Gotas oculares de suero autólogo para el ojo seco
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: 2‐period cross‐over, randomized controlled trial Unit of randomization: individual Number randomized: Total: 20 participants Per sequence: 10 randomized to each sequence Unit of analysis: individual Number analyzed: Total: 20 participants Per sequence: 10 per sequence | |
| Participants | Country: Turkey Mean age: 56 years Gender: 18 women; 2 men Underlying conditions: severe, refractory dry eye Inclusion criteria: Exclusion criteria: | |
| Interventions | Sequence 1: washout ‐ 20% AS ‐ washout ‐ artificial tears AS protocol: 20% AS solution used 4 times a day for 1 month Artificial tears protocol: artificial tears (Refresh) used 4 times a day for 1 month Length of follow‐up: Planned: 3 months Actual: 3 months | |
| Outcomes | Participant questionnaire: Ocular Surface Disease Index (OSDI) Tear function:Tear BUT and Schirmer’s test | |
| Notes | Trial registration: not reported Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each patient was randomized to one of the two treatment groups. Using the random number table method, a random treatment assignment code was created and every patient was given a sealed opaque envelope with their secret code" |
| Allocation concealment (selection bias) | Low risk | "Each patient was randomized to one of the two treatment groups. Using the random number table method, a random treatment assignment code was created and every patient was given a sealed opaque envelope with their secret code" |
| Masking of participants of the allocated intervention (Performance bias). | Low risk | "Only the biochemistry specialist (CU), who was not responsible for patient evaluation, knew which patient received which treatment during two different study periods" Vials for both AS and artificial tears were "likewise wrapped with aluminum foil by a biochemistry specialist (CU) for blinding the patients. The droppers were also wrapped with aluminum foil for further blinding the patient while applying the eye drops" |
| Masking of study personnel of the allocated intervention (Performance bias) | Low risk | "Only the biochemistry specialist (CU), who was not responsible for patient evaluation, knew which patient received which treatment during two different study periods" |
| Masking of outcome assessors during follow‐up – patient reported symptoms (Detection bias) | Low risk | "Only the biochemistry specialist (CU), who was not responsible for patient evaluation, knew which patient received which treatment during two different study periods" |
| Masking of outcome assessors during follow‐up – clinical examination (Detection bias) | Low risk | "Only the biochemistry specialist (CU), who was not responsible for patient evaluation, knew which patient received which treatment during two different study periods" |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the trial |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported as described in the Methods section; however, although the study reported outcome data between treatment groups, investigators reported only P values, and thus we were unable to extract paired data for quantitative analysis |
| Other bias | Low risk | We believed the cross‐over design was appropriate given the relative stability of dry eye, eliminating the potential for a temporal treatment effect, and given that participants received their treatments in clearly random order. Use of a 2‐week washout between treatment periods ensured no carry‐over effect from one treatment period to the next. |
| Methods | Study design: parallel‐group, randomized controlled trial Unit of randomization: individual Number randomized: Total: 20 participants (37 eyes) Per group: 10 participants Unit of analysis: individuals Number analyzed: Total: 20 participants Per group: 10 participants | |
| Participants | Country: Japan Mean age: AS: 62 years Artificial tears: 65 years Gender: AS: 8 women; 2 men Artificial tears: 8 women; 2 men Underlying conditions: 8 of 10 participants in the AS group and 9 of 10 participants in artificial tears group had Sjögren's syndrome Inclusion criteria: All participants met diagnostic criteria of the Japanese Dry Eye Research Group: Schirmer's 1 test < 5 mm, or tear film BUT < 5 seconds Exclusion criteria: History of punctal occlusion, ocular or systemic disease, or history of drug or contact lens use that would alter the ocular surface | |
