Fototerapia ultravioleta B de banda corta versus fotoquimioterapia ultravioleta A‐psoraleno o ultravioleta B de banda ancha para la psoriasis
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009481.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 23 octubre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Ming Yang and Xiaomei Chen are joint first authors as they contributed equally to this review.
Min Zhang was the contact person with the editorial base, co‐ordinated contributions from the co‐authors, and wrote the final draft of the review.
Xiaomei Chen, Yan Cheng, and Ming Yang screened papers against eligibility criteria.
Xiaomei Chen and Yan Cheng appraised the quality of the papers.
Xiaomei Chen and Ming Yang extracted data for the review and sought additional information about papers.
Ming Yang entered data into RevMan.
Ming Yang, Xiaomei Chen, and Guanjian Liu analysed and interpreted data.
Xiaomei Chen and Ming Yang worked on the methods sections.
Xiaomei Chen and Min Zhang drafted the clinical sections of the background.
Yan Cheng was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
Min Zhang is the guarantor of the update.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK.
Sources of support
Internal sources
-
Dermatology Department, West China Hospital of Sichuan University, China.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
None known.
Acknowledgements
The authors appreciate the kind help given by Hywel Williams, Finola Delamere, Elizabeth Doney, and Laura Prescott from the Cochrane Skin Group during the process of drafting the review.
The Cochrane Skin Group editorial base wishes to thank Luigi Naldi who was the Key Editor for this review; Matthew Grainge and Ching‐Chi Chi who were the Statistical and Methods Editors, respectively; the clinical referee, Robert Dawe and another clinical referee who wishes to remain anonymous; and the consumer referee, who also wishes to remain anonymous.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Oct 23 | Narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen‐ultraviolet A photochemotherapy for psoriasis | Review | Xiaomei Chen, Ming Yang, Yan Cheng, Guan J Liu, Min Zhang | |
| 2011 Dec 07 | Narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen‐ultraviolet A photochemotherapy for psoriasis | Protocol | Xiaomei Chen, Yan Cheng, Ming Yang, Guan J Liu, Min Zhang | |
Differences between protocol and review
We added some useful information to the background regarding the categories of psoriasis, and the treatments PUVA and BB‐UVB. These additional pieces of information might help readers understand the relevance of the contents more easily.
We moved one of our prespecified secondary outcomes, namely 'Percentage of participants who achieved complete clearance in the clinician's opinion', to a primary outcome, and we also renamed it 'Clearance rate'. The reason we did this is because 'clearance rate' is an important outcome for both clinicians and people with psoriasis, and during the process of working on the review, we identified some studies that reported 'complete clearance' and 'minimal residual activity (MRA)' as an independent outcome named 'clearance', and in our opinion, it was a reasonable change to make.
We also added further outcomes to our secondary outcomes, which we identified while working on the review, and we thought they might be valuable for users to make an optimal treatment choice.
In the protocol, we planned to search for information regarding adverse events from non‐RCTs. However, we did not carry out these further searches for three reasons:
-
The included RCTs revealed that phototherapy is generally well‐tolerated although some mild adverse events might exist.
-
The included RCTs had paid much attention to the adverse events.
-
According to the Cochrane Handbook for Systematic Reviews of Interventions, it is reasonable to use either identical or different eligibility criteria for selecting studies that address beneficial effects and adverse effects.
In addition, in the protocol, we planned to use an alpha of 0.05 for the Chi² test. However, during the process of drafting, we found that the number of trials included in meta‐analyses was few. In this case, we used a P value of 0.10 for the Chi² test.
Notes
There were no ongoing studies listed in the last published review, and a search of MEDLINE and PubMed in December 2014 did not find any relevant results. A new search in January 2016 did not reveal any new relevant trials. This review has been deemed stable as an update has not been considered necessary for two successive years. Our Information Specialist will run a new search in 2017 to re‐assess whether an update is needed.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram

'Risk of bias' summary: Review authors' judgements about each 'Risk of bias' item for each included study
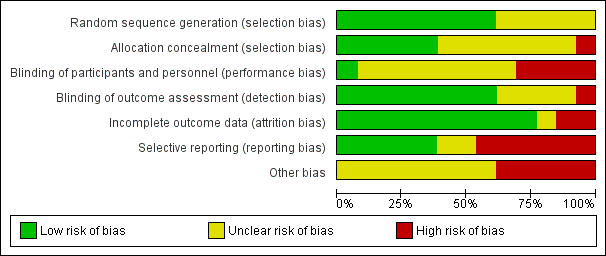
'Risk of bias' graph: Review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 1 PASI 75.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 2 PASI 75 (ITT analysis).
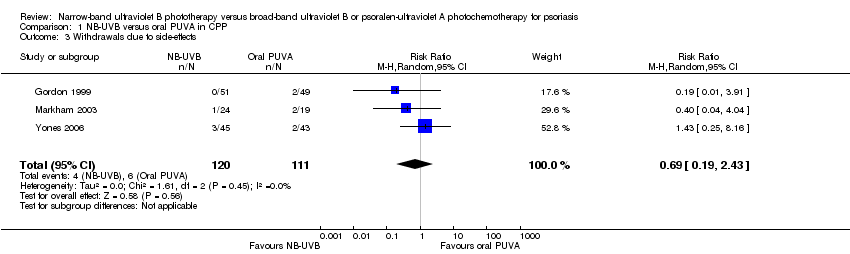
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 3 Withdrawals due to side‐effects.
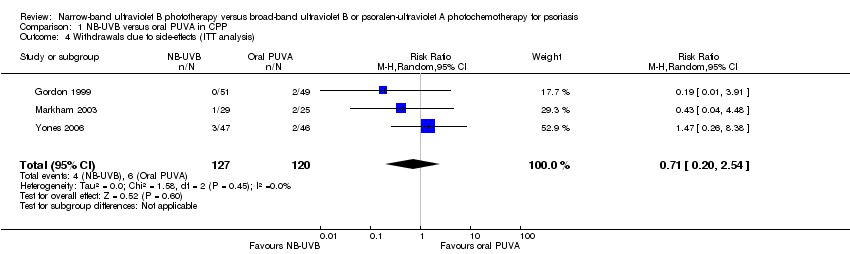
Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 4 Withdrawals due to side‐effects (ITT analysis).

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 5 Clearance rate.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 6 Clearance rate (ITT analysis).

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 7 Clearance lasting 6 months.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 8 Time to PASI 75.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 9 Relapse rate at 6 months after treatment completion.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 10 Withdrawals due to poor response.

Comparison 1 NB‐UVB versus oral PUVA in CPP, Outcome 11 Adverse events.
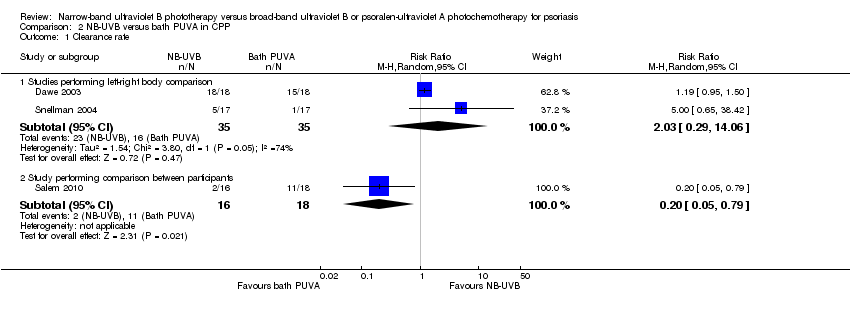
Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 1 Clearance rate.

Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 2 Clearance rate (ITT analysis).

Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 3 PASI score reduction.

Comparison 2 NB‐UVB versus bath PUVA in CPP, Outcome 4 Adverse events.
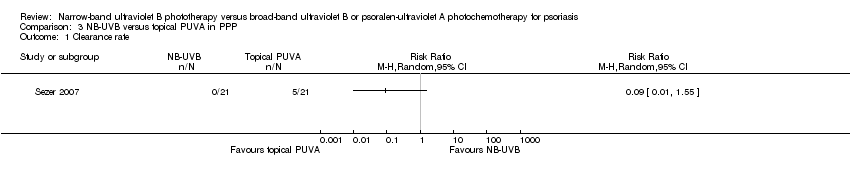
Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 1 Clearance rate.

Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 2 Clearance rate (ITT analysis).
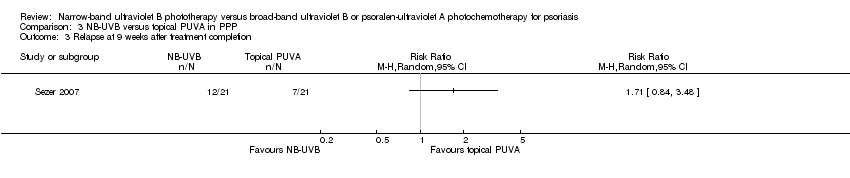
Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 3 Relapse at 9 weeks after treatment completion.
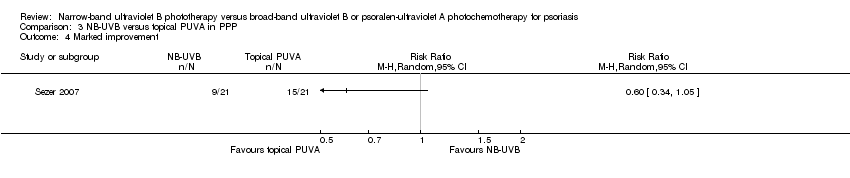
Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 4 Marked improvement.

Comparison 3 NB‐UVB versus topical PUVA in PPP, Outcome 5 Adverse events.
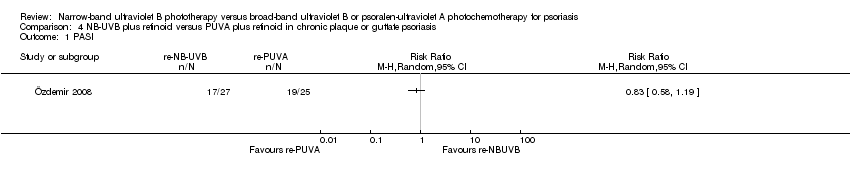
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 1 PASI.

Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 2 PASI 75 (ITT analysis).
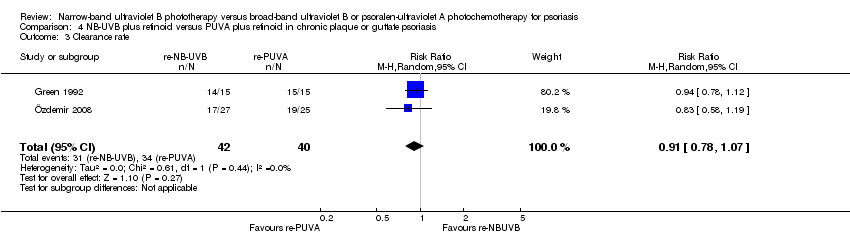
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 3 Clearance rate.

Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 4 Clearance rate (ITT analysis).
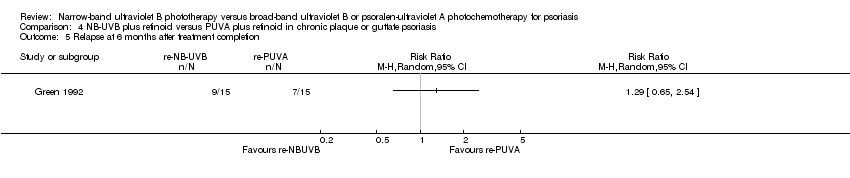
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 5 Relapse at 6 months after treatment completion.
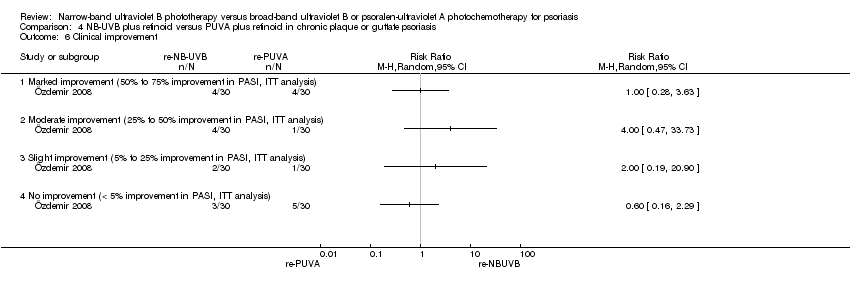
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 6 Clinical improvement.
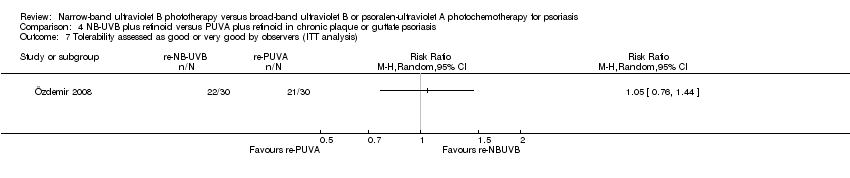
Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 7 Tolerability assessed as good or very good by observers (ITT analysis).

Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 8 Tolerability assessed as good or very good by participants (ITT analysis).

Comparison 4 NB‐UVB plus retinoid versus PUVA plus retinoid in chronic plaque or guttate psoriasis, Outcome 9 Adverse events.
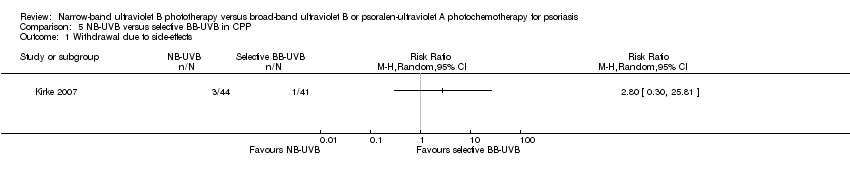
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 1 Withdrawal due to side‐effects.

Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 2 Withdrawals due to side‐effects (ITT analysis).
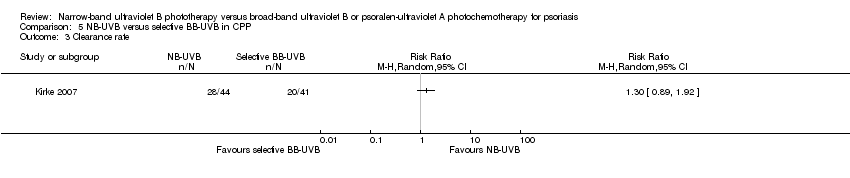
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 3 Clearance rate.
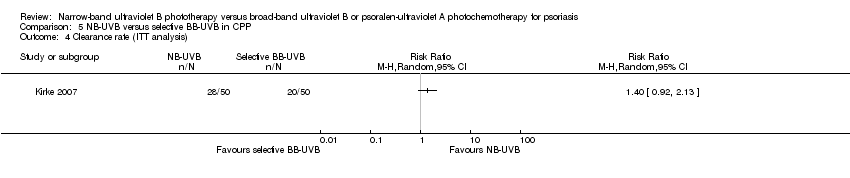
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 4 Clearance rate (ITT analysis).
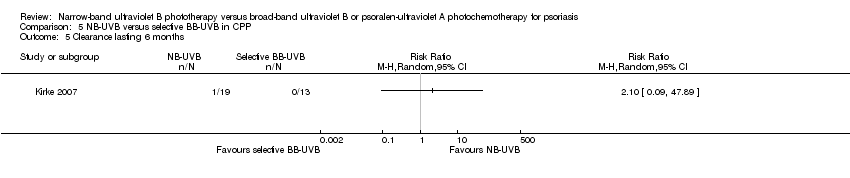
Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 5 Clearance lasting 6 months.

Comparison 5 NB‐UVB versus selective BB‐UVB in CPP, Outcome 6 Adverse events.

Comparison 6 NB‐UVB versus conventional BB‐UVB in different types of psoriasis, Outcome 1 Cumulative UV dose during the study.

