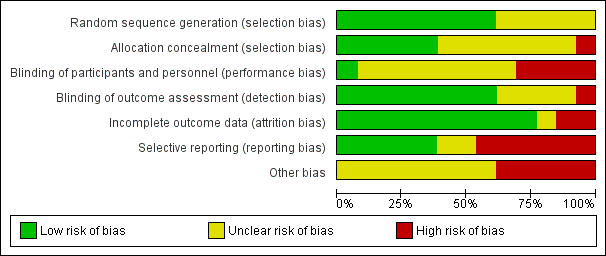
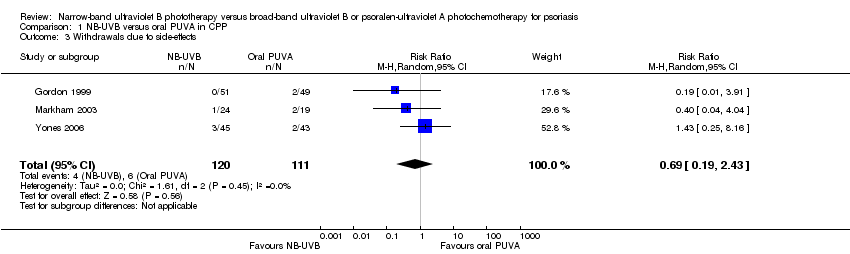
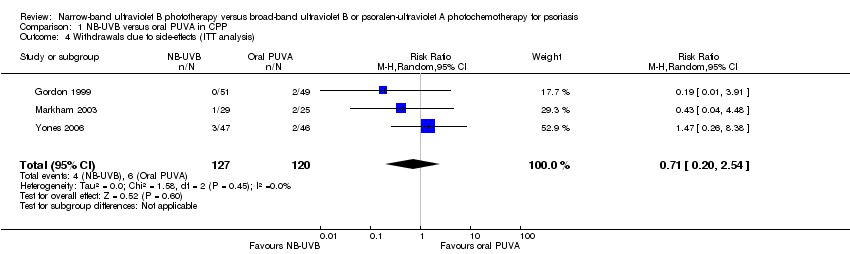
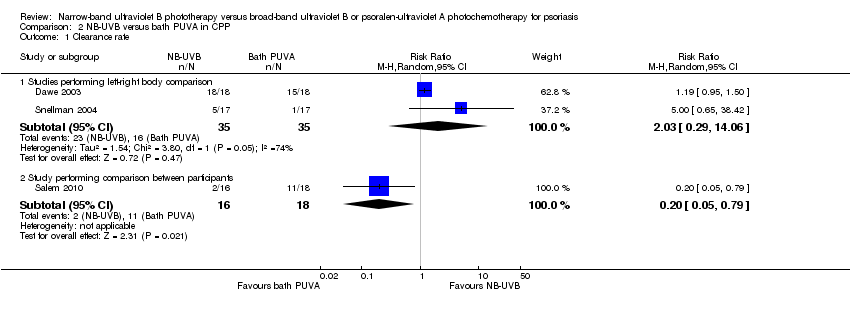
Contenido relacionado
Revisiones y protocolos relacionados
Frank Peinemann, Marco Harari, Sandra Peternel, Thalia Chan, David Chan, Alexander M Labeit, Thilo Gambichler | 5 mayo 2020
Aditya K Gupta, Maryse Paquet, Elmer Villanueva, William Brintnell | 12 diciembre 2012
Jason Thomson, Sarah Hogan, Jo Leonardi-Bee, Hywel C Williams, Fiona J Bath-Hextall | 17 noviembre 2020
Arash Valipoura, Manuel Jägera, Peggy Wu, Jochen Schmitt, Charles Bunch, Tobias Weberschock | 7 julio 2020
Grace Obeid, Giao Do, Lisa Kirby, Carolyn Hughes, Emilie Sbidian, Laurence Le Cleach | 20 enero 2020
John R Ingram, Pick‐Ngor Woo, Ser Ling Chua, Anthony D Ormerod, Nemesha Desai, Anneke C Kai, Kerry Hood, Tara Burton, Francisco Kerdel, Sarah E Garner, Vincent Piguet | 7 octubre 2015
Fiona J Bath‐Hextall, Rubeta N Matin, David Wilkinson, Jo Leonardi‐Bee | 24 junio 2013
Chun Shing Kwok, Sam Gibbsa, Cathy Bennett, Richard Holland, Rachel Abbott | 12 septiembre 2012
Gwendy Dupire, Catherine Droitcourt, Carolyn Hughes, Laurence Le Cleach | 5 marzo 2019
Monica Novoa, Eulalia Baselga, Sandra Beltran, Lucia Giraldo, Ali Shahbaz, Hector Pardo‐Hernandez, Ingrid Arevalo‐Rodriguez | 18 abril 2018