| Interventions | Intervention 1: 20% AS (saline) Intervention 2: preservative‐free artificial tears Length of follow‐up: Planned: 2 weeks Actual: 2 weeks | |
| Outcomes | Participant questionnaire: Absence of any pain constituted a score of 0 points on visual analogue pain scales; intense, unbearable pain was considered a full pain score of 100 points Tear function:Tear film BUT was measured 3 times, and the mean value was calculated. Tear film BUT was considered abnormal if < 5 seconds. Schirmer's test was considered abnormal if < 5 mm. Ocular surface: The ocular surface was examined by the double vital staining method. 2 mL of a preservative‐free combination of 1% Rose Bengal and 1% fluorescein dye was instilled in the conjunctival sac: “According to the study protocol, tear film BUT analysis was performed initially, followed by fluorescein and Rose Bengal vital staining of the ocular surface. The Schirmer 1 test was then performed. Tear film BUT, vital staining of the ocular surface, and visual analog pain symptom scores were compared before and after treatment” | |
| Notes | Trial registration: not reported Source of funding: Japanese Ministry of Education and Science (Tokyo) and Hightech Research Center at Tokyo Dental College (Chiba, Japan) Study author provided additional information not included in published report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After washout, all patients were randomly assigned to two groups..." |
| Allocation concealment (selection bias) | Unclear risk | "After washout, all patients were randomly assigned to two groups..." |
| Masking of participants of the allocated intervention (Performance bias). | Unclear risk | Study authors described the collection and production of autologous serum, including venipuncture, as well as storage requirements, but it was unclear whether only the autologous serum group underwent the necessary collection procedures or received the same storage instructions |
| Masking of study personnel of the allocated intervention (Performance bias) | Unclear risk | No information provided in the published report revealed whether study personnel were aware of each participant's treatment assignment. Specific instructions were given to study participants regarding proper care and storage of autologous serum vials |
| Masking of outcome assessors during follow‐up – patient reported symptoms (Detection bias) | Unclear risk | "patients were asked to check a point on the line corresponding to their degree of pain" |
| Masking of outcome assessors during follow‐up – clinical examination (Detection bias) | Low risk | “The examiner who carried out the tear function and ocular surface evaluations was masked to the type of the eyedrops prescribed to the patients in this study” |
| Incomplete outcome data (attrition bias) | Unclear risk | A total of 39 eyes from 20 participants were analyzed and reported in a conference abstract; 37 eyes from 20 patients were analyzed in the full‐text publication. No explanation was provided for eyes excluded from the full‐text report |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial registry record was found; all outcomes were reported as described in the Methods section |
| Other bias | Low risk | No other potential sources of bias were identified |
| Methods | Study design: parallel‐group, randomized controlled trial Unit of randomization: individual Number randomized: Total: 27 participants (54 eyes) Per group: AS: 12 participants (24 eyes) Saline: 15 participants (30 eyes) Unit of analysis: eyes Number analyzed: 1 month BUT: AS 20, saline 23 Schirmer’s: AS 20, saline 19 Rose Bengal: AS 20, saline 15 Fluorescein: AS 20, saline 23 3 months BUT: AS 18, saline 15 Schirmer’s: AS 16, saline 15 Rose Bengal: AS 16, saline 11 Fluorescein: AS 18, saline 15 6 months BUT: AS 8, saline 10 Schirmer’s: AS 8, saline 10 Rose Bengal: AS 6, saline 10 Fluorescein: AS 8, saline 10 | |
