (n = 30) Group 2: morphine iv (loading dose 0.1 mg/kg, bolus 0.02 mg/kg/10min) (n = 30) Administration time: after surgery | |
| Outcomes | ‐ Documentation of haemodynamic and respiratory parameters during and after anaesthesia ‐ Postoperative pain scales (CHEOPS) (0, 15, 30, 60 min; 2, 4, 6, 24 h postoperation) ‐ Documentation of sedation score ‐ Cumulative opioid consumption ‐ Number of patients with adverse events (urinary retention, nausea) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "This prospective, randomized, double‐blind study was designed..." "Randomization was performed consecutively." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information Quote: "The patients were then randomly allocated to receive..." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Thus both observers and patients were blinded to the drug used." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured. Pain intensity score (CHEOPS) was described, but no values were reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Tonsillectomy with or without adenoidectomy, n = 50 (23 males, 27 females), children aged 4 to 7 years Group 1: 10 males, 15 females; mean age 5.7 years (SD 1.5) Group 2: 13 males, 12 females; mean age 6.8 years (SD 1.6) | |
| Interventions | Group 1: tramadol 1 mg/kg iv (n = 25) Group 2: meperidine 1 mg/kg iv (n = 25) Administration time: after induction of anaesthesia | |
| Outcomes | ‐ Documentation of MAP perioperatively ‐ Postoperative pain score (FPS) at 0 min, 10 min, 20 min, 45 min postoperation) ‐ Opioid related adverse events like nausea and vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "Fifty children ... were randomly assigned... ." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: " The nurse in the PACU who was unaware of the nature of the test drug assessed the agitation score. The same nurse evaluated analgesia..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Tonsillectomy with or without adenoidectomy, n = 45 (27 males, 18 females), children aged 2 to 11 years) Group 1: 8 males, 7 females; mean age 5.1 years (SD 2.8) Group 2: 10 males, 5 females; mean age 5.3 years (SD 2.9) Group 3: 9 males, 6 females; mean age 5.6 years (SD 3.2) | |
| Interventions | Group 1: tramadol 0.5 mg/kg iv (n = 15) Group 2: tramadol 1 mg/kg iv (n = 15) Group 3: placebo iv (n = 15) Administration time: after induction of anaesthesia | |
| Outcomes | ‐ Postoperative pain score (FPS ≤ 6 years, VAS > 7 years; 15 min, 30 min, 60 min postoperation) ‐ Number of patients with need for additional analgesia ‐ Opioid related adverse events ‐ Documentation of HR and MAP perioperatively | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "... a randomized, double‐blind and placebo‐controlled protocol." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information Quote: "...these syringes were consecutively used." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The syringes were marked only with a coded label ... ." "...the author who was unaware of group assignment... assessed pain ... . " |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Dental extraction, n = 50 (26 males, 24 females), children aged 4 to 7 years Group 1: 21 males, 19 females; mean age 5.3 years (SD 1.1) Group 2: 5 males, 5 females; mean age 4.8 years (SD 1.2) | |
| Interventions | Group 1: tramadol 3 mg/kg + 0.5 mg/kg midazolam po (n = 40) Group 2: placebo + 0.5 mg/kg midazolam po (n = 10) Administration time: after induction of anaesthesia All children received midazolam | |