Comparison 7 NB‐UVB plus dithranol versus conventional BB‐UVB plus dithranol in different types of psoriasis, Outcome 1 Cumulative UV dose during the study.
| NB‐UVB compared with oral PUVA for chronic plaque psoriasis | ||||||
| Patient or population: People with chronic plaque psoriasis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral PUVA | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | 720 per 1000 | 655 per 1000 | RR 0.91 | 51 | ⊕⊕⊝⊝ | This is the result of ITT analysis |
| Withdrawal due to side‐effects | 32 per 1000 | 50 per 1000 (7 to 82) | RR 0.71 | 247 | ⊕⊕⊝⊝ | This is the result of ITT analysis |
| Clearance rate | Study population | Not estimable | 0 | See comment | The results of 3 small RCTs are contradictory. Because of the significant statistical heterogeneity, the data were not pooled | |
| See comment | See comment | |||||
| Moderate | ||||||
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ The study was of small sample size. | ||||||
| NB‐UVB compared with bath PUVA for chronic plaque psoriasis | ||||||
| Patient or population: People with chronic plaque psoriasis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bath PUVA | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Clearance rate | 348 per 1000 | 623 per 1000 | RR 1.79 | 92 | ⊕⊕⊝⊝ | 1. On the basis of studies performing left‐right body comparison. 2. This is the result of ITT analysis |
| Clearance rate | 611 per 1000 | 110 per 1000 | RR 0.18 | 36 | ⊕⊕⊝⊝ | 1. On the basis of the study performing comparison between participants. 2. This is the result of ITT analysis |
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ Both of the studies were of small sample size and at high risk of bias, and the result was based on less than 300 participants. | ||||||
| NB‐UVB compared with topical PUVA for palmoplantar psoriasis | ||||||
| Patient or population: People with palmoplantar psoriasis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical PUVA | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Clearance rate | 200 per 1000 | 18 per 1000 | RR 0.09 | 50 | ⊕⊕⊝⊝ | This is the result of ITT analysis |
| * Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ This study was at unclear risk of bias. | ||||||
| NB‐UVB plus retinoid compared with PUVA plus retinoid for chronic plaque or guttate psoriasis | ||||||
| Patient or population: People with chronic plaque or guttate psoriasis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PUVA plus retinoid | NB‐UVB plus retinoid | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | RR 0.89 | 60 | ⊕⊕⊝⊝ | This is the result of ITT analysis | |
| 633 per 1000 | 564 per 1000 | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Clearance rate | 756 per 1000 | 688 per 1000 | RR 0.93 | 90 | ⊕⊕⊝⊝ | This is the result of ITT analysis |
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ This study was at high risk of bias. | ||||||
| NB‐UVB compared with selective BB‐UVB for chronic plaque psoriasis | ||||||
| Patient or population: People with chronic plaque psoriasis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Selective BB‐UVB | NB‐UVB | |||||
| Participant‐rated global improvement | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Percentage of participants reaching PASI 75 | Study population | Not estimable | 0 | See comment | No included RCT addressed this outcome | |
| See comment | See comment | |||||
| Moderate | ||||||
| Withdrawal due to side‐effects | Study population | RR 3.00 | 100 | ⊕⊕⊝⊝ | This is the result of ITT analysis | |
| 20 per 1000 | 60 per 1000 | |||||
| Moderate | ||||||
| Clearance rate | 400 per 1000 | 560 per 1000 | RR 1.40 | 100 | ⊕⊕⊝⊝ | This is the result of ITT analysis |
| *Comment: The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ The study was at unclear risk of bias. | ||||||
| Medical term and abbreviations | Explanation |
| Apoptosis | The process of programmed cell death that occurs during growth and development of multicellular organisms. It is generally considered a part of normal cell aging, but it can also be a response to cellular injury |
| BB‐UVB | Broad‐band ultraviolet B |
| Collagenase | An enzyme that breaks the peptide bonds in collagen |
| CPP | Chronic plaque psoriasis |
| Cytokines | Small protein molecules that are secreted by cells of the nervous system or the immune system. They are used in intercellular communication |
| Defective maturation of epidermal keratinocytes | Incomplete formation of keratin (the horny material in nails) due to rapid growth of cells in the epidermal layer of the skin |
| Dilatation of dermal capillaries | Dilation of small blood vessels in the skin |
| Erythrodermic psoriasis | A subtype of psoriasis that affects nearly all body sites |
| Erythrogenic response | Redness of the skin caused by light exposure |
| Extensor aspects | An anatomical term ‐ when a joint bends, the parts of the skin on the opposite side of the joint are called the extensor aspects |
| Hyperkeratosis | Thickening of the stratum corneum (outermost layer of the skin) usually associated with an abnormality of the keratin and an increase of the granular layer of the skin |
| Hyperplasia | An increase in the number of cells |
| Hyperproliferation | An abnormally high rate of proliferation of cells by rapid division |
| Hypertriglyceridaemia | High levels of triglyceride fatty acids |
| ITT | Intention‐to‐treat: An ITT analysis is often recommended as the least biased way to estimate intervention effects in RCTs. The principals of ITT analysis are as follows: 1. keep participants in the intervention group to which they were randomised, regardless of the intervention they actually received; 2. measure outcome data on all participants; and 3. include all randomised participants in the analysis |
| MRA | Minimal residual activity |
| MOP | Methoxypsoralen |
| NB‐UVB | Narrow‐band ultraviolet B |
| Paronychia | Swelling of the skin over the nail |
| PASI | Psoriasis Area and Severity Index. The higher the score, the more severe the lesions are |
| PASI 75 | Equal to or more than 75% reduction in PASI score |
| PPP | Palmoplantar psoriasis |