| Participants | Country: Japan Mean age: 30 years Gender: 100% men Underlying conditions: All participants had LASIK surgery 1 week before the start of the study Concurrent dry eye treatments: One week after LASIK surgery, all participants received topical steroids, antibiotics, and hyaluronic acid eye drops 5 times per day and discontinued use at 1 week postoperatively Inclusion criteria: post‐LASIK, men “All patients revealed normal findings by routine preoperative ophthalmologic examination including tear function and vital staining. None of the patients had worn contact lenses before LASIK” | |
| Interventions | Intervention 1: 20% AS (saline) Intervention 2: preservative‐free, saline‐based tears (Soft Santear, Santen) Length of follow‐up: Planned: 1 week post LASIK to 6 months post LASIK Actual: 1 week post LASIK to 6 months post LASIK | |
| Outcomes | Participant questionnaire: Dry eye symptoms were graded by participants, who used a written questionnaire according to the following criteria: 0, none; 1, mild; 2, moderate; 3, strong; and 4, very strong Tear function:Schirmer's test with anesthesia, tear clearance rate, and tear BUT Ocular surface staining:Fluorescein staining was graded from 0 to 3 for each of the upper, middle, and lower thirds of the cornea. Rose Bengal staining was graded from 0 to 3 for the temporal conjunctiva, cornea, and nasal conjunctiva. The grading scale was decided according to the extent of staining: 0, negative; 1, minute scattering; 2, moderately spotty; and 3, diffuse blotchy staining. Total of scores in the 3 areas was defined as fluorescein or Rose Bengal score | |
| Notes | Trial registration: not reported Source of funding: not reported Contacted study author for additional information but did not receive information not included in the published report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All candidates for the study were selected, and patients were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | "All candidates for the study were selected, and patients were randomly divided into two groups" |
| Masking of participants of the allocated intervention (Performance bias). | Unclear risk | Study authors described the collection and production of autologous serum, including venipuncture, as well as storage requirements, but it was unclear whether only the autologous serum group underwent the necessary collection procedures or received the same storage instructions |
| Masking of study personnel of the allocated intervention (Performance bias) | Unclear risk | No information provided in the published report revealed whether study personnel were aware of each participant's treatment assignment, but specific instructions were given to study participants regarding proper care and storage of autologous serum vials |
| Masking of outcome assessors during follow‐up – patient reported symptoms (Detection bias) | Unclear risk | "Typical dry eye symptoms were graded by the patients using a written questionnaire according to the following criteria: 0, none; 1, mild; 2, moderate; 3, strong; and 4, very strong" |
| Masking of outcome assessors during follow‐up – clinical examination (Detection bias) | Unclear risk | "To evaluate tear function, Schirmer test with anesthesia, tear clearance rate, and tear break‐up time (BUT) were measured as previously described." No other description revealed whether outcome assessment was done by a masked investigator |
| Incomplete outcome data (attrition bias) | Unclear risk | Study authors reported the number of eyes for each outcome at all time points across both treatment arms but did not provide reasons for missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial registry record was found. Reported all outcomes at all time points for outcomes described in the Methods section, although reported information was insufficient for review authors to extract usable data for quantitative summary analysis |
| Other bias | Unclear risk | Unit of randomization was the participant, and results were reported per eye |
| Methods | Study design: paired‐eye, randomized controlled trial Unit of randomization: eyes Number randomized: Total: 26 eyes of 13 participants Per group: 13 eyes of 13 participants Unit of analysis: eyes Number analyzed: Total: 12 eyes of 12 participants Per group: 12 eyes of 12 participants | |