| Outcomes | ‐ Postoperative pain score (Oucher face pain scale) (15, 30, 60, and 120 min postoperation) ‐ Documentation of oxygen saturation ‐ Opioid related adverse events (respiratory, cardiovascular, or emetic incidents) ‐ Documentation of HR and MAP perioperatively | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "...were included in a double‐blind, randomized, placebo‐controlled trial..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The anaesthetist who did the anaesthetic assessments and the research sister who did the postanaesthetic recovery and pain assessments were not informed which study medication was administered." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Dental extraction, n = 60 (31 males, 29 females), children aged 4 to 7 years Group 1: 18 males, 14 females, mean age 5.3 years (SD 1.1) Group 2: 13 males, 15 females, mean age 5.1 years (SD 1.2) | |
| Interventions | Group 1: tramadol 1.5 mg/kg + midazolam po (n = 31) Group 2: placebo + midazolam po (n = 29) Administration time: after induction of anaesthesia All children received midazolam | |
| Outcomes | ‐ Postoperative pain score (Oucher face pain scale) (15, 30, 60, and 120 min postoperation) ‐ Opioid related adverse events (emesis) ‐ Documentation of postoperative recovery ‐ Number of patients with the need for rescue analgesia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "... were included in a double‐blind, randomized, placebo‐controlled trial.." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Neither, anaesthetist, ..., nor research sister who did the post‐anaesthetic recovery and pain assessments, were informed which study medication ..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Herniotomy, orchidopexy, circumcision, hydrocelectomy, n = 60, children aged 1 to 9 years Group 1: 25 males, 5 females; mean age 4.5 years (SD 1.9) Group 2: 26 males, 4 females; mean age 4.87 years (SD 2.4) | |
| Interventions | Group 1: 0.15 to 0.2 mg/kg nalbuphine im Group 2: 0.75 to 1 mg/kg tramadol im Administration time: after surgery (at the arrival in the recovery area) | |
| Outcomes | ‐ VAS pain scale (0 to 10 cm) (1, 2, 3, 4, 6, 8, 24 h postoperation) ‐ Number of adverse events (vomiting, headache, singultus, hallucination, dry mouth) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...each group received in a randomized and double‐blind manner ..."; "... received by an randomization plan..." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "...each group received in a randomized and double‐blind manner ..." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Adeno‐tonsillectomy, n = 60 (32 males, 28 females), children aged 5 to 12 years Group 1: 5 males, 10 females; mean age 6.9 (SD 2.1) Group 2: 9 males, 6 females; mean age 7.13 (SD 2.51) Group 3: 7 males, 8 females; mean age 6.06 (SD 2.51) Group 4: 7 males, 8 females; mean age 6.96 (SD 2.08) | |
| Interventions | Group 1: ketamine 0.5 mg/kg iv (n = 15) Group 2: morphine 0.1 mg/kg iv (n = 15) Group 3: tramadol 1.5 mg/kg iv (n = 15) Group 4: saline iv (n = 15) Administration time: during induction of anaesthesia | |
| Outcomes | ‐ Postoperative pain score (5‐P‐NRS, CHEOPS); 1 min, 5 min, 10 min, 15 min, 20 min, 30 min, 45 min, 60 min, 120 min, 240 min, 360 min postoperation ‐ Number of patients with need for additional analgesia ‐ Duration of postoperative analgesia, time to first rescue analgesia ‐ Opioid related adverse events (nausea, vomiting, sedation) ‐ Documentation of heart rate and oxygen saturation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information Quote: "A randomized, prospective, double‐blind and placebo‐controlled study design was used." |
