Bronchoscopy‐guided antimicrobial therapy for cystic fibrosis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre (8 CF centres in Australia and New Zealand), randomized controlled study. | |
| Participants | 170 Infants younger than 6 months age, with confirmed diagnosis of CF, diagnosed through newborn screening programs. 84 infants were randomized to receive BAL‐directed therapy (80 completed study) and 86 randomized to receive standard therapy (77 completed study). BAL‐directed group Mean age (SD) 3.8 (1.6) years. Gender split: 44 male/40 female. Mean (SD) weight at enrolment: 5.7 kg (1.40). Number of participants with homozygous ΔF508 mutation: 57 (68%). Number of participants with pancreatic insufficiency: 73 (87%). Number of participants with meconium ileus: 17 (20%). Number of participants born pre‐term (under 37 week gestation): 8 (10%). History of exposure to tobacco smoke during pregnancy present in: 22 (26%). History of concurrent smoking in the household present in: 30 (36%). Standard therapy group Mean age (SD) 3.7 (1.7) years. Gender split: 44 male/42 female. Mean weight (SD) at enrolment: 5.6 kg (1.5). Number of participants with homozygous ΔF508 mutation: 54 (64%). Number of participants with pancreatic insufficiency: 71 (85%). Number of participants with meconium ileus: 16 (19%). Number of participants born pre‐term (under 37‐week gestation): 9 (11%). History of exposure to tobacco smoke during pregnancy present in: 13 (15%). History of concurrent smoking in the household present in: 23 (28%). | |
| Interventions | Intervention: BAL‐directed therapy for pulmonary exacerbations until age 5 years. The participants in the BAL‐directed therapy groups underwent BAL at following times:
Control: Standard therapy (directed by clinical features and oropharyngeal swab cultures) for pulmonary exacerbations until age 5 years. The standard therapy included oropharyngeal swab at following time points:
| |
| Outcomes | Reported at at 5 years age. Primary outcome measures
Secondary outcome measures
| |
| Notes | The BAL‐directed therapy group had BAL before age 6 months when well, when hospitalized for pulmonary exacerbations, when P. aeruginosa was cultured from their oropharyngeal specimens and following P. aeruginosa therapy. The standard therapy included taking oropharyngeal swabs when having pulmonary exacerbation and at the end of antibiotic therapy. Children in both groups had BAL and HRCT scan of chest at 5 years age. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After consent, the participants were randomly assigned in 1:1 ratio to 2 groups by a centralized computer‐generated schedule with stratification by site and sex. |
| Allocation concealment (selection bias) | Low risk | The allocation was done by a centralized computer‐generated schedule; the randomization key was concealed and held remotely. Allocation was revealed by telephone after confirmed recruitment. |
| Incomplete outcome data (attrition bias) | Low risk | Although the study was set up to be analysed on intention‐to‐treat basis, the participants with missing outcomes were not included in the primary analysis. The risk of bias is considered moderate to low as less than 10% of the data were missing and the reasons of exclusions were balanced across both groups. |
| Selective reporting (reporting bias) | Low risk | The economic analysis was planned but was not reported initially but now has been published. It has been included in the updated version of the review. All other outcomes planned to be assessed in the protocol were reported. |
| Other bias | Low risk | No other potential source of bias was identified. |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and the personnel were not blinded to the randomization (which might not have been possible in this study setting). However, the risk of bias is low as the primary outcome measures were unlikely to be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | Risk of bias is low as the outcome assessors were blinded for both the primary outcome measures. |
BAL: bronchoalveolar lavage
BMI: body mass index
CF: cystic fibrosis
CFU: colony forming units
CT: computer tomography
P. aeruginosa: Pseudomonas aeruginosa
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study of induced sputum and not bronchoscopy. | |
| 3‐way cross‐over study of single sample from sputum induction, bronchoalveolar lavage and expectorated sputum to identify pathogens; did not lead to comparison of treatment. | |
| A different intervention (cough plates) was studied. | |
| A different intervention (cough plates) was studied. | |
| 2‐way cross‐over study of induced sputum and BAL to compare inflammatory markers; no comparison of treatment. | |
| Study of the effect of dornase alfa on lungs using bronchoalveolar lavage; all participants underwent bronchoalveolar lavage. | |
| Study to establish levels of tobramycin, not comparison of therapy depending on sampling technique. | |
| Comparison of throat swabs and nasopharyngeal suction specimens not bronchoscopy. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Z score FEV1 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 1 Z score FEV1. | ||||
| 1.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Z score FVC Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 2 Z score FVC. | ||||
| 2.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total CF‐CT score (Brody‐II) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 3 Total CF‐CT score (Brody‐II). | ||||
| 3.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Individual CF‐CT scores (at 5 years) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 4 Individual CF‐CT scores (at 5 years). | ||||
| 4.1 Bronchiectstasis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Parencymal disease | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Mucus plugging | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Airway wall thickening | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Air trapping | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Z score for weight Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 5 Z score for weight. | ||||
| 5.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Z score BMI Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 6 Z score BMI. | ||||
| 6.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Positive P.aeruginosa isolates per patient per year Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 7 Positive P.aeruginosa isolates per patient per year. | ||||
| 7.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Prevalence of P. aeruginosa in BAL at 5 years age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 8 Prevalence of P. aeruginosa in BAL at 5 years age. | ||||
| 9 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (40% vs 5%) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 9 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (40% vs 5%). | ||||
| 10 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (5% vs 40%) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 10 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (5% vs 40%). | ||||
| 11 Clearance of P.aeruginosa after 1 or 2 eradication treatments Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.11  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 11 Clearance of P.aeruginosa after 1 or 2 eradication treatments. | ||||
| 11.1 At 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Age at first acquisition of P. aeruginosa infection Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 12 Age at first acquisition of P. aeruginosa infection. | ||||
| 12.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Number of hospital admissions per patient per year Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 13 Number of hospital admissions per patient per year. | ||||
| 13.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Number of hospitalizations per person per year due to non‐P. aeruginosa exacerbations Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 14 Number of hospitalizations per person per year due to non‐P. aeruginosa exacerbations. | ||||
| 14.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Duration of hospital admissions due to non‐P.aeruginosa exacerbations Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.15  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 15 Duration of hospital admissions due to non‐P.aeruginosa exacerbations. | ||||
| 15.1 New Subgroup | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Days as hospital inpatient per patient per year Show forest plot | 1 | Risk Difference (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.16  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 16 Days as hospital inpatient per patient per year. | ||||
| 16.1 New Subgroup | 1 | Risk Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Total cost of care per participant (Australian Dollars) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.17  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 17 Total cost of care per participant (Australian Dollars). | ||||
| 18 Mean hospital admissions cost per patient Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.18  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 18 Mean hospital admissions cost per patient. | ||||
| 19 Number of pulmonary exacerbations (requiring oral or intravenous antibiotics) per patient per year Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 1.19  Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 19 Number of pulmonary exacerbations (requiring oral or intravenous antibiotics) per patient per year. | ||||
| 19.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 1 Z score FEV1.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 2 Z score FVC.
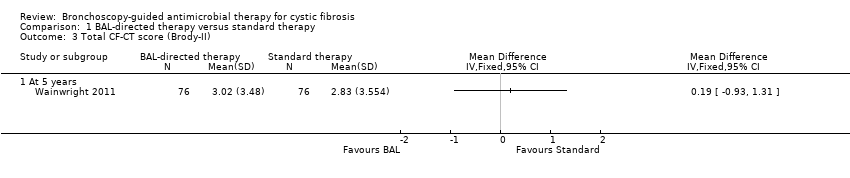
Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 3 Total CF‐CT score (Brody‐II).