| Participants | Country: Australia Mean age: 60 years Gender: Men: 5 Women: 7 Underlying conditions: 5 participants had Sjögren’s syndrome: 2 had primary Sjögren’s syndrome and 3 had secondary Sjögren’s syndrome. Non‐Sjögren’s‐type dry eyes included non‐Hodgkin’s lymphoma (n = 1), graft‐versus‐host disease (n = 1), Stevens‐Johnson syndrome (n = 1), rheumatoid arthritis (n = 1), and idiopathic (n = 3) Concurrent dry eye treatments: artificial tears as needed Inclusion criteria: Exclusion criteria: | |
| Interventions | Intervention 1: 20% AS Intervention 2: unpreserved saline solution and dilute fluorescein solution Length of follow‐up: Planned: 2 months Actual: 2 months | |
| Outcomes | Participant questionnaire: Symptoms of dry eye (discomfort, foreign body sensation, dryness, and photophobia) were recorded at every visit and were graded according to severity as grade 0, no symptoms; 1, mild; 2, moderate; and 3, severe Tear function: assessed by Schirmer’s test with anesthesia and tear BUT at baseline and 2 months after treatment Ocular surface: examined with vital dye staining with fluorescein and Rose Bengal. Fluorescein staining was rated from 0 to 3 but only on the cornea. For Rose Bengal staining, the degree of staining was recorded separately for temporal and nasal conjunctivae and cornea on a scale of 0 to 3. Maximum score for each area was 3. Scores for each area were added together to obtain the total score for each eye. Therefore, maximum score for each eye was 9. Conjunctival impression cytology and slit‐lamp photography were performed to document the change in ocular surface Corrected visual acuity and slit‐lamp examinations were performed on each visit, and application of additional topical lubricants was recorded for both treatment groups | |
| Notes | Trial registration: not reported Source of funding: not reported Study author provided additional information for assessing risk of bias not included in published report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Investigators used blocks of 2 for randomization when the right eye of participant 1 was assigned to be the study eye and the fellow eye was the control eye. For participant 2, the left eye was assigned as the study eye and the fellow eye was the control. (correspondence from study investigator) |
| Allocation concealment (selection bias) | High risk | Investigators used blocks of 2 for randomization when the right eye of participant 1 was assigned to be the study eye and the fellow eye was the control eye. For participant 2, the left eye was assigned as the study eye and the fellow eye was the control. (correspondence from study investigator) |
| Masking of participants of the allocated intervention (Performance bias). | Low risk | Participants were masked to treatment assignment (correspondence from study investigator) |
| Masking of study personnel of the allocated intervention (Performance bias) | High risk | The investigator who assessed outcomes was not masked to treatment assignments (correspondence from study investigator) |
| Masking of outcome assessors during follow‐up – patient reported symptoms (Detection bias) | High risk | The investigator who assessed outcomes was not masked to treatment assignments (correspondence from study investigator) |
| Masking of outcome assessors during follow‐up – clinical examination (Detection bias) | High risk | The investigator who assessed outcomes was not masked to treatment assignments (correspondence from study investigator). Although these were objective clinical tests, detection bias was possible if investigators conducting the test and interpreting the results were aware of participants' treatment assignments |
| Incomplete outcome data (attrition bias) | Low risk | "One patient with ocular cicatricial pemphigoid was excluded after enrollment because of asymmetry of the severity of dry eye between the two eyes." No other missing data were reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial registry record was found. Reported all outcomes at all time points for outcomes described in the Methods section, although reported information was insufficient for review authors to extract usable data for quantitative summary analysis |
| Other bias | Unclear risk | Participants used lubricant artificial tears as needed during the study. This may have had an effect on results. Frequency and quantity of application of the drops for each participant were unknown |
| Methods | Study design: 2‐period cross‐over, randomized controlled trial Unit of randomization: individual Number randomized: 12 participants Number analyzed: 12 participants | |