| Allocation concealment (selection bias) | Low risk | Quote: "A sealed envelope method was used for randomization." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "A randomized, prospective, double‐blind and placebo‐controlled study design was used." "pain scores... were assessed by an independent observer." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "pain scores... were assessed by an independent observer." |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Tonsillectomy with and without adenoidectomy; n = 152 (99 males, 53 females), children and young adults aged 8 to 21 years Group 1: 25 males, 12 females; mean age 13 (SD 15) Group 2: 25 males, 13 females; mean age 14 (SD 15) Group 3: 26 males, 12 females; mean age 16 (SD 14) Group 4: 23 males, 16 females; mean age 15 (SD 14) | |
| Interventions | Group 1: saline (n = 37) Gropu 2: tramadol 3 mg/kg iv (n = 38) Group 3: pethidine 1.5 mg/kg iv (n = 38) Group 4: nalbuphine 0.3 mg/kg iv (n = 39) Administration time: 30 sec before induction of anaesthesia | |
| Outcomes | ‐ Postoperative pain score (restlessness‐pain score in PACU) ‐ Number of patients with need for postoperative additional analgesia ‐ Time to extubation ‐ Documentation of heart rate, blood pressure ‐ Opioid related adverse events (emesis, sedation) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A prospective, double‐blind, randomized, controlled study ... .", "Each patient was block randomized ... ." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The test drugs were injected by the investigating anaesthetist, who was not blind to the test drug injected." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Nurses in the PACU, blind to the test drug administered ..., assessed residual analgesia ... ." |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes were measured and completely reported |
| Other bias | Unclear risk | No apparent bias |
| Methods | RCT Parallel design | |
| Participants | Adenoidectomy, n = 80, children aged 1 to 3 years | |
| Interventions | Group 1: tramadol 2 mg/kg iv (n = 40) Group 2: saline iv (n = 40) Administration time: after induction of anaesthesia All children received rectal ibuprofen (10 mg/kg) before start of surgery | |
| Outcomes | ‐ Postoperative cumulative pethidine consumption ‐ Number of patients with the need for rescue analgesia ‐ Duration of postoperative analgesia, time to first rescue analgesia ‐ Opioid related adverse events (nausea, vomiting) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eighty children were allocated randomly... .", "According to a computer‐generated table of random numbers... ." |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both study drugs were prepared and administered by a nurse who did not otherwise participate in the care of the child." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "... assessed by a trained nurse who was blinded... ." |
| Incomplete outcome data (attrition bias) | Low risk | All patients were evaluated. There were no missing data |
| Selective reporting (reporting bias) | High risk | All outcomes were measured, but pain score values were not reported |
| Other bias | Unclear risk | No apparent bias |
iv = intravenously
po = per mouth
im = intramuscularly
SD = standard deviation
PONV = postoperative nausea and vomiting