| Psoralen | A compound that can be used as a kind of photosensitiser to improve the influence of natural or artificial light |
| PUVA | Psoralen plus ultraviolet A |
| Photosensitiser | Chemical treatments that are used to sensitise the skin and enhance the effect of light treatments |
| Pustular | Lesions containing purulent materials |
| QOL | Quality of life |
| Re‐NB‐UVB | NB‐UVB combined with retinoid |
| Re‐PUVA | PUVA combined with retinoid |
| Severity index of PPP | A tool developed by Hofer 2006 to evaluate the severity of palmoplantar psoriasis. The separate scores of erythema, scaling, pustulation, and infiltration for palms and soles were added to calculate the severity index (0 = absent; 1 = slight; 2 = moderate; 3 = marked; and 4 = very marked) |
| Xerophthalmia | Dryness of the eye, especially the cornea and conjunctiva |
| Xerosis | Extreme dryness of the skin |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PASI 75 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 PASI 75 (ITT analysis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Withdrawals due to side‐effects Show forest plot | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.19, 2.43] |
| 4 Withdrawals due to side‐effects (ITT analysis) Show forest plot | 3 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.20, 2.54] |
| 5 Clearance rate Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Clearance rate (ITT analysis) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Clearance lasting 6 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Time to PASI 75 Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Relapse rate at 6 months after treatment completion Show forest plot | 3 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.58] |
| 10 Withdrawals due to poor response Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Adverse events Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 erythema | 3 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.47, 2.09] |
| 11.2 nausea | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.94] |
| 11.3 pruritus | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.31, 2.43] |
| 11.4 PMLE | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 6.77] |
| 11.5 grade 1 erythema | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.68, 1.26] |
| 11.6 grade 2 erythema | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.79] |
| 11.7 any adverse events | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.40, 2.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance rate Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Studies performing left‐right body comparison | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.29, 14.06] |
| 1.2 Study performing comparison between participants | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.79] |
| 2 Clearance rate (ITT analysis) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Studies performing left‐right body comparison | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.46, 6.91] |
| 2.2 Studies performing comparisons between participants | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.71] |
| 3 PASI score reduction Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 erythema | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 pruritus | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 grade 1 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 grade 2 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 grade 3 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 folliculitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 any adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clearance rate Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Clearance rate (ITT analysis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Relapse at 9 weeks after treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Marked improvement Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 palmar hyperpigmentation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PASI Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 PASI 75 (ITT analysis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Clearance rate Show forest plot | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.07] |
| 4 Clearance rate (ITT analysis) Show forest plot | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
| 5 Relapse at 6 months after treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Clinical improvement Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Marked improvement (50% to 75% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Moderate improvement (25% to 50% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Slight improvement (5% to 25% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 No improvement (< 5% improvement in PASI, ITT analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Tolerability assessed as good or very good by observers (ITT analysis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Tolerability assessed as good or very good by participants (ITT analysis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 diffuse hair loss | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 reversible hypertriglyceridaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 withdrawal due to pruritus and burning | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 nausea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Withdrawal due to side‐effects Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Withdrawals due to side‐effects (ITT analysis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Clearance rate Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Clearance rate (ITT analysis) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Clearance lasting 6 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 severe erythema | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 PMLE | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 pruritus | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative UV dose during the study Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative UV dose during the study Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