| Participants | Country: Chile Mean age: 52 years Gender: 11 women; 1 man Underlying conditions: severe non‐Sjögren's dry eye Inclusion criteria: At least 18 years old with severe dry eye, as defined by OSDI score ≥ 40, tear BUT < 5 seconds, cornea‐conjunctival epithelial defects measured by fluorescein staining and evaluation using Oxford score and Schirmer's score < 5 mm/5 min; "all had used previous treatment with artificial tears with preservative" Exclusion criteria: Ocular surface disease other than dry eye, severe anemia, previous use of autologous serum or concomitant use of other topical ocular drug (i.e., topical steroids or cyclosporine), hypersensibility to any proposed interventions, inability to complete study protocol | |
| Interventions | Sequence 1: 20% AS ‐ washout ‐ artificial tears AS protocol: 20% AS solution used 4 times a day for 2 weeks Artificial tears protocol: artificial tears (Systane) used 4 times a day for 2 weeks Length of follow‐up: Planned: 5 weeks Actual: 5 weeks | |
| Outcomes | Participant questionnaire: score reduction in OSDI Tear function:tear BUT (in seconds) | |
| Notes | Trial registration: NCT00779987 Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using random number tables method, a random treatment assignment code was created and every patient was given a sealed opaque envelope with the secret code" |
| Allocation concealment (selection bias) | Low risk | "Using random number tables method, a random treatment assignment code was created and every patient was given a sealed opaque envelope with the secret code. Then, the patient handed the envelope to the Cell Therapy Laboratory operator who delivered the treatment set" |
| Masking of participants of the allocated intervention (Performance bias). | Low risk | "Both groups of treatments were given a set of 14 identical, opaque flasks (containing either AS or artificial tears) with instructions of keeping them frozen at ‐20°C" |
| Masking of study personnel of the allocated intervention (Performance bias) | Low risk | "Both DES patient groups, clinical evaluators, and data analyst were masked to group intervention assignment through the whole completion of the protocol (double‐masked design)" |
| Masking of outcome assessors during follow‐up – patient reported symptoms (Detection bias) | Low risk | "Both DES patient groups, clinical evaluators, and data analyst were masked to group intervention assignment through the whole completion of the protocol (double‐masked design)" "Clinical evaluation of each patient (OSDI, BCVA, TBUT, and OXFORD) was assessed at baseline, beginning and end of treatment by two researchers (Cristhian A. Urzua and Dario H. Vasquez) in a masked way" |
| Masking of outcome assessors during follow‐up – clinical examination (Detection bias) | Low risk | "Both DES patient groups, clinical evaluators, and data analyst were masked to group intervention assignment through the whole completion of the protocol (double‐masked design)" "Clinical evaluation of each patient (OSDI, BVCA, TBUT, and OXFORD) was assessed at baseline, beginning and end of treatment by two researchers (Cristhian A. Urzua and Dario H. Vasquez) in a masked way" |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Reported data for all outcomes described in CT.gov record; however, although the study report described paired analyses to take advantage of the within‐participant design, outcome data were reported according to treatment group; thus we were not able to extract paired data |
| Other bias | Unclear risk | Differences in eligibility criteria between the CT.gov record and the published report, including non‐Sjogren's syndrome and Schirmer’s score < 5 mm/5 min We believed the cross‐over design was appropriate given the relative stability of dry eye, eliminating the potential for a temporal treatment effect, and that participants received their treatments in a clearly random order. Use of a 1‐week washout between treatment periods ensured no carry‐over effect from one treatment period to the next. |
APL: autologous platelet lysate.
AS: autologous serum eye drops.
BCVA: best‐corrected visual acuity.
DES: dry eye syndrome.
BUT: break‐up time.
LASIK: laser‐assisted in situ keratomileusis.
OSDI: ocular surface disease index.