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Intravenous patient‐controlled analgesia | |
| Peritonsillar infiltration of tramadol | |
| Only neonates were studied, continuous application of tramadol on an intensive care unit | |
| No children included | |
| Retrospective study | |
| Conference abstract | |
| Propofol injection pain was assessed | |
| Intravenous patient‐controlled analgesia | |
| Children and adult included, no differentiation possible | |
| Epidural injection | |
| Tested different dosages of tramadol (1 mg/kg versus 2 mg/kg) | |
| No RCT | |
| Caudal application | |
| Comparison with ilioinguinal and iliohypogastric block | |
| Retrospective study | |
| Conference abstract | |
| No RCT | |
| No RCT | |
| Retrospective study | |
| No RCT | |
| Versus paracetamol | |
| No RCT |
RCT = randomized controlled trial
iv = intravenous
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
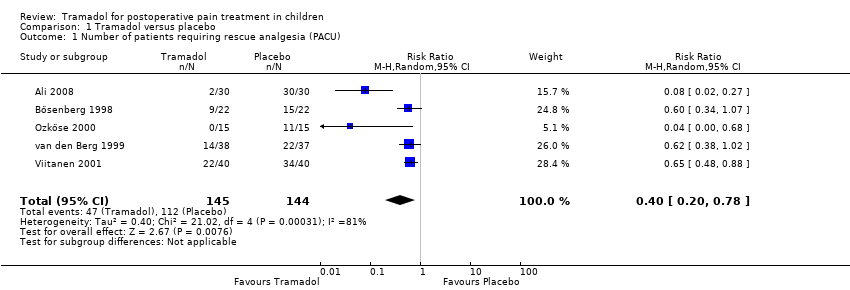
| 1 Number of patients requiring rescue analgesia (PACU) Show forest plot | 5 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.20, 0.78] |
| Analysis 1.1 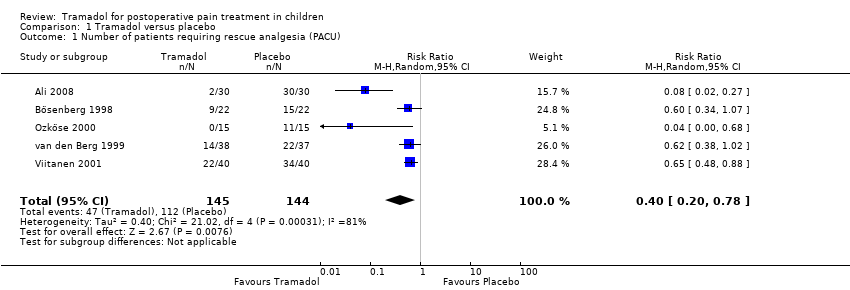 Comparison 1 Tramadol versus placebo, Outcome 1 Number of patients requiring rescue analgesia (PACU). | ||||
| 2 Number of patients requiring rescue analgesia (PACU) (Subgroup: Surgery) Show forest plot | 5 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| Analysis 1.2 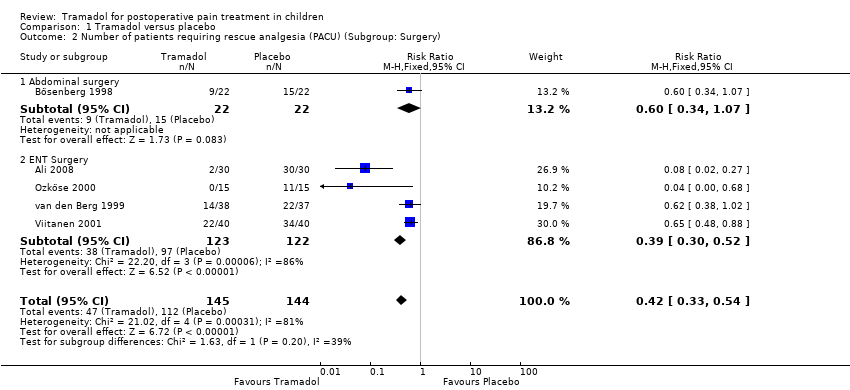 Comparison 1 Tramadol versus placebo, Outcome 2 Number of patients requiring rescue analgesia (PACU) (Subgroup: Surgery). | ||||
| 2.1 Abdominal surgery | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.34, 1.07] |
| 2.2 ENT Surgery | 4 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.30, 0.52] |
| 3 Number of patients requiring rescue analgesia (PACU) (Subgroup: Dose) Show forest plot | 5 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| Analysis 1.3  Comparison 1 Tramadol versus placebo, Outcome 3 Number of patients requiring rescue analgesia (PACU) (Subgroup: Dose). | ||||
| 3.1 Tramadol 1mg iv. | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.21] |
| 3.2 Tramadol 2mg iv. | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.48, 0.83] |
| 3.3 Tramadol 3mg iv. | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 4 Time to first rescue analgesic (min) Show forest plot | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 44.66 [‐24.26, 113.58] |
| Analysis 1.4  Comparison 1 Tramadol versus placebo, Outcome 4 Time to first rescue analgesic (min). | ||||
| 5 Number of patients with PONV (PACU) Show forest plot | 3 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.28, 2.52] |
| Analysis 1.5  Comparison 1 Tramadol versus placebo, Outcome 5 Number of patients with PONV (PACU). | ||||
| 6 Number of patients with PONV (24h postop) Show forest plot | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.12] |
| Analysis 1.6  Comparison 1 Tramadol versus placebo, Outcome 6 Number of patients with PONV (24h postop). | ||||
| 7 Number of patients with respiratory depression Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.7  Comparison 1 Tramadol versus placebo, Outcome 7 Number of patients with respiratory depression. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients requiring rescue analgesia (PACU) Show forest plot | 3 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.83, 1.89] |
| Analysis 2.1 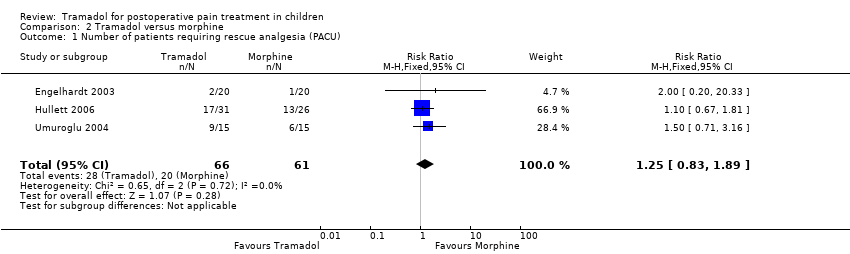 Comparison 2 Tramadol versus morphine, Outcome 1 Number of patients requiring rescue analgesia (PACU). | ||||