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 4 Individual CF‐CT scores (at 5 years).

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 5 Z score for weight.
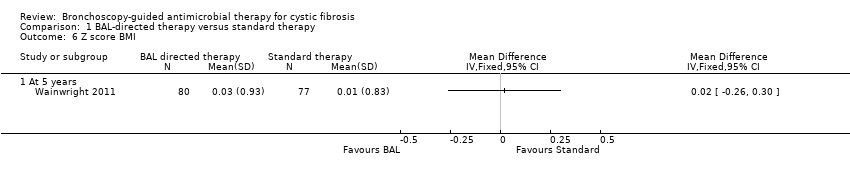
Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 6 Z score BMI.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 7 Positive P.aeruginosa isolates per patient per year.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 8 Prevalence of P. aeruginosa in BAL at 5 years age.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 9 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (40% vs 5%).

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 10 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (5% vs 40%).

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 11 Clearance of P.aeruginosa after 1 or 2 eradication treatments.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 12 Age at first acquisition of P. aeruginosa infection.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 13 Number of hospital admissions per patient per year.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 14 Number of hospitalizations per person per year due to non‐P. aeruginosa exacerbations.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 15 Duration of hospital admissions due to non‐P.aeruginosa exacerbations.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 16 Days as hospital inpatient per patient per year.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 17 Total cost of care per participant (Australian Dollars).

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 18 Mean hospital admissions cost per patient.