TBUT: tear film break‐up time.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomized controlled trial | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Randomized trial comparing autologous platelet lysate drops with artificial tears | |
| Non‐randomized case report | |
| Overview about efficacy and recommendations for autologous serum for dry eye disease; not a randomized controlled trial | |
| Non‐randomized case series | |
| Review of autologous blood products in the treatment of dry eye; not a randomized controlled trial | |
| Retrospective study investigating allogenic (donor) serum | |
| Non‐randomized case series | |
| Retrospective study | |
| Randomized trial comparing 50% autologous serum drops with 100% autologous serum drops | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Randomized trial comparing autologous serum eye drops with bandage contact lenses | |
| Not a randomized controlled trial | |
| Non‐randomized case series | |
| Randomized trial in participants with Hansen's disease (leprosy); comparison of cord blood serum eye drops vs autologous serum eye drops vs artificial tears | |
| Randomized trial in participants using isotretinoin (vitamin A derivative); comparison of autologous serum eye drops vs artificial tears | |
| Conventional treatment arm with different pharmacological agents for each participant | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Non‐randomized case series | |
| Comparison group for umbilical cord serum did not meet the criteria for our included studies |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐reported symptoms (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 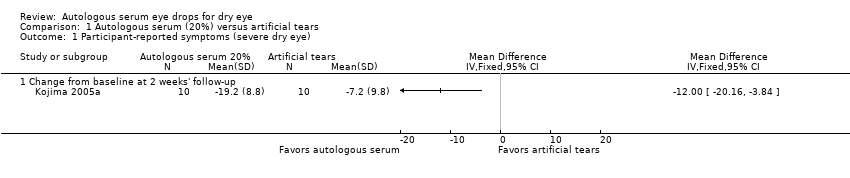 Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 1 Participant‐reported symptoms (severe dry eye). | ||||
| 1.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fluorescein (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2 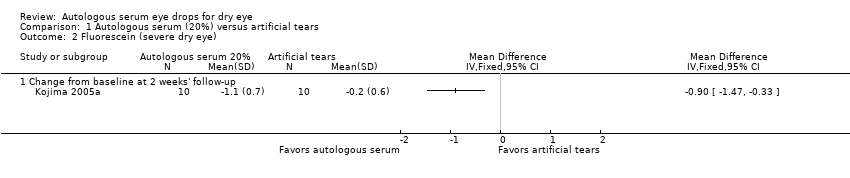 Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 2 Fluorescein (severe dry eye). | ||||
| 2.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Rose Bengal (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 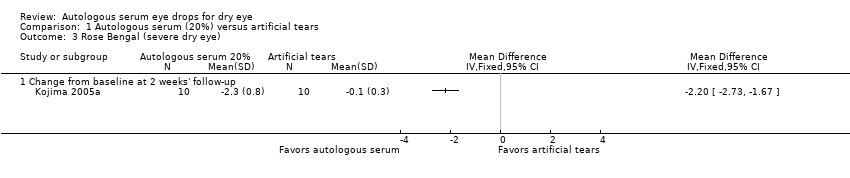 Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 3 Rose Bengal (severe dry eye). | ||||
| 3.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 TBUT (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 4 TBUT (severe dry eye). | ||||
| 4.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Schirmer's 1 test (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 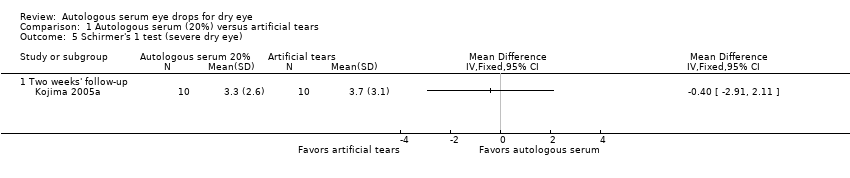 Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 5 Schirmer's 1 test (severe dry eye). | ||||
| 5.1 Two weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rose Bengal (post‐LASIK dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 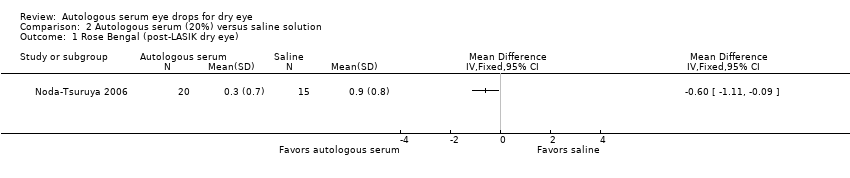 Comparison 2 Autologous serum (20%) versus saline solution, Outcome 1 Rose Bengal (post‐LASIK dry eye). | ||||
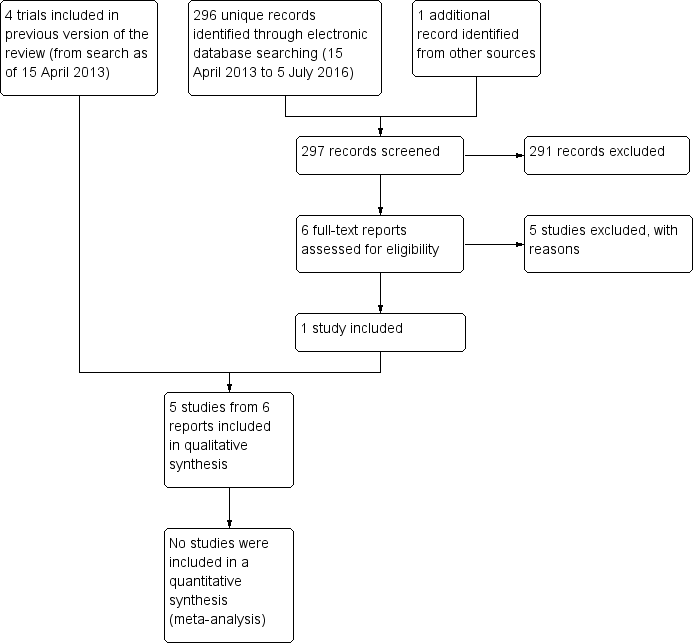
Results obtained by searching for studies for inclusion in the review.