| 2 Number of patients requiring rescue analgesia (24h postop) Show forest plot | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.65, 4.04] |
| Analysis 2.2  Comparison 2 Tramadol versus morphine, Outcome 2 Number of patients requiring rescue analgesia (24h postop). | ||||
| 3 Number of patients with respiratory depression Show forest plot | 4 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.51] |
| Analysis 2.3 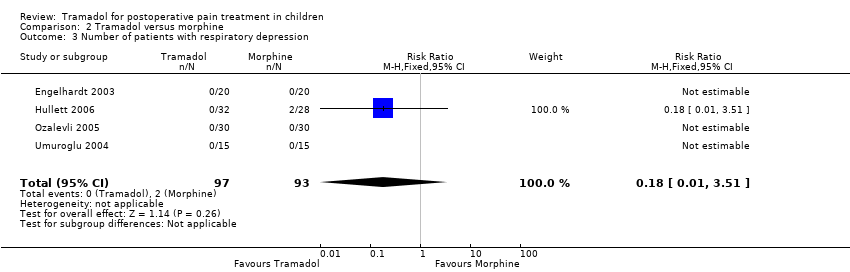 Comparison 2 Tramadol versus morphine, Outcome 3 Number of patients with respiratory depression. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients requiring rescue analgesia (24h postop) Show forest plot | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.16, 2.45] |
| Analysis 3.1  Comparison 3 Tramadol versus nalbuphine, Outcome 1 Number of patients requiring rescue analgesia (24h postop). | ||||
| 2 Number of patients with PONV (PACU) Show forest plot | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 2.01] |
| Analysis 3.2  Comparison 3 Tramadol versus nalbuphine, Outcome 2 Number of patients with PONV (PACU). | ||||
| 3 Number of patients with respiratory depression Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.3  Comparison 3 Tramadol versus nalbuphine, Outcome 3 Number of patients with respiratory depression. | ||||
| 4 Number of patients with bradycardia Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.4  Comparison 3 Tramadol versus nalbuphine, Outcome 4 Number of patients with bradycardia. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients requiring rescue analgesia (PACU) Show forest plot | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.43, 2.02] |
| Analysis 4.1  Comparison 4 Tramadol versus pethidine, Outcome 1 Number of patients requiring rescue analgesia (PACU). | ||||
| 2 Number of patients with moderate/severe pain (PACU) Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.16] |
| Analysis 4.2  Comparison 4 Tramadol versus pethidine, Outcome 2 Number of patients with moderate/severe pain (PACU). | ||||
| 3 Number of patients with PONV (PACU) Show forest plot | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.02] |
| Analysis 4.3 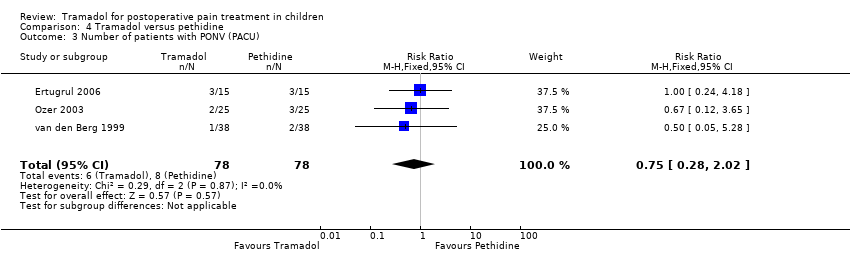 Comparison 4 Tramadol versus pethidine, Outcome 3 Number of patients with PONV (PACU). | ||||
| 4 Number of patients with respiratory depression Show forest plot | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.03] |
| Analysis 4.4  Comparison 4 Tramadol versus pethidine, Outcome 4 Number of patients with respiratory depression. | ||||
| 5 Number of patients with bradycardia Show forest plot | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.5 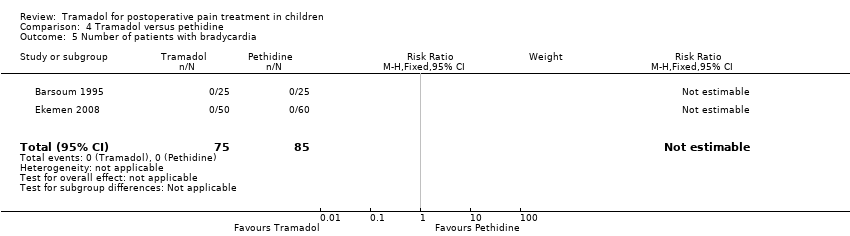 Comparison 4 Tramadol versus pethidine, Outcome 5 Number of patients with bradycardia. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
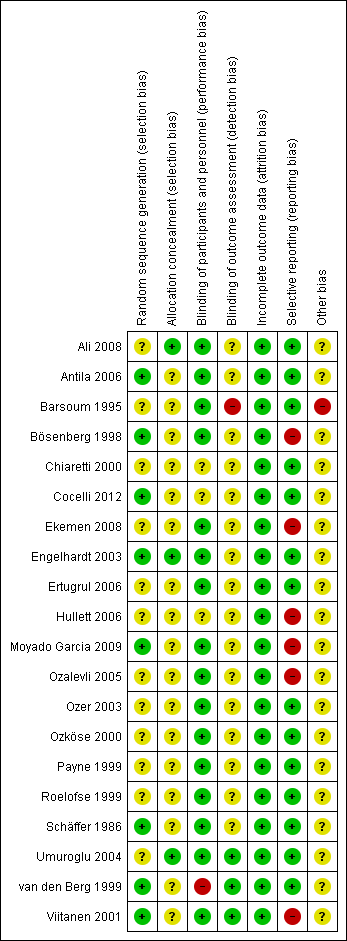
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Tramadol versus placebo, Outcome 1 Number of patients requiring rescue analgesia (PACU).
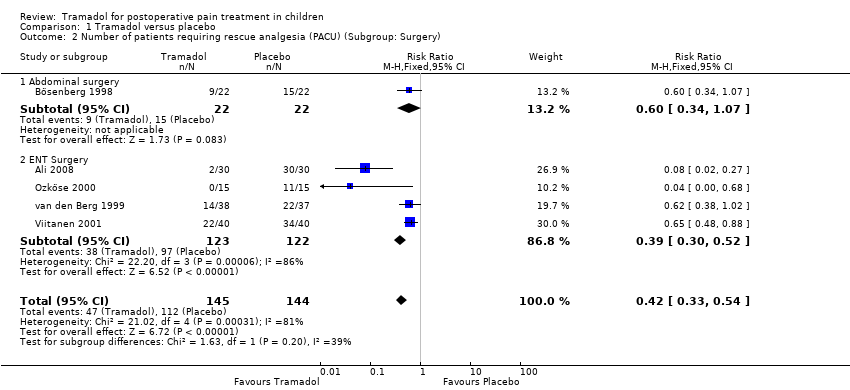
Comparison 1 Tramadol versus placebo, Outcome 2 Number of patients requiring rescue analgesia (PACU) (Subgroup: Surgery).

Comparison 1 Tramadol versus placebo, Outcome 3 Number of patients requiring rescue analgesia (PACU) (Subgroup: Dose).

Comparison 1 Tramadol versus placebo, Outcome 4 Time to first rescue analgesic (min).

Comparison 1 Tramadol versus placebo, Outcome 5 Number of patients with PONV (PACU).