Comparison 1 BAL‐directed therapy versus standard therapy, Outcome 19 Number of pulmonary exacerbations (requiring oral or intravenous antibiotics) per patient per year.
| BAL‐directed therapy versus standard therapy for cystic fibrosis | ||||||
| Patient or population: people with pulmonary exacerbations in cystic fibrosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard therapy | BAL‐directed therapy | |||||
| Z score FEV1 | The mean z score for FEV1 in the standard therapy group was ‐0.41 (SD 1.23). | The mean z score FEV1 in the intervention group ‐0.56 (SD 1.25) that was 0.15 lower (0.54 lower to 0.24 higher) than the standard therapy group. | 157 | ⊕⊕⊕⊝ | FEV1 and FVC were measured using standard spirometer after bronchodilatation. The mean difference between the two groups represents the difference in the mean z scores for each parameter. Z scores for FEV1, FVC were calculated from British reference values (www.lungfunction.org/growinglungs). | |
| Z score FVC | The mean z score for FVC in the standard therapy group was 0.01 (SD 1.2). | The mean z score FVC in the intervention groups was ‐0.04 (SD 1.31) that was 0.05 higher (0.44 lower to 0.34 higher) than the standard therapy group. | 157 | ⊕⊕⊕⊝ | FEV1 and FVC were measured using standard spirometer after bronchodilatation. The mean difference between the two groups represents the difference in the mean z scores for each parameter. Z scores for FEV1, FVC were calculated from British reference values (www.lungfunction.org/growinglungs). | |
| HRCT score (Brody‐II) | The mean HRCT score in the standard therapy group was 2.83 (SD 3.5). | The mean HRCT score (Brody‐II) in the intervention group was 3.02 (SD 3.48) 0.19 higher (0.93 lower to 1.31 higher) than the standard therapy group. | 152 | ⊕⊕⊕⊕ | The study had adequate power to detect a difference in HRCT score. HRCT scans were assesses by an independent assessor who was blinded to subject allocation using an updated version of Brody‐II score. | |
| Z score for weight | The mean z score for weight was ‐0.21 (SD 0.82) in the standard therapy group. | The mean z score for weight in the intervention group was ‐0.15 that was 0.06 higher (0.21 lower to 0.33 higher) than the standard therapy group. | 157 | ⊕⊕⊕⊝ | The z scores for weight and BMI were calculated from the 2000 CDC Growth Reference Charts (http://cdc.gov/growthcharts). The mean difference between the two groups represents the difference in their z scores for each parameter. | |
| Z score BMI | The mean z score for BMI was 0.01 (SD 0.83) in the standard therapy group. | The mean z score BMI in the intervention group was 0.03 (SD0.93) that was 0.02 higher (0.26 lower to 0.30 higher) than the standard therapy group. | 157 | ⊕⊕⊕⊝ | The z scores for weight and BMI were calculated from the 2000 CDC Growth Reference Charts (http://cdc.gov/growthcharts). The mean difference between the two groups represents the difference in their z scores for each parameter. | |
| Number of hospitalizations per participant per year | The number of hospitalizations per participant per year was 1.08 in the standard therapy group. | The number of hospitalizations per participant per year in the intervention group was 1.52 that was 1.4 times higher (1.08 lower to 1.82 higher) than the standard therapy group. | 157 | ⊕⊕⊕⊝ | ||
| Overall cost of care per participant (AUD) Follow up: 5 years | The total cost of care for each participant was 90,958 AUD (SD 110,255) in the standard therapy group. | The total cost of care in the intervention group was 92,860 AUD (SD 73,378) that was 1902 AUD higher (27,508.98 lower to 31,312.98 higher) than the standard therapy group. | 157 (1 study) | ⊕⊕⊕⊕ | The cost of hospital admissions per participant over the total study duration in the intervention group was 9288 AUD lower in the intervention group (34,996.37 lower to 16,420.37 higher). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The study had low statistical power and research is needed to provide definitive answers. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Z score FEV1 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Z score FVC Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total CF‐CT score (Brody‐II) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Individual CF‐CT scores (at 5 years) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Bronchiectstasis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Parencymal disease | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Mucus plugging | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Airway wall thickening | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Air trapping | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Z score for weight Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Z score BMI Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Positive P.aeruginosa isolates per patient per year Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 7.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Prevalence of P. aeruginosa in BAL at 5 years age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (40% vs 5%) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Sensitivity analysis ‐ Prevalence of P. aeruginosa in BAL at 5 years age (5% vs 40%) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Clearance of P.aeruginosa after 1 or 2 eradication treatments Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 At 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Age at first acquisition of P. aeruginosa infection Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 12.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Number of hospital admissions per patient per year Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 13.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Number of hospitalizations per person per year due to non‐P. aeruginosa exacerbations Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 14.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Duration of hospital admissions due to non‐P.aeruginosa exacerbations Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 New Subgroup | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Days as hospital inpatient per patient per year Show forest plot | 1 | Risk Difference (Fixed, 95% CI) | Totals not selected | |
| 16.1 New Subgroup | 1 | Risk Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Total cost of care per participant (Australian Dollars) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18 Mean hospital admissions cost per patient Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19 Number of pulmonary exacerbations (requiring oral or intravenous antibiotics) per patient per year Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 19.1 At 5 years | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