Methodological quality summary: risk of bias review authors' judgements about each risk of bias item for each included study.
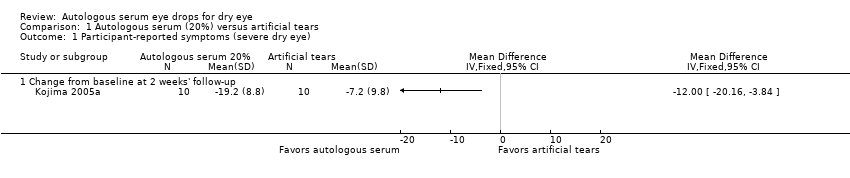
Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 1 Participant‐reported symptoms (severe dry eye).
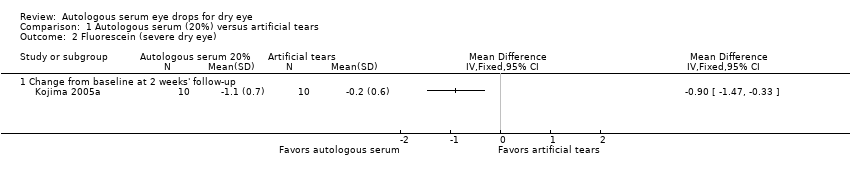
Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 2 Fluorescein (severe dry eye).

Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 3 Rose Bengal (severe dry eye).

Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 4 TBUT (severe dry eye).

Comparison 1 Autologous serum (20%) versus artificial tears, Outcome 5 Schirmer's 1 test (severe dry eye).

Comparison 2 Autologous serum (20%) versus saline solution, Outcome 1 Rose Bengal (post‐LASIK dry eye).
| Autologous serum compared with artificial tears for dry eye | ||||||
| Patient or population: participants with dry eye Settings: eye clinics Intervention: autologous serum 20% Comparison: artificial tears | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Artificial tears | Autologous serum | |||||
| Participant‐reported symptoms Range of scale: 0‐100, where a higher score is worse Follow‐up: 2‐4 weeks | Mean change in symptom score in the control group was 7.2 point improvement | Mean change in symptom score in the autologous serum group was 12.0 points more improved (20.16 to 3.84 more improved) | 20 | ⊕⊕⊝⊝ | Trial investigators of 2 other studies reported more symptomatic improvement in the autologous serum group than in the artificial tears group; however, studies used a cross‐over design and did not provide sufficient data for comparison of treatments between groups | |
| Tear hyperosmolarity Follow‐up: 2‐4 weeks | Not reported | |||||
| Fluorescein staining Range of scale: 0‐9, where a higher score is worse Follow‐up: 2‐4 weeks | Mean change in fluorescein score in the control group was 0.2 point improvement | Mean change in fluorescein score in the autologous serum group was 0.9 points more improved (1.47 to 0.33 more improved) | 20 | ⊕⊕⊝⊝ | Trial investigators of 2 other studies reported a non‐significant difference in Oxford Scale scores; however, studies used a cross‐over design and did not provide sufficient data for comparison of treatments between groups | |
| Rose Bengal staining Range of scale: 0‐9, where a higher score is worse Follow‐up: 2‐4 weeks | Mean change in Rose Bengal score in the control group was 0.1 point improvement | Mean change in Rose Bengal score in the autologous serum group was 2.2 points more improved (2.73 to 1.67 more improved) | 20 | ⊕⊕⊝⊝ | Trial investigators of 2 other studies did not report data for this outcome | |
| Tear film break‐up time Follow‐up: 2‐4 weeks | Mean change in tear film break‐up time in the control group was 0.1 seconds | Mean change in tear film break‐up time in the autologous serum group was 2.00 seconds longer (0.99 to 3.01 longer) | 20 | ⊕⊕⊝⊝ | Trial investigators of 2 other studies reported the difference in TBUT between groups as 1 and 2 seconds; however, studies used a cross‐over study design and did not provide sufficient data for comparison of treatments between groups | |