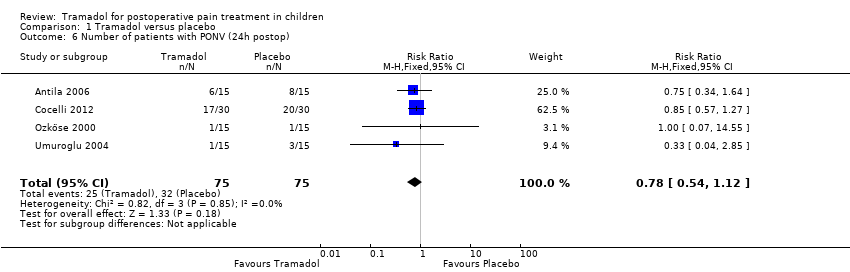
Comparison 1 Tramadol versus placebo, Outcome 6 Number of patients with PONV (24h postop).
Comparison 1 Tramadol versus placebo, Outcome 7 Number of patients with respiratory depression.

Comparison 2 Tramadol versus morphine, Outcome 1 Number of patients requiring rescue analgesia (PACU).
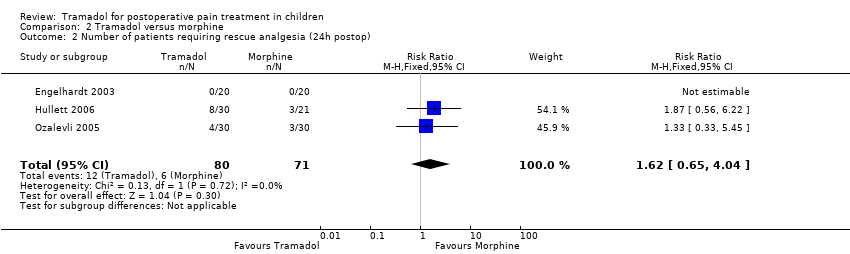
Comparison 2 Tramadol versus morphine, Outcome 2 Number of patients requiring rescue analgesia (24h postop).
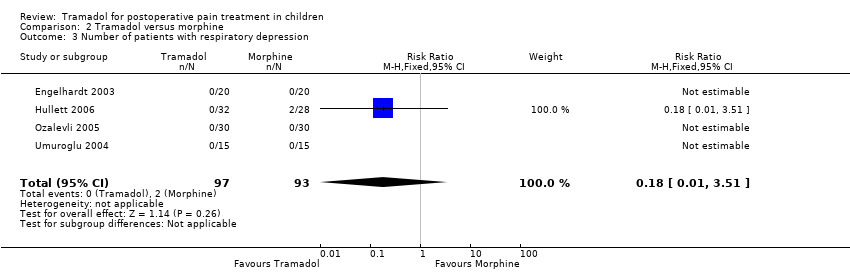
Comparison 2 Tramadol versus morphine, Outcome 3 Number of patients with respiratory depression.
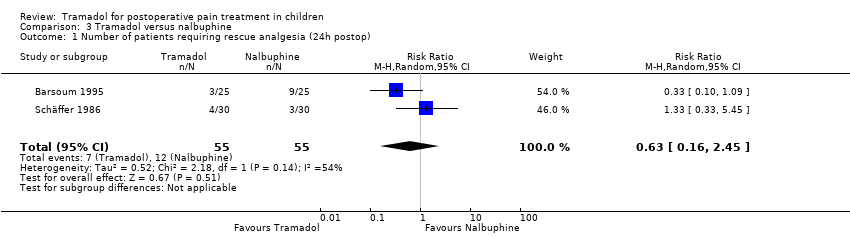
Comparison 3 Tramadol versus nalbuphine, Outcome 1 Number of patients requiring rescue analgesia (24h postop).

Comparison 3 Tramadol versus nalbuphine, Outcome 2 Number of patients with PONV (PACU).

Comparison 3 Tramadol versus nalbuphine, Outcome 3 Number of patients with respiratory depression.

Comparison 3 Tramadol versus nalbuphine, Outcome 4 Number of patients with bradycardia.
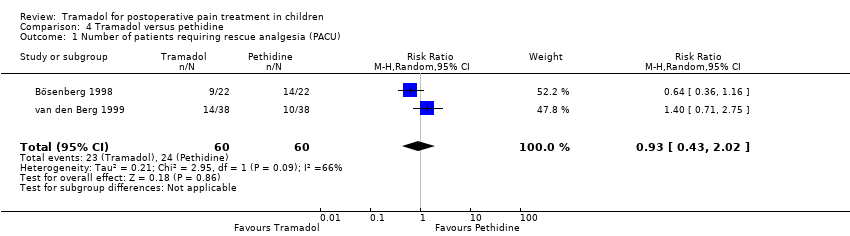
Comparison 4 Tramadol versus pethidine, Outcome 1 Number of patients requiring rescue analgesia (PACU).

Comparison 4 Tramadol versus pethidine, Outcome 2 Number of patients with moderate/severe pain (PACU).
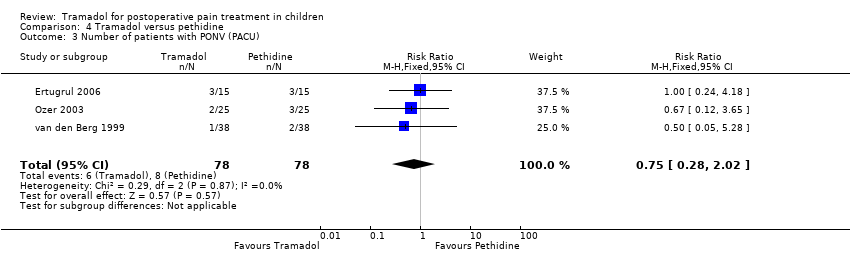
Comparison 4 Tramadol versus pethidine, Outcome 3 Number of patients with PONV (PACU).
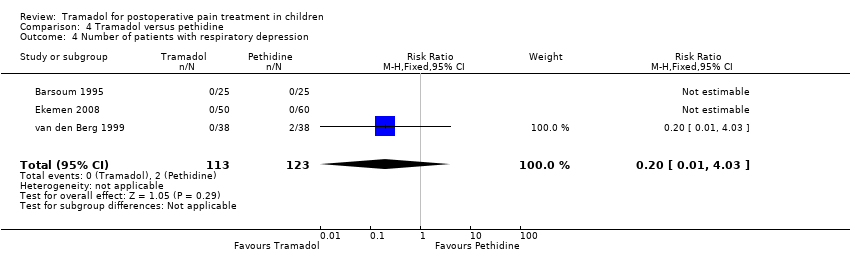
Comparison 4 Tramadol versus pethidine, Outcome 4 Number of patients with respiratory depression.
Comparison 4 Tramadol versus pethidine, Outcome 5 Number of patients with bradycardia.
| Tramadol compared with placebo for postoperative pain in children | ||||
| Patient or population: children undergoing surgery Settings: hospital Intervention: 1 to 3 mg/kg tramadol intravenously Comparison: placebo | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Number of patients requiring rescue analgesia (PACU) | RR 0.40 (0.20 to 0.78) | 289 (5) | ⊕⊕⊝⊝ | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| Number of patients with moderate to severe pain (PACU) | 44 (1) | ⊕⊝⊝⊝ | triple‐downgraded, due to high risk of publication bias, limitations in the study design (use of non‐validated pain scale) and imprecision of results (wide CIs) | |
| Number of patients with PONV (PACU) | RR 0.84 (0.28 to 2.52) | 215 (3) | ⊕⊕⊕⊝ | downgraded due to imprecision of results (wide CIs) |
| Number of patients with PONV (24 hours postoperatively) | RR 0.78 (0.54 to 1.12) | 150 (4) | ⊕⊕⊕⊝ | downgraded due to imprecision of results (wide CIs) |
| GRADE Working Group grades of evidence | ||||
| RR = relative risk CI = confidence interval PONV = postoperative nausea and vomiting PACU = postoperative care unit | ||||
| Tramadol compared with morphine for postoperative pain in children | ||||
| Patient or population: children undergoing surgery Settings: hospital Intervention: 1 to 2 mg/kg tramadol intravenously Comparison: 0.1 mg/kg morphine intravenously | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Number of patients requiring rescue analgesia (PACU) | RR 1.25 (0.83 to 1.89) | 127 (3) | ⊕⊕⊝⊝ | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| Number of patients with moderate to severe pain (PACU) | no data available | |||
| Number of patients requiring rescue analgesia (24 hours postoperatively) | RR 1.62 (0.65 to 4.04) | 151 (3) | ⊕⊕⊝⊝ | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| GRADE Working Group grades of evidence | ||||
| RR = relative risk CI = confidence interval PONV = postoperative nausea and vomiting PACU = postoperative care unit | ||||
| Tramadol compared with nalbuphine for postoperative pain in children | ||||
| Patient or population: children undergoing surgery Settings: hospital Intervention: 0.75 to 3 mg/kg tramadol intravenously or intramuscularly Comparison: 0.1 to 0.3 mg/kg nalbuphine intravenously or intramuscularly | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Number of patients requiring rescue analgesia (24 hours postoperatively) | RR 0.63 (0.16 to 2.45) | 110 (2) | ⊕⊝⊝⊝ | triple‐downgraded due to unexplained heterogeneity, limitations in the study design (use of different pain scales and triggers for rescue analgesia) and imprecision of results (wide CIs) |
| Number of patients with moderate to severe pain (PACU) | 50 (1) | ⊕⊝⊝⊝ | triple‐downgraded, due to high risk of publication bias, limitations in the study design (use of non‐validated pain scale) and imprecision of results (wide CIs) | |
| Number of patients with PONV (PACU) | RR 1.00 (0.50 to 2.01) | 137 (2) | ⊕⊕⊝⊝ | double‐downgraded due to unexplained heterogeneity and imprecision of results (wide CIs) |
| GRADE Working Group grades of evidence | ||||
| RR = relative risk CI = confidence interval PONV = postoperative nausea and vomiting PACU = postoperative care unit | ||||
| Tramadol compared with pethidine for postoperative pain in children | ||||
| Patient or population: children undergoing surgery Settings: hospital Intervention: 1 to 3 mg/kg tramadol intravenously, per mouth or intramuscularly Comparison: 1 to 1.5 mg/kg pethidine intravenously, per mouth or intramuscularly | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Number of patients requiring rescue analgesia (PACU) | RR 0.93 (0.43 to 2.02) | 120 (2) | ⊕⊝⊝⊝ | triple‐downgraded due to unexplained heterogeneity, limitations in the study design (use of different pain scales and triggers for rescue analgesia) and imprecision of results (wide CIs) |