| Schirmer’s test Score < 4 mm indicates severe dry eye Follow‐up: 2‐4 weeks | Mean Schirmer’s test score in the control group was 3.7 mm | Mean Schirmer’s test score in the autologous serum group was | 20 | ⊕⊕⊝⊝ | Trial investigators of 1 other study reported no difference in Schirmer’s test scores between groups; however, the study used a cross‐over design and did not provide sufficient data for comparison of treatments between groups | |
| Adverse events | Not reported | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded (‐1) for imprecision (wide confidence intervals) | ||||||
| Autologous serum compared with saline for dry eye | ||||||
| Patient or population: participants with dry eye Settings: eye clinics Intervention: autologous serum 20% Comparison: saline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Saline | Autologous serum | |||||
| Participant‐reported symptoms Follow‐up: 2‐4 weeks | See comment | Trial investigators of 2 studies reported no difference in symptom scores between groups; however, studies did not provide sufficient data for comparison of treatments between groups | ||||
| Tear hyperosmolarity Follow‐up: 2‐4 weeks | Not reported | |||||
| Fluorescein staining Range of scale: 0‐9, where a higher score is worse Follow‐up: 2‐4 weeks | See comment | Trial investigators of 2 studies reported no difference in fluorescein staining scores between groups; however, studies did not provide sufficient data for comparison of treatments between groups | ||||
| Rose Bengal staining Range of scale: 0‐9, where a higher score is worse Follow‐up: 2‐4 weeks | Mean Rose Bengal score in the control group was 0.9 points | Mean Rose Bengal score in the autologous serum group was 0.60 points lower (1.11 to 0.09 lower) | 35 | ⊕⊝⊝⊝ | Trial investigators of 1 other study reported no difference in Rose Bengal staining scores between groups; however, the study did not provide sufficient data for comparison of treatments between groups | |
| Tear film break‐up time Follow‐up: 2‐4 weeks | See comments | Trial investigators of 1 study reported no difference in tear film break‐up time between groups; however, the study did not provide sufficient data for comparison of treatments between groups | ||||
| Schirmer’s test Score < 4 mm indicates severe dry eye Follow‐up: 2‐4 weeks | See comments | Trial investigators of 1 study reported no difference in Schirmer's test scores between groups; however, the study did not provide sufficient data for comparison of treatments between groups | ||||
| Adverse events | See comments | One study reported that 2 of 12 participants had signs of conjunctivitis with negative culture; in both cases, symptoms resolved later with proper treatment. It was not stated whether affected eyes were assigned to the AS group or the control group | ||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded (‐3) for high or unclear risk of selection, performance, detection, and reporting bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant‐reported symptoms (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fluorescein (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Rose Bengal (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 TBUT (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Change from baseline at 2 weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Schirmer's 1 test (severe dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Two weeks' follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rose Bengal (post‐LASIK dry eye) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