| Number of patients with moderate to severe pain (PACU) | RR 0.64 (0.36 to 1.16) | 94 (2) | ⊕⊕⊝⊝ | double‐downgraded due to unexplained heterogeneity and limitations in the study design (use of different pain scales and triggers for rescue analgesia) |
| Number of patients with PONV (PACU) | RR 0.75 (0.28 to 2.02) | 156 (3) | ⊕⊕⊝⊝ | double‐downgraded due to unexplained heterogeneity and imprecision of results (wide CIs) |
| GRADE Working Group grades of evidence | ||||
| RR = relative risk CI = confidence interval PONV = postoperative nausea and vomiting PACU = postoperative care unit | ||||
| Tramadol compared with fentanyl for postoperative pain in children | ||||
| Patient or population: children undergoing surgery Settings: hospital Intervention: 0.5 mg/kg bolus followed by 150 μg/kg/h tramadol intravenously Comparison: 2 µg/kg/h fentanyl intravenously | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Number of patients requiring rescue analgesia (24 hours postoperatively) | 28 (1) | ⊕⊝⊝⊝ | triple‐downgraded, due to high risk of publication bias, limitations in the study design and imprecision of results (wide CIs) | |
| Number of patients with moderate to severe pain (PACU) | no data available | |||
| Number of patients with PONV (24 hours postoperatively) | 28 (1) | ⊕⊝⊝⊝ | triple‐downgraded, due to high risk of publication bias, limitations in the study design and imprecision of results (wide CIs) | |
| GRADE Working Group grades of evidence | ||||
| RR = relative risk CI = confidence interval PONV = postoperative nausea and vomiting PACU = postoperative care unit | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients requiring rescue analgesia (PACU) Show forest plot | 5 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.20, 0.78] |
| 2 Number of patients requiring rescue analgesia (PACU) (Subgroup: Surgery) Show forest plot | 5 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.1 Abdominal surgery | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.34, 1.07] |
| 2.2 ENT Surgery | 4 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.30, 0.52] |
| 3 Number of patients requiring rescue analgesia (PACU) (Subgroup: Dose) Show forest plot | 5 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 3.1 Tramadol 1mg iv. | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.21] |
| 3.2 Tramadol 2mg iv. | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.48, 0.83] |
| 3.3 Tramadol 3mg iv. | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 4 Time to first rescue analgesic (min) Show forest plot | 3 | 154 | Mean Difference (IV, Random, 95% CI) | 44.66 [‐24.26, 113.58] |
| 5 Number of patients with PONV (PACU) Show forest plot | 3 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.28, 2.52] |
| 6 Number of patients with PONV (24h postop) Show forest plot | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.12] |
| 7 Number of patients with respiratory depression Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients requiring rescue analgesia (PACU) Show forest plot | 3 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.83, 1.89] |
| 2 Number of patients requiring rescue analgesia (24h postop) Show forest plot | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.65, 4.04] |
| 3 Number of patients with respiratory depression Show forest plot | 4 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients requiring rescue analgesia (24h postop) Show forest plot | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.16, 2.45] |
| 2 Number of patients with PONV (PACU) Show forest plot | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 2.01] |
| 3 Number of patients with respiratory depression Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of patients with bradycardia Show forest plot | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients requiring rescue analgesia (PACU) Show forest plot | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.43, 2.02] |
| 2 Number of patients with moderate/severe pain (PACU) Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.16] |
| 3 Number of patients with PONV (PACU) Show forest plot | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.02] |
| 4 Number of patients with respiratory depression Show forest plot | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.03] |
| 5 Number of patients with bradycardia Show forest plot | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